Peptides-modified polystyrene-based polymers as high-performance substrates for the growth and propagation of human embryonic stem cells
Fen Yng,D Zhng,Qunming Zhou,Mengchu Li,Chengling Xie,Shoyun Li,Xun Wng,Wei Wng,Ying Guo,Qici Xio,∗,Yong Wng,Liqin Go,∗
a School of Pharmaceutical Sciences (Shenzhen),Sun Yat-sen University,Shenzhen 518107,China
b Institute of Bioengineering and Bioimaging,Singapore 138669,Singapore
Keywords:Extracellular matrix Stem cell Peptide Coverslip Polystyrene
ABSTRACT As human stem cells with the special pluripotency play important roles in the innovative drug discovery and regenerative medicine,development of extracellular matrix (ECM) mimetics or functional materials that can support stem cell growth and propagation is of high significance.Despite numerous efforts spent,one major limitation restricting the wide applications of stem cells to the clinical translation is the lack of efficient strategies for low cost and large-scale stem cell production under xeno-free culture conditions.Herein,we reported a new strategy with peptides-modified polystyrene-based polymers coated onto the surface of coverslips for the growth and reproduction of human embryonic stem cells (hESCs).The modified peptides are the active parts of proteins which has been shown to contribute to the pluripotent stem cell attachment or proliferation.The peptides were linked to the glass coverslips coated by the polymer materials via chemical crosslinking,and the composite substrates successfully maintain the longterm growth of HUES-7,H7 and DF699.Our study shows that the coating of polystyrene-derived polymer modified by our developed peptides is a good matrix for long-term growth and reproduction of stem cells.This polystyrene-derived polymer substrate can be produced in large scale and stored for a long time.The most important thing is that it can support the growth of undifferentiated human pluripotent stem cells (hPSCs) for more than ten passages,which could provide a new and relatively easy way to amplify hESCs in vitro.
It is strongly believed that maintaing the pluripotency in human stem cells and directing their controlled differentiation is quite important [1,2].In particular,the human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs),which are believed to have the potential to indefinitely grow in the culture medium and selectively differentiate into any type of cells in the adult human body represent important resources in regenerative medicine and disease modeling,aiming to reproduce the disease pathogenesis of patients in the laboratory [3–5].Therefore,growing stem cells in an undifferentiated state is important for large-scale applications [6,7].Traditional methods for culturing hiPSCs normally include growing the cells on a feeder cell layer of mitotically inactivated mouse embryonic fibroblast,or in the medium containing fetal bovine serum (FBS) or serum replacement to provide an extracellular matrix (ECM)-rich environment for cell adhesion [8–14].Feeder cells can be avoided by culturing hESCs on Matrigel,a basement membrane material prepared from mouse sarcoma,human serum or purified ECM proteins [10,15].However,most of these biological materials are expensive to manufacture,which may also have high batch-to-batch variability,thus likely to restricting them from broad applications [16–18].Besides,animalderived materials must be subject to expensive tests to ensure that they are pathogens-free and non-immunogenic [16,17].Consequently,great efforts have been spent in developing newly synthetic materials as good substrates for long-term growth and proliferation of pluripotent stem cells.Polymer-based materials have been recently paid more and more attention because they are widely applied as carriers for the delivery of targeted drugs [19–21].Besides,peptides-based biological materials have also played important roles in lots of biomedical applications [22–27].The growth of H1,H7 and H9 hESCs can be passed for 10-25 generations when the polymer materials were coated on cell culture dishes [17,28].Modification of cell culture matrix,such as treatment of substrates under ultraviolet (UV) light or conjugation with template peptides,can also promote the growth and differentiation of stem cells [29–31].
