Microfluidics-based technologies for the analysis of extracellular vesicles at the single-cell level and single-vesicle level
Fengjio Zhu,Yhui Ji,Jiu Deng,Linmei Li,Xue Bi,Xinming Liu,Bingcheng Lin,Yo Lu,∗
a Department of Biotechnology,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
b University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:Microfluidics Single-cell EV analysis Single EV analysis Extracellular vesicle Heterogeneity
ABSTRACT Extracellular vesicles (EVs) are membrane vesicles secreted by cells,playing critical roles in mediating intercellular communications for various physiological and pathological processes.Most of the EV analysis is currently performed at the bulk level,obscuring the origin of the EVs and diverse characteristics of the individual extracellular vesicle.Technologies to analyze the extracellular vesicles at the single-cell and single-vesicle levels are needed to evaluate EV comprehensively and decode the heterogeneity underlying EV secretion.Microfluidic platforms that could control and manipulate fluids at the microscale provide an efficient way to achieve the aims.Various microfluidics-based technologies are emerging to realize single-cell EV secretion analysis and single EV analysis,which would be summarized in this mini-review.
1.Introduction
Extracellular vesicles (EVs),including exosomes (30–200 nm),microvesicles (MVs,200–1000 nm),and apoptotic bodies(>1000 nm),are cell-derived membrane vesicles that are abundant and stable in various body fluids.EVs could carry a variety of biological molecules from their parental cells,including proteins[1–2],microRNA (miRNA),RNA [3]and DNA [4],to mediate cellto-cell communication in a range of physiological and pathological contexts,e.g.,immunological system,tumor microenvironment.For example,programmed death ligand-1 (PD-L1) on the surface of EV secreted by tumor cells can bind to programmed cell death protein-1 (PD1) on T cells to help tumor cells’immune evasion [5].Cell-derived EVs could also provide a non-invasive way to obtain molecular information of their parental cells,making EV a potential biomarker for clinical diagnosis [6–12].For example,exosomal folate receptor alpha could potentially be used as a biomarker for early detection and progression monitoring of ovarian cancer[9].Prostate cancer patients had an increased level of the SUM signature (sum of the levels of seven individual markers of EVs,including cluster of differentiation 63,protein tyrosine kinase 7,epithelial cell adhesion molecule,liver cancer membrane antigen targeted by LZH8 aptamer sequence,human epidermal growth factor receptor 2;prostate-specific antigen,cancer antigen 125) of EVs compared with patients with benign disease and the value of the SUM signature of EVs recurrence cancer patients was relative higher to the no recurrence group [10].
Various methods have been developed to characterize the molecular components of EVs for a better understanding of EV and its related functions,including surface proteins,miRNAs,mRNAs,which have been well summarized in recent reviews [13–17].However,heterogeneity is always present between cells.EV secretion from cells is not an exception.For example,EVs show significant diversity in size,morphology,and molecular compositions.The heterogeneity underlying EV secretion could be categorized into two ways: EV itself and cell of origin.Bulk measurements with mixed EV phenotypes from population cells could mask the distinct characteristics of individual vesicles or individual cells of origin.Thus,profiling EV at single cell or single EV resolution is necessary to characterize EV comprehensively to uncover the heterogeneity averaged in bulk analysis.
Microfluidics,a miniaturized “lab on a chip”,can precisely control fluids and particles in dimensions ranging from picoliter to nanoliter [18].Microfluidics has advantages in high throughput,minimal sample consumption,rapid sample processing,and increased analysis sensitivity,making significant contributions to biology and clinical research.These advantages could help compartment extracellular vesicle samples into single-cell or single-EV level resolution,reducing sample consumption and improving throughput and sensitivity.Microfluidics could also provide automated and integrated platforms to reduce labor and costs requirements.A variety of microfluidic devices have been developed for the analysis of extracellular vesicles at the single-cell level and single-vesicle level,including microwell/chamber-based microfluidics [19–23],microchannel-based microfluidic [24,25],and droplet-based microfluidic [26,27].This review would summarize the recent development of microfluidics-based technologies to analyze EVs at the single-cell and single-vesicle levels,discuss the advantages and limitations of these methods,and provide our perspectives for the field.
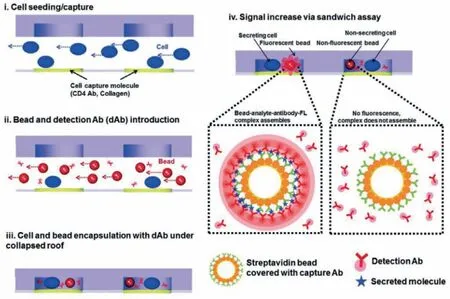
Fig.1.A reconfigurable microwell array captures and encapsulates single cells and sensing beads for dynamic monitoring of single-secreted EVs.Reproduced with permission[22].Copyright 2016,Royal Society of Chemistry.
