Single-cell metabolite analysis on a microfluidic chip
Chenlong Wng,Wnting Hu,Lindi Gun,Xioping Yng,Qionglin Ling,∗
a Center for Synthetic and Systems Biology,Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology,Ministry of Education,Department of Chemistry,Tsinghua University,Beijing 100084,China
b State Key Laboratory of Chemical Oncogenomics,The Graduate School,Tsinghua University,Shenzhen 518055,China
Keywords:Single-cell Metabolomics Microfluidic Cell trapping Cell manipulation Detection
ABSTRACT Metabolites can directly reflect and modulate cell responses and phenotypical changes by influencing energy balances,intercellular signals,and many other cellular functions throughout the lifespan of cells.Taking into account the heterogeneity of cells,single-cell metabolite analysis offers an insight into the functional process within one cell.Microfluidics as a powerful tool has attracted significant interest in the single-cell metabolite analysis field.The microfluidic platform is possible to observe,classify,and stimulate individual cells.It can also transport single-cell to subsequent analysis steps in a fast and controllable way to determine and analyze the composition and content of metabolites.The reviews of topics in microfluidics for single-cell metabolite analysis have been published in the past few years.However,most of them focused on metabolite analysis with mass spectrometry.Here,we covered the advances of microfluidic devices for single-cell metabolite analysis,with a focus on single-cell isolation and manipulation.What is more,we summarized the detection methods and applications of single-cell metabolites.
1.Introduction
Metabolites can directly reflect and modulate cell responses and phenotypical changes by influencing energy balances,intercellular signals,and many other cellular functions throughout the lifespan of cells [1–4].It becomes clear that tissue-scale metabolite,or even population-based metabolite,yields averaged data that can oftentimes be misleading [5].More specifically,dysregulation of one single cell in multicellular organisms can lead to human diseases cancers or neurological disorders [6],which is often ignored in tissue-scale metabolites.Taking into account the heterogeneity of cells,single-cell metabolite analysis offers an insight into the functional process within one cell [7].The ability to detect,identify,and quantify metabolites within one single cell will open new doors to understanding the reason behind cell-to-cell heterogeneity,even within a seemingly homogeneous population [8–10].Therefore,single-cell metabolite analysis has great potential in metabolic pathway discovery,pharmacological research,disease diagnosis,cancer screening,and other fields.
However,single-cell metabolite analysis is difficult to apply because of the large chemical diversity between the molecules,fast metabolic turnover rates,and inability to amplify small-molecule metabolites.The goal of single-cell metabolite analysis is to make a comprehensive qualitative and quantitative analysis of metabolites existing intracellular or extracellular at a defined time and under specific physiological conditions [11].Microfluidic chip is an ideal tool to achieve the goal.It has many advantages like high throughput,low sample consumption,and fast analysis.Microfluidic now has been dramatically developed in many disciplines,such as chemistry [12],physics [13–16],biology [17],and others[18–20].Microfluidics can efficiently isolate and process a large number of single cells in a microdevice.What is more,it brings unique advantages,including significant enhancements in throughput performance and cost-effectiveness,simplification of the workflow,and improvement of assay consistency [2].
Since the first method of manipulating single cells on a microfluidic chip has published in 1997 [21],microfluidic chips have been widely used in single-cell analysis.Kralyet al.firstly systematically summarized applications of microfluidic technology in single-cell metabolite analysis,including micro-separations,sample preparation,and microfluidics for cellular analysis,where five concepts related to metabolites had been defined and explained[22].Nowadays,the vast majority of microfluidic systems have focused on coupling selective separation and detection systems for the determination of biological samples.Several microfluidic systems have been developed that integrate biological systems with a microfluidic system to perform metabolite studies.Furthermore,microfluidic has its unique strengths in single-cell analysis.The dimensions of microfluidics (10–100 μm) are similar to the cell size(3–30 μm),which makes microfluidics a good choice for single-cell analysis.Microfluidics can act as a 3D cell culture matrix to simulate the extracellular environment for long-term single-cell culture.In addition,the microfluidic chip can integrate many units,such as cell culture unit,stimulating unit,cell sorting unit,and cell membranes dissolve unit.Nowadays,a few comprehensive reviews can be found in the literature on microfluidics for single-cell metabolite analysis.However,most of them focused on metabolite analysis with mass spectrometry (MS) [23].Here,we covered the advances of microfluidic devices for single-cell metabolite analysis for the first time,with a focus on single-cell isolation and manipulation.What is more,we summarized the detection methods of singlecell metabolite and their developments (Fig.1).Because single-cell metabolite analysis was in the early stages of research,many researches mentioned in this review were restricted to only a few kinds of metabolite,which was far from omics.However,we believe that it will be achieved shortly.
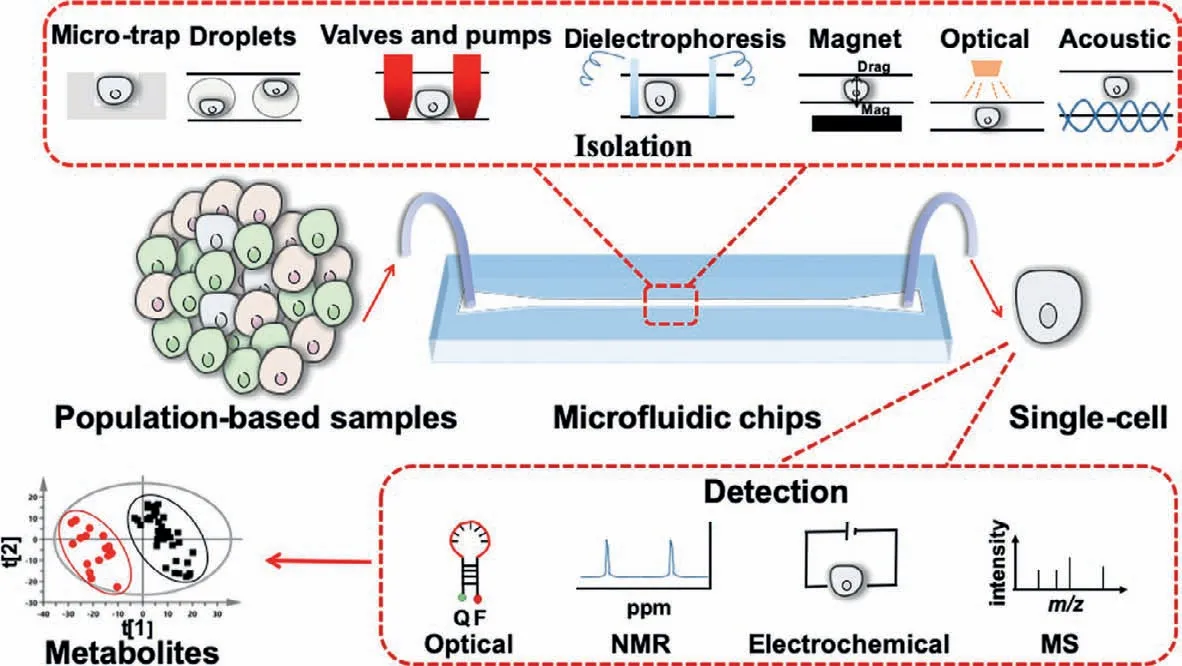
Fig.1.The conceptual description shows that,compared with the commonly used population-based analysis methods,single-cell separation can be achieved by a variety of separation methods on microfluidic chips,and different detection methods can be used for single-cell metabolite