In addition,the active peptides of self-assembling proteins are not only used as high-performance substrates for embryonic stem cell self-renewal [32],but also widely used in many other biomedical fields [33–39].Up to now,hundreds of polymers have been synthesized to support the cell growth [29,40–45];however,most of these materials are only applicable on the cell culture plates or dishes,which can not meet the requirements for the large-scalein vitroculture of human pluripotent stem cells (hPSCs).In this study,we modified the cell culture plate by initializing polymerization on the surface of the plate.Polystyrene (PS) derived polymers,including poly(vinyl benzoic acid-co-styrene) (P(VBA-co-St))and poly(vinyl benzoic acid-co-vinyl benzyl methoxy triethylene glycol ether) (P(VBA-co-mTEGSt)),were synthesizedvianitroxidemediated polymerization (NMP).The polymeric substrates for cell culture were sieved from P(VBA-co-St) and P(VBA-co-mTEGSt) with different monomer ratios.NMP plays an important role in synthesizing polymers with three characteristics,including narrow polymer dispersity index (PDI),well-controlled molecular weight and exact monomer ratio [46–49].Of note,the copolymer,P(VBA-co-St),coated on a glass coverslip substrate,can be used for stem cell growth.The short peptides representing different protein active fragments were linked to the polymer-coated coverslips by chemical crosslinking,and subsequently followed by functionalization on the polymer-coated coverslip.By screening twenty active peptides,three suitable peptides for hESCs growth were obtained and characterized,and the peptides were successfully incorporated to the polymers.Biological characteristics such as high-level expression of stem cell (H7,HUES-7 and DF699) marker proteins,Nanog,Oct3/4,Sox2 and Tra160,gene expression and karyotype analysis after more than 10 passages indicate that this material is suitable the growth and proliferation of hESCs.Importantly,this PS-derived polymer substrate can be produced in large scale,and it can support the growth of undifferentiated hPSCs for a long time,which could provide a new and relatively easy way to amplify hESCsin vitrofor PSC-based therapies.
As shown in Scheme 1a,the chemical structures of the two polymers were synthesized for coverslip coating.Among the chemical structures,vinyl benzoic acids with carboxylic acid groups were used for peptide conjugation and glasses surface coating.Styrene and 4-vinyl benzyl methoxy triethylene glycol ether(mTEGSt) could be used to adjust the hydrophilicity of coated coverslips because hydrophilicity is one of the important factors to affect the stem cell attachment [28].As shown in Tables S1 and S2 (Supporting information),various types of polymers including P(VBA-co-St) and P(VBA-co-mTEGSt) with different monomer ratios were synthesized for tests.For P(VBA-co-St),the ratios of VBA units in the polymers were obtainedvia1H-NMR ranging from 100% to 35.3% while the ratios of the styrene units changed from 0% to 64.7% accordingly (Table S1).To investigate the hydrophilicity of coated coverslips in a broader range,styrene was replaced by mTEGSt monomer to further improve the hydrophilicity.During the process of synthesizing polymers,the ratios of the mTEGSt units could range from 20.7% to 73.1% while VBA changed from 79.3% to 26.9% (Table S2).These polymers were applied to be coated onto the coverslips for screening in stem cell culture.
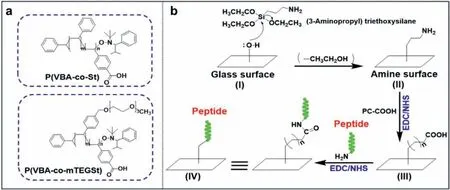
Scheme 1.(a) Chemical structures of the copolymers;(b) Representative scheme of the polymers coated onto the coverslips [PC-COOH: P(VBA-co-St) or P(VBA-co-mTEGSt),EDC: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride,NHS: N-hydroxysuccinimide].
The whole process of the coverslip coating involved three steps:the first step was to activate the coverslip by (3-aminopropyl) triethoxysilane (APTES);the second step was to coat the coverslip with a polymer;in the third step,peptides were used to modify the surface of the polymer-coated coverslip (Scheme 1b).Contact angle measurements,Fourier transform infrared (FTIR) spectroscopy,X-ray photoelectron spectroscopy (XPS),atomic force microscope (AFM) and toluidine blue O (TBO) staining experiments were performed to characterize each step to ensure the successful coating.The contact angle measurement of the water can straightly illustrate the change of hydrophilic ability before and after coating.The contact angles of coverslip after coating with P(VBA-co-St) and P(VBA-co-mTEGSt) were shown in Fig.1.The results showed that the hydrophobicity of the surface coated by the polymers increased compared to the control coverslips,of which the water contact angle is around 50o.For P(VBA-co-St) coating coverslips,the contact angle changed in a broad range from 60oto 80o.In addition,the contact angle increased with the ratios of the styrene in the polymers increasing (Fig.1a).However,for P(VBA-co-mTEGSt),the contact angle changed in a relatively narrow range from around 55oto 60o,which may be due to the similar polarity of mTEGSt and VBA(Fig.1b).According to the study previously reported,the optimal contact angle for stem cell attachment is around 70o[50].Therefore,it can be suggested that the most optimal materials were P(VBA-co-St) together with ratio of the VBA:St either 50%:50% or 30%:70%.