2.Analysis of EV at the single-cell level
Profiling EV information from single cells provides a direct way to evaluate the cell heterogeneity in their parental cells.However,single-cell resolution EV analysis is challenging since the amount of EVs secreted by single cells is limited and could diffuse rapidly.Therefore,trapping single cells in a confined environment is needed to isolate the secretion information from individual cells and prohibit secretion diffusion.Besides,the relative EV secretome concentration could also be improved significantly in the nanoliter volume confined space,realizing high-sensitivity detection.Various microfluidic platforms,including microwell,micromesh,and microchamber,have been used for single-cell EV assay,which would be summarized below.
2.1.Microwell
Microwell is the most widely used microfluidic platform that can be easily adapted to trap single cells for various downstream assays,including imaging [28,29],epigenome [20,21],genome [22–24],transcriptome [25,26],proteome [27,28],metabolome [29,30]and cytokine secretion detection [30,31].Sonet al.[22]developed a reconfigurable microwell device to encapsulate single hepatocellular carcinoma cells to realize single-cell EV analysis.Microbeads functionalized with antibodies against CD63 detection antibodies were used for EV capture within a 20 pL micro-compartment(Fig.1).A fluorescence-based sandwich assay was conducted for dynamic monitoring of single cell-secreted EVs with a fluorescence microscope.Time-lapse images of fluorescence signals on microbeads co-entrapped with cells were acquired at 30 min intervals for a total of 12 h using the fluorescence microscope.The average amount of CD63+EVs secreted by single HepG2 cells was(0.17–0.89) × 102particles in 12 h,according to the titration curve,whose linear range and limit of detection (LOD) is 0-60 ng/mL(R2=0.904) and 3.3 ng/mL,respectively.
2.2.Micromesh
Polydimethylsiloxane (PDMS) micromesh is another commonly used microfluidic platform for single-cell EV analysis.Compared with microwell design,it provides an open interface for facile cell perturbation and manipulation.Due to the open structure design,PDMS mesh can also facilitate massively parallel single-cell transfer for downstream analysis after single-cell EV secretion assay.Caiet al.[19]demonstrated a single-cell translocation and secretion assay (TransSeA).The platform (Fig.2A) consists of three parts:(1) a polyester thin film filter and a PDMS mesh chip for singlecell isolation and culture [20],(2) an antibody-coated glass slide to capture target-specific EV and microwells to collect holistic EV secretion for digital droplet polymerase chain reaction (ddPCR),(3)UV patterned PDMS through-holes to transfer targeted cells to another single-cell culture chip.The platform was used to profile the EV secretion rate and EV miR-21 expressed from five single glioblastoma cells,reveals positive correlations between the EV secretion rate and the miR-21 copy numbers.Moreover,the authors investigated whether the EV secretion rate of a cell is hereditary.By quantifying the CD63+EV secretion rate,the CMK3 single cells were classified as hyposecretory cells or hypersecretory cells.The single cells were cultured for an additional 72 h to generate 2 to 6 progeny cells.Using TransSeA,each of the progeny cells could be translocated from the original cell plate to a new cell plate to culture them individually.The CD63+EV secretion rate was then determined for each progeny cell and compared to the parental rate.The result shows no obvious genetic hereditary trait of EV secretion rate between parental and progeny cells,demonstrating the capabilities of TransSeA in profiling the relations between the behavior and its genealogy.
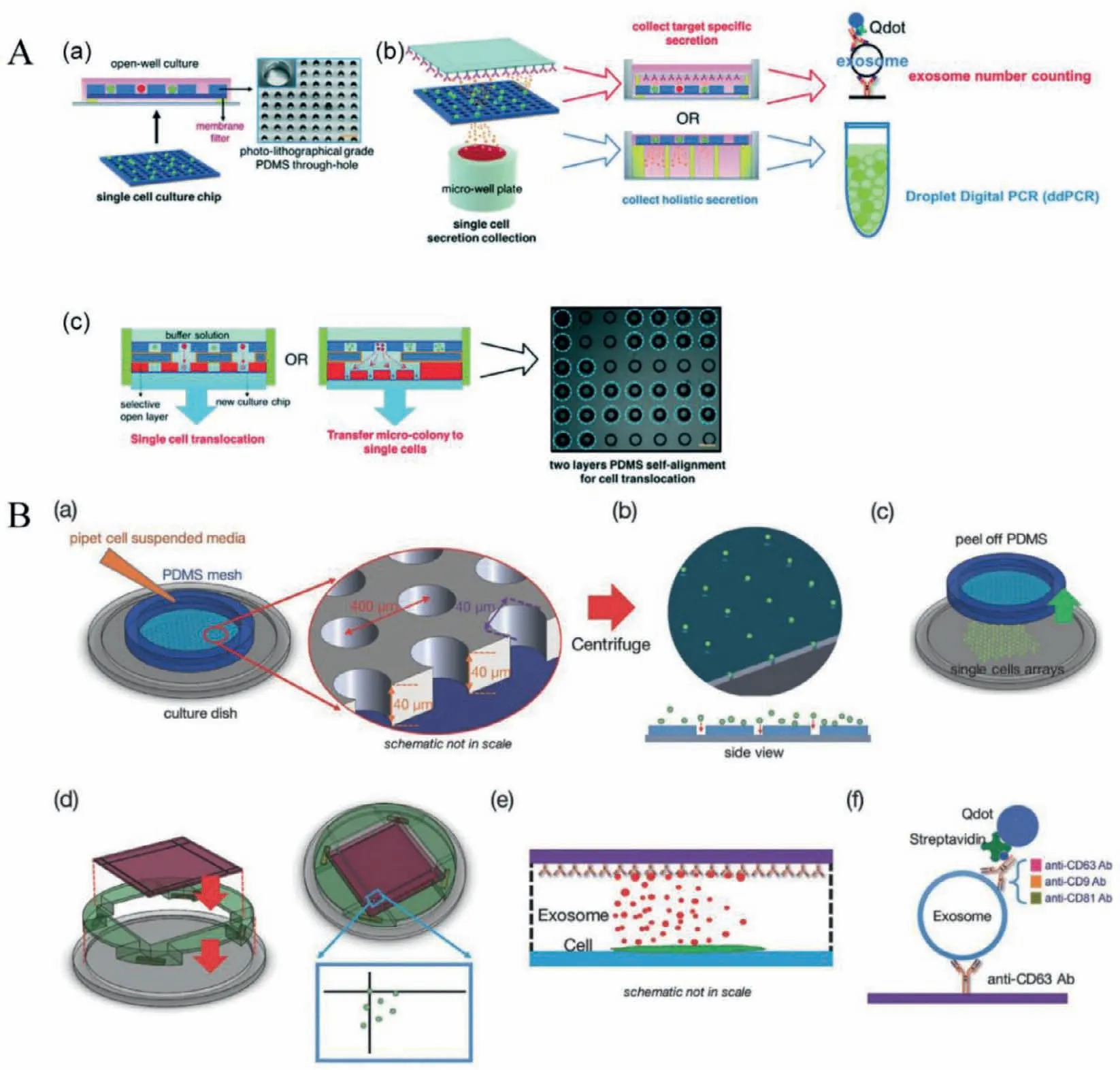
Fig.2.PDMS mesh used for single-cell EV secretion analysis.(A) A single-cell translocation and secretion assay was used for single-cell culture,EV collection,targeted and non-targeted EV detection,and parallel cell transfer.Reproduced with permission [19].Copyright 2018,Royal Society of Chemistry.(B) The platform consists of high throughput PDMS micromesh to guide cell loading and isolate individual cells and an antibody-coated glass to capture EV secretion,including biotinylated CD9,CD63,and CD81 antibodies.Streptavidin labeled quantum dots (Qdots) were bounded with biotinylated antibodies to visualize captured EVs.An inverted fluorescent microscope was used to count quantum dots that reflect the amount of EV secreted by individual cells.Reproduced with permission [20].Copyright 2016,WILEY-VCH.
The highly versatile micromesh single-cell assay (Fig.2B) can be used for time-lapse studies of single-cell EV secretion [20].Authors found the CD63+EV secretion rate of three breast cell lines at single-cell resolution,revealing that the secretion rates of EV of both human breast cancer cell lines MCF-7 and MDA-MB-231 are 60–65 h−1per cell.In comparison,the secretion rate of human non-neoplastic human breast cell line MCF10A is about 170 h−1per cell.They also investigated the secretion rates of CD63+EVs,CD63+CD9+EVs,and CD63+CD81+EVs under different culture conditions such as drug treatments,pH changes and growth factors.