To investigate whether polymers have been successfully coated onto the coverslips,FTIR experiment was adopted here.As shown in Fig.1c,the results showed that,for the coverslips coated by two series of polymers synthesized,the peaks for carbonyl stretching vibration clearly appeared,while no peaks in the control coverslips.These results unambiguously confirmed that the polymers have been successfully coated onto the coverslips.To detect the element changes during the coating process,XPS experiments were carried out.As shown in Fig.1d and Table S3 (Supporting information),our results showed that control coverslips did not contain any nitrogen elements.However,after coating by APTES,there were around 7.56% nitrogen atoms.In addition,when coated by polymers and peptides,the nitrogen elements slightly decreased because parts of nitrogen atoms from APTES were probably covered by polymers and peptides.
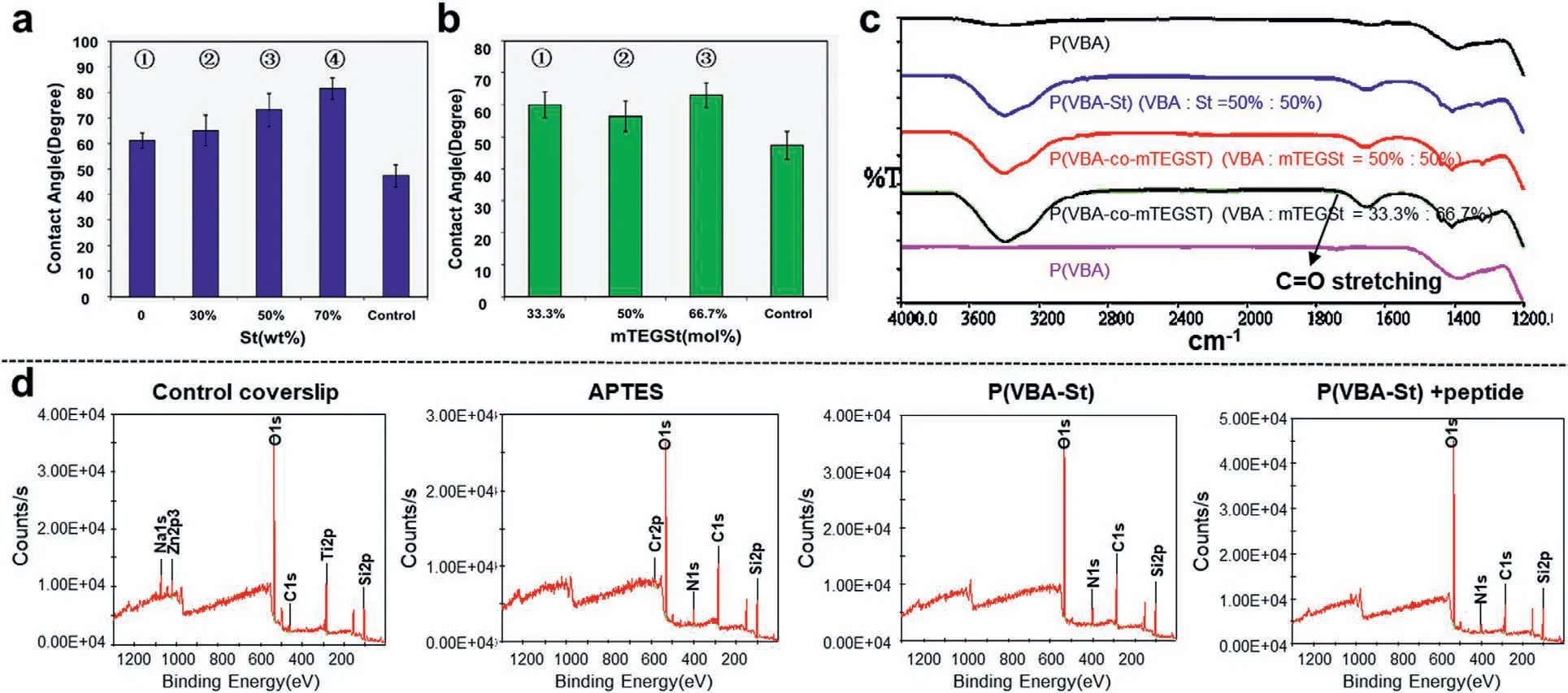
Fig.1.Contact angles of the coverslips after coating by the copolymers.(a) VBA:St (wt%): 1○=100%:0,2○=70%:30%,3○=50%:50%,4○=30%:70%.Data are presented as mean ± standard deviation (SD), n=3;(b) VBA:mTEGSt (mol%): 1○=66.7%:33.3%;2○=50%:50%;3○=33.3%:66.7%.Data are presented as mean ± SD, n=3;(c) FTIR spectra of the coverslips coated by the copolymers of P(VBA-co-St) and P(VBA-co-mTEGSt);(d) XPS spectra of the coverslips at different steps of coating.
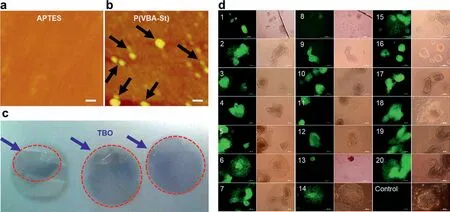
Fig.2.(a) AFM images of coverslip coated with APTES,Scale bar=10 μm;(b) AFM images of coverslip coated with P(VBA-co-St) further,Scale bar=10 μm;(c) P(VBA-St)coated coverslip stained by Toluidine Blue O (TBO) dye;(d) Fluorescence of BG0V1 cells on peptide-conjugated polymer P(VBA-co-St) (VBA:St (wt%)=50%:50%) at day 6 for peptides 1–20 and control P(VBA-co-St).Scale bar=200 μm.
To further confirm whether the polymers have been coated onto coverslips,AFM experiments were performed to directly characterize the topographic changes before and after coatings by the polymers.It was found that,after coating by APTES,there was no obvious difference compared to control coverslips (Fig.2a).However,after coating by the polymers,a coating layer with thickness around 5 nm was clearly observed (Fig.2b),thus clearly indicating that the transparent coverslips have been coated by a thin layer of polymers,which can not be observed by naked eyes.To quantitatively detect the amounts of carboxylic acids,TBO staining,as one of the suitable detection methods,can