2.3.Multiplexed single-cell EV profiling based on PDMS microchamber
Microwell-based and micromesh-based platforms could be easily designed and modified for single-cell EV analysis.However,they also suffer from limited proteomic parameters for EV analysis from every cell due to the spectral overlap.Thus,they’re not sufficient to dissect EV secretion heterogeneity comprehensively.To address the need,Jiet al.[21]used the spatially patterned antibody barcodes and high throughput microchamber array to realize multiplexed EV secretion with oral squamous cell carcinoma (OSCC) cell lines and primary cells at single-cell resolution (Fig.3A).High throughput elongated-shaped microchambers were designed to capture singlecell and concentrate the concentration of detection targets.A corresponding microchip with a highly parallel microchannel array was used to pattern spatially resolved antibody barcodes onto a poly-L-lysine glass slide for multiplexed profiling.Up to 9 different antibodies can be patterned simultaneously to capture different EV phenotypesin situwithout purifying EVs.Besides,the antibody barcode makes it possible to realize multiplexed detection of EV secretion and cytokines secretion simultaneously from the same single cells to investigate the multidimensional spectrum of cellular communications.They applied the platform to profile human oral squamous cell carcinoma (OSCC) cell lines,which led to previously undifferentiated heterogeneity and structured clusters within the cell population underlying EV secretion (Fig.3B).They also observed the decrement of certain EV phenotypes (e.g.,CD63+EV)were associated with the invasive feature of both OSCC cell lines and primary OSCC cells.
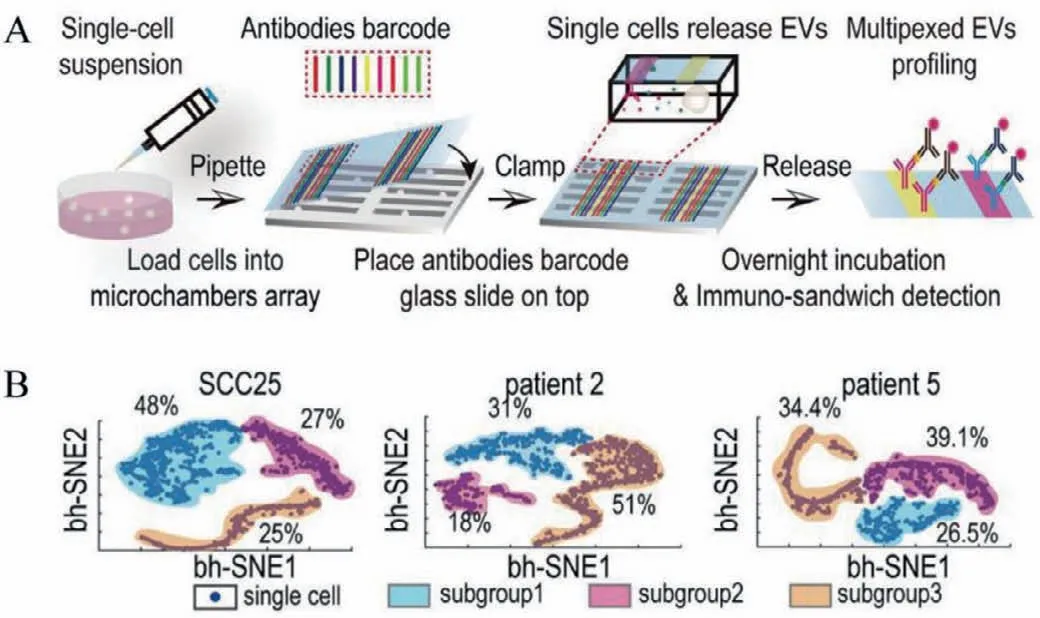
Fig.3.Multiplexed profiling of single-cell EV secretion with microchamber and antibody barcode.(A) Illustration of single-cell EV secretion assay workflow.(B)Tiered functional subgroups with distinct secretion profiles were differentiated in the SCC25 cell line and patient samples.Reproduced with permission [21].Copyright 2019,National Academy of Sciences.
3.Analysis of EV at the single-vesicle level
Another direction to evaluate the heterogeneity associated with EV secretion would focus on the vesicle itself,which shows great diversity in size,shape,and molecular contents.The detailed information would also be averaged when EVs are mixed in the bulk assay,precluding the deep understanding of EV phenotypes[23,25,26,32].Profile EV at single-vesicle resolution is highly challenging owing to their nanometer sizes.Recently,various methods have been developed to profile EV at single-vesicle resolution,including digital methods [25,33],imaging [25,32,34],fluorescent nanoparticle tracking analysis [35,36],flow cytometry [37,38],nano-plasmonic sensor [39,40].This section will focus on the microfluidics-based methods for the analysis of EV at the singlevesicle level.
3.1.Imaging
Imaging is a simple and straightforward way to observe and profile a single EV.Fraser et al.realized single EV analysis by immobilizing biotinylated EVs on a streptavidin-glass slide and labeled with fluorescent antibodies,followed by imaging using the BX-63 Upright Fluorescent Microscope (Olympus) with a 100× oil objective [32].They optimized these experimental steps on a microfluidic chip imaged by an inverted microscope equipped with an sCMOS camera with 20× (Fig.4A) [25].The on-chip analysis allows better control of experimental conditions such as flow rate and staining time compared with off-chip analysis.The biotinylated EVs were immobilized on the microfluidic chamber’s surface and stained with a mixture of fluorescent antibodies.For the subsequent staining round,the prior fluorescence was quenched by quench fluorochromes after acquiring the image.Authors successfully realized multiplex profiling (11 kinds of surface proteins) of individual EV (three at a time) using repeated imaging stain/cycles(46 min per cycle).The average signal to noise of this method is∼3.Based on this multiplexed single EV analysis method,authors profiled EVs derived from the glioblastoma cell line.t-Distributed stochastic neighbor embedding (t-SNE) was used to visualize the EV subgroups based on 11 different protein markers,revealing the underlying heterogeneity of EV.The Single EV analysis technology has the potential application in identifying biomarkers on the surface of EVs.
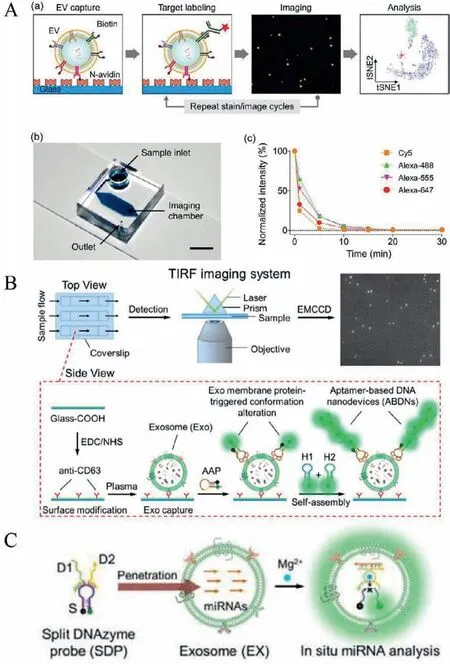
Fig.4.Imaging the single EVs directly with microscopy.(A) Steps for EV imaging and the microfluidic chip used for EV capture.Reproduced with permission [25].Copyright 2018,American Chemical Society.(B) TIRF imaging system was used to quantify single EVs.Reproduced with permission [41].Copyright 2019,American Chemical Society.(C) Illustration of analysis of EV miRNAs by using the SDP.Reproduced with permission [24].Copyright 2019,Hung-Wing He et al.
Heet al.[41]visualized and quantified EVs derived from tumor patients’plasma using total-internal-reflection-fluorescence (TIRF)microscopy (Fig.4B).EVs in plasma were captured by the CD63 antibodies immobilized on the glass coverslip.PTK7+EV were detected by activatable aptamer probes (AAP) and the hybridization chain reaction (HCR).TIRF imaging system was used to capture and quantify single EV images.The authors found that the content of CD63+PTK7+EVs in plasma samples of human acutelymphoblastic leukemia cancer patients is significantly higher than healthy controls,demonstrating the potential application for early disease diagnosis.This platform was also used to quantitative and stoichiometric profile disease-associated EV marker (miRNA-21) at single EV resolution in cell culture medium and clinic serum sample from cancer patients and healthy donors [24].The experimental procedures are similar to those procedures described in Fig.4B.Before the EV was fixed by CD63 antibodies,the Mg2+-dependent split DNAzyme probes (SDP) penetrated EVs,which can hybridize with target miRNAs to produce fluorescence signals with the help of Mg2+(Fig.4C).The fluorescence signals were recorded by TIRF microscopy to quantify the miRNA content of individual EV.The LOD is 378 copies/μL.Authors proved that the contents of miR-21 in tumor cells-derived EVs are significantly higher than in normal cells.In addition,TIRF assay can discriminate cancer patients from healthy subjects by the contents miR-21 in EV.the miR-21 copies in health donors’EVs is lower than cancer patients.Surprisingly,the contents of patients’miR-21 in EV were decreased after good treatment.The heterogeneity of EV hinders their application in clinical medicine.The platform based on TIRF microscopy was used to profile disease-associated EV markers,showing the application potential to monitoring tumor progression in the clinic.However,the imaging methods work only on fixed EVs,which may introduce potential artifacts.