be adopted because TBO can be equivalent moles to the carboxylic groups and gave blue color staining on the surface containing carboxylic acids.As shown in Fig.2c,the stained coverslips coated by P(VBA-co-St)showed blue color,unambiguously indicating that there were numerous carboxylic acid groups on the coverslips.The amounts of carboxylic acid groups quantitatively measured on coverslips were(1.2 ± 0.1) × 10−3μmol per coverslip.
To screen and identify the optimal polymers and peptides for stem cell culture,BG0V1,one of hESC lines containing green fluorescence protein (GFP) expressing gene and driven by the Oct3/4 promoter,was used to evaluate the studied materials.As shown in Tables S1,S2 and S4 (Supporting information),the polymers screening data and the peptides used for polymer conjugation and screening were summarized.By virtue of the expertise in highthroughput screening [51,52],we constructed a combinatorial peptide library resulting from the active part of proteins,which have been shown to contribute to the attachment or proliferation for pluripotent stem cells.After the peptides were conjugated to the copolymers at N-terminus,screened The BG0V1 cells were used for the screening to test these conjugatesviathe green fluorescence of the GFP,which could indicate that the hESCs were maintained in their pluripotency state.Due to its suitable contact angle,the polymer of the P(VBA-co-St) with the ratio of 50%:50%(VBA:St,wt%) was chosen tentatively as the polymer substrate to modify with twenty peptides listed in Table S4,respectively.The qualitative analysis of cell growth and GFP expression for twenty peptides were shown in Table S4.Basically,after 24 h of seeding,hESCs could be adhered to the surface of the coverslips coated by P(VBA-co-St) (VBA:St (wt%),50%:50%) conjugated with different peptides (Table S4 and Fig.S4 in Supporting information).It was also observed that the control of P(VBA-co-St) itself was a very good substrate for hESCs adherence.However,the hESCs began to differentiate or differentiated severely,which was supported by the very weak expression of GFP fluorescence after continuous culture of cells onto the surfaces of coverslips coated by some peptides for up to 6 days,which were shown in Fig.2d.Specifically,for the control P(VBA-co-St) substrate without any peptides,the hESCs almost completely differentiated after 5 days.This indicated that these 20 peptides helped at a different extent to maintain the pluripotency of the stem cells.