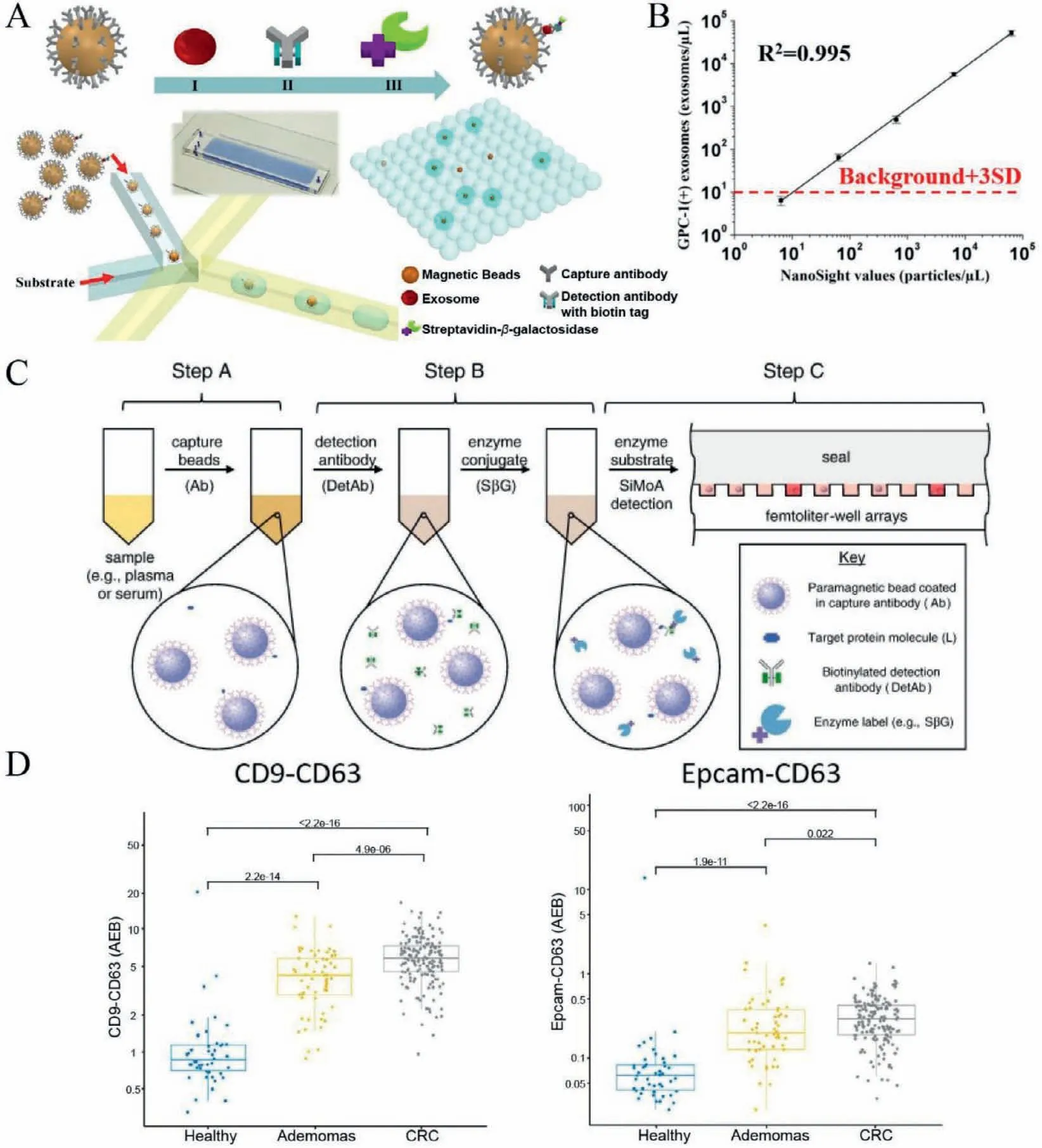
Fig.5.Profiling vesicles at the single-vesicle level based on digital methods.(A) The workflow of droplet digital ExoELISA for the profiling of individual EV.(B) The calibration curve of droplet digital ExoELISA.Reproduced with permission [27].Copyright 2018,American Chemical Society.(C) The workflow of Single-molecule arrays (SiMoA) for single EV analysis.Reproduced with permission [47].Copyright 2012,Elsevier B.V.(D) Analysis of CD9+CD63+ EVs and CD63+ EV from plasma samples (healthy donors and cancer patients).Reproduced with permission [33].Copyright 2020,Dawei Li et al. Published by Informa UK Limited,trading as Taylor &Francis Group on behalf of the International Society for Extracellular Vesicles.