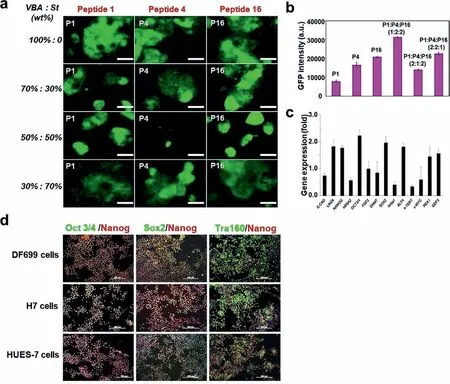
Fig.3.(a) Fluorescence and attachment of BG0V1 cells on P(VBA-co-St) coated coverslip conjugated with P1,P4 and P16 respectively on Day 6.(b) Fluorescent intensity of BG0V1 cells on P(VBA-co-mTEGSt) coated coverslip conjugated with combined P1,P4 and P16 of different ratio on Day 6.Data are presented as mean ± SD, n=3.(c)Pluripotent gene expression analysis of HUES-7 cells grown for 10 passages on peptide coated polymer surface.The fold difference was calculated with the expression value in Matrigel as one fold.The expression values were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH).Data are presented as mean ± SD, n=3.(d)Stained Nanog,Oct3/4,Sox2,Tra160 of DF699 cells,H7 cell and HUES-7 cells after 10 passages.Scale bar=200 μm.
To identify which peptide/peptides could greatly maintain the pluripotency of the stem cells,the image results of the stem cells grown on the coverslips with different substrates from Day 1 to Day 6 were shown in Fig.2d and Fig.S4 as the fate of the stem cells cultured on different substrates began to differentiate on Day 6.Based on the image results,it clearly showed that our stem cells could be efficiently attached to the surface of coverslips except peptides 8 and 13,which did not significantly promote the attachment of stem cells.More interestingly,peptides 2,12,14,17 and 19 not only showed a very good ability for stem cell attachment and proliferation,but also showed a compromised ability of maintaining the pluripotency of the stem cells.Besides,peptides 6,8 and 10 were quite powerful to maintain the pluripotency of the stem cells,but weak in promoting cell growth.To our surprise,peptide 1 (GRGDSP{propargyl-Gly}),peptide 4 (YAVTGRGDSPASSKPIA)and peptide 16 (GRGESPK) showed the best ability for both cell attachment and pluripotency maintenance among these peptides.Therefore,peptides 1,4 and 16 were chosen for the further studies together with the copolymers of P(VBA-co-St) and P(VBA-comTEGSt).