3.2.Digital methods
Digital methods,including digital PCR [42,43]and digital enzyme-linked immunosorbent assay (ELISA) [44–46],are ultrasensitive detection methods that could realize single-molecule counting.Digital methods count the number of fluorescence signals from spatially isolated labeled beads.Digital strategies are usually based on diluting the sample extensively to distribute molecules evenly into tens of thousands of microchambers.Infinite dilution would ensure that the single molecules are dispersed in each chamber and follow the Poisson distribution,enabling the absolute quantitation of the target molecules (nucleic acids or proteins) in a sample.For example,Liuet al.[27]developed a droplet digital microfluidic platform,named droplet digital single-exosome-counting enzyme-linked immunoassay (droplet digital ExoELISA),for absolute counting of cancer-specific EVs.EVs were firstly captured on magnetic microbeads modified with CD63 antibodies (Fig.5A).The captured EV was further labeled with the biotinylated detection antibody and streptavidin-β-galactosidase successively to form an immunocomplex.According to the Poisson distribution,the constructed magnetic beads and enzymatic substrate were encapsulated into 1 million microdroplets to ensure that only one or no magnetic beads were encapsulated in a droplet.The enzymatic substrate was catalyzed byβ-galactosidase to emit fluorescein,and the number of fluorescent droplets correlated with the number of target EVs.Droplet digital ExoELISA could achieve a LOD of 10 enzyme-labeled EV complexes per microliter (∼10−17mol/L) with a dynamic range of 5 logs (Fig.5B).Using droplet digital ExoELISA,the researchers quantified the GPC-1+EV in plasm samples from breast cancer patients and found that the number of GPC-1+EV of breast cancer patients’serum samples was significantly higher than that of the normal and benign breast disease samples,while the number of GPC-1+EV significantly reduced after surgery,showing the potential clinical value of this method.
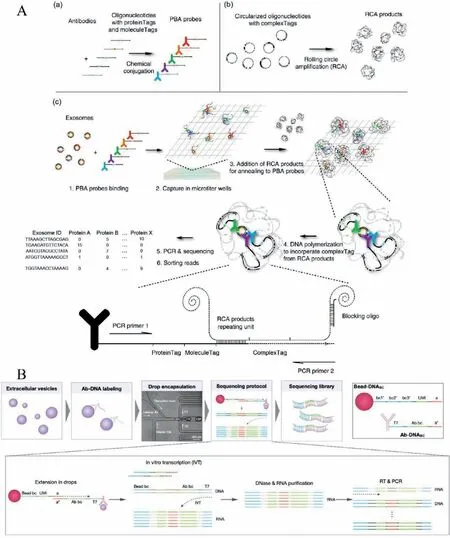
Fig.6.Profiling vesicles at the single-vesicle level based on sequencing methods.(A) The procedures of PBA.Reproduced with permission [23].Copyright 2019,Di Wu et al.(B) The workflow of seiSEQ.Reproduced with permission [26].Copyright 2021,American Chemical Society.
Single-molecule arrays (SiMoA) is an ultrasensitive digital ELISA technology that could detect single protein molecules in complex samples [45–47].The workflow of SiMoA is illustrated in Fig.5C.In brief,protein molecules were captured by a magnetic bead coated with antibodies.Then the captured protein was labeled with a biotinylated detection antibody and an enzyme conjugate.The beads and enzyme-substrate were loaded into 50 fL reaction chambers following the Poisson distribution to isolate and detect single protein molecules.The LOD of SiMoA,which is fully automated,is∼10−15mol/L when detecting clinically relevant proteins in serum.Weiet al.[33]used the SiMoA technology to detect universal EV and tumor-derived EV (T-EV) in cancer cell culture medium and plasma samples at single EV resolution.They found that the number of CD9+CD63+EVs and Epcam+CD63+EVs,the independent prognosis factors of colorectal cancer,in plasma samples of colorectal cancer patients is higher than that in healthy and benign controls (Fig.5D),demonstrating the potential clinical significance.
3.3.Sequencing
The abundance of specific surface proteins in individual single EV is relatively low.Therefore,signal amplification methods are needed to profile surface proteins of the single EV.Unlike PCR,which can amplify the target gene exponentially,there is still no practical way to amplify the protein to date.Transforming protein profiles to DNA sequences followed by sequencing methods would be a viable strategy to amplify and characterize the surface protein of single EVs.Wuet al.[23]demonstrated a proximitydependent barcoding assay (PBA) for profiling the combination and abundance of the surface protein of individual EV utilizing PBA probe conjugation and next-generation sequencing.In PBA,EVs are incubated with PBA probes to form complexes (Fig.6A).The PBA probes compose of antibody and DNA oligonucleotide,containing protein Tag to identify specific proteins and unique molecular identifier (UMI) for counting the number of the particular protein.The EVs complexes were captured by 96-well microtiter wells modified with cholera toxin subunit B (CTB).Then rolling circle amplification (RCA) products containing repeated circularized DNA sequences (complex Tag) were added to wells to interact with a single EV,resulting in the same EV shares the same complex Tag were barcode in each EV.After amplification by PCR and sequencing,the abundance of a specific protein on a single EV can be quantified by counting the molecule Tag.PBA realized the profiling of up to 38 surface proteins of a single EV from human serum and seminal fluid sample and conditioned media of different cell lines as a high throughput method.By t-SNE analysis,EVs from various sources that have specific surface protein combinations were nicely differentiated.
Coupled with single-cell RNA sequencing (scRNAseq),Koet al.[26,48]developed a microfluidic droplet platform for multiplexed profiling surface proteins of EVs based on the antibody immunosequencing method,named single EV immunosequencing (seiSEQ).The procedure is illustrated in Fig.6B [26].Firstly,the EVs were labeled with different antibody conjugated DNA barcodes (Ab-DNABC) that define different proteins to form complexes.Second,Ab-DNABClabeled EVs,and barcoded beads were encapsulated into droplets,as different bead-DNABCmeans different EVs.Through optimizing the size of beads and channel,flow rates,EV input concentration,and drop volume,the authors achieved ∼8.1% of droplets containing both a single EV and a single bead.Third,amplicons were synthesized through multiple extension and amplification steps.The protein combination of individual EV was determined through sequencing of the amplicons.As a proof of concept,the authors profiled eight surface proteins in 1100 EVs and determined the EV protein coexpression levels covered up in bulk analysis.SeiSEQ skillfully converted protein analysis to DNA sequencing,realizing high-throughput multiplexed analysis of proteins on the surface of individual EV,which could be helpful in the field of vesicle biology and clinical applications.
In summary,these techniques based on microfluidic can provide surface and intracellular content information of single EVs,which uncover the underlying heterogeneity of EV.Imaging is a straightforward method to profile single EVs by combing immunology with optics.EVs were immobilized on a surface by immune recognition.Then surface markers and intracellular contents(such as miRNA) are quantified by detecting the fluorescence signal emitted by the labeled EVs.Imaging methods provide singlemolecule information of EVs.However,fluorophore-induced dimerization or photobleaching may produce misleading results.Ultrasensitive digital strategies can achieve absolute quantification of EVs in complex plasma or samples.However,the overlapping of spectra limits the multiplexed profiling of the single EV.Therefore,the methods based on sequencing overcomes the limitation of spectral overlap by tagging surface proteins of EVs using anchoring molecules and transforming protein profiles to DNA sequences to realize high-throughput multiplexed profiling.Compared with direct imaging and the absolute quantification digital method,the sequencing-based analysis method is complex and costly.
4.Conclusion and perspective
Great strides have been made in microfluidics-based technologies for extracellular vesicle analysis at the single-cell [19–22]and single-vesicle levels [8,23–27,32,33,41,48,49],leading to a deep understanding of the heterogeneity associated with EV secretion.However,the research in the field is still at the beginning with limited technologies.Many technical challenges are still waiting to be improved: (1) The throughput and sensitivity of these platforms still have room for improvement to obtain more abundant information.(2) Most current single-cell EV analysis and single EV analysis technologies mainly focus on the heterogeneity in surface proteins,which is not enough to comprehensively evaluate the EVs.New technologies are needed to analyze the molecular compositions inside EVs and surface proteins simultaneously at the single-cell and single-vesicle resolution,including miRNA,mRNA,proteins.(3) Through single-cell EV analysis and single EV analysis,different levels of heterogeneity and different EV phenotypes can be observed.However,the mechanism behind these heterogeneities is unclear,which could be solved by coupling with multiomics analysis technologies or other single-cell analysis technologies to uncover the mystery behind them.(4) The technology to combine single-cell EV analysis and single EV analysis technologies to realize single-cell EV secretion analysis at single EV resolution is still lacking,which could be a promising direction to uncover the EV heterogeneity to a greater extent.(5) The platforms reviewed above show great potential for clinical uses.However,the low availability of these platforms still hinders their broad applications in clinical settings.Current single-cell EV analysis and single EV analysis technologies rely heavily on bulky and expensive instruments.Robust,user-friendly,and low-cost platforms are needed to make them easily accessible and affordable for widespread use.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The project was supported by theNational Natural Science Foundation of China (Nos.21874133,31927802),Youth Innovation Promotion Association CAS (No.2018217),and funds from the Dalian Institute of Chemical Physics,CAS (No.I201908).
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