To further optimize the polymers to maintain the pluripotency and proliferation of the stem cells,the polymers entitled P(VBAco-St) and P(VBA-co-mTEGSt) with different monomer ratios were conjugated with peptides 1,4 and 16,respectively.As the conjugation,BG0V1 stem cells were grown on different monomer ratios of P(VBA-co-St) and P(VBA-co-mTEGSt).As shown in Fig.3a and Fig.S5 (Supporting information),the results showed that P(VBA-co-St)promoted stem cell proliferation better than P(VBA-co-mTEGSt) although cell attachment was effective for all of them.After carefully comparing the growth and GFP expression of BG0V1 on P(VBA-co-St) with four of different monomer ratios on Day 6,P(VBA-co-St) of VBA:St (wt%,50%:50%) was chosen for further studies,because it proved to be the best substrate for cell growth and GFP expression in all ratios.Based on the aforementioned studies,P(VBA-co-St) of VBA:St (wt%,50%:50%) was modified by the combined peptides 1,4 and 16 to obtain the optimal results.The P(VBA-St) was conjugated by different ratios of peptides 1,4 and 16.To optimize the ratio of the polymers modified by the peptides,the quantitative analysis of the GFP intensity was carried out for hESCs cultured on substrates modified by combined peptides.As shown in Fig.3b,the results indicated that the mass ratio of P1:P4:P16=1:2:2 exhibited the highest GFP intensity.Therefore,P(VBA-co-St) materials with VBA:St (wt%,50%:50%) and peptides 1,4 and 16 with mass conjugation ratio of 1:2:2 were chosen for extensive studies with various stem cell lines and long-term propagation.Two human embryonic stem cell lines (HUES-7 and H7) and one hiPSC line (DF699)were used for long-term propagation studies.Typically,ten passages were considered to be long-term culture.After 10 passages,the typical pluripotency markers such as Nanog,Oct3/4,Sox2 and Tra160 were analyzed either by immunostaining or FACS (antibody information shown in Table S5 in Supporting information).
As shown in Fig.3d,the immunostaining results showed that,after 10 passages,hESCs lines (HUES-7 and H7) and the hiPSC line(DF699) were non-ignorable positive for the pluripotency marker proteins,indicating that the stemness of the stem cells was well maintained.Quantitatively,the percentage of pluripotency maintenance was studied by flow cytometry with Nanog and Oct3/4 antibodies.As shown in Fig.S6 (Supporting information),the results showed that more than 90% of the cells (HUES-7,H7 and DF699)were noted to be positive for these marker proteins.To further confirm and evaluate the ability of the pluripotency maintenance by our developed materials,the gene expression analysis experiments were adopted.As shown in Fig.3c,it suggested that the expression of pluripotent markers including Lin28,Nanog,Oct3/4,Sox2,KLF4,Rex1 and GDF3 were higher than the expression level in Matrigel,while some other genes such as E-Cad,NR5A2,Nr6a1,h-Tert and c-MYC were comparatively lower than Matrigel.Our results unambiguously indicated that we have successfully optimized the ratios of polymers and peptides coated onto the surfaces of the coverslips for long-term stem cell growth and propagation.
In this study,we have successfully constructed peptidesconjugated-polymers for coating the surface of the coverslips for long-term stem cell growth and propagation.To optimize the peptide sequences for the conjugation with the polymers to be coated onto the surface of the coverslips,we constructed a combinatorial peptide library for the stem cell screening,which resulted from the active parts of proteins beneficial to the attachment and proliferation for pluripotent stem cells.Based on the screening,three peptides were successfully identified including peptides 1 (with sequence GRGDSP{propargyl-Gly}),4 (with sequence YAVTGRGDSPASSKPIA) and 16 (with sequence GRGESPK).To optimize the polymers for the stem cell growth and proliferation,various types of polymers including P(VBA-co-St) and P(VBAco-mTEGSt) with different monomer ratios were synthesized for screening tests,and the results showed that P(VBA-co-St) with the ratio of VBA:St (wt%,50%:50%),conjugated by selected peptides(P1:P4:P16=1:2:2,mass ratio),can be suitable substrates for stem cell growth and propagation to coat the surface of coverslips for stem cell culture.More interestingly,peptides-conjugated polymers coating coverslips could sustain the growth of both hESCs (such as HUES-7 and H7) and hiPSC (such as DF699) for more than 10 passages without differentiation.This polystyrene-derived polymer substrate can be produced in large scale and stored for a long time.The most important thing is that it can support the growth of undifferentiated hPSCs for a long time,which could provide a new and relatively easy way to amplify hESCsin vitroin the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21877130 and U1801681),The Key Field Research and Development Program of Guangdong Province (No.2019B020235001),Basic and Applied Basic Research Foundation of Guangdong Province (No.2019A1515110451),Science and Technology Program of Guangzhou (No.201803010009) and Natural Science Foundation of Guangdong Province (Nos.2018A030313600,2021A1515010357).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.028.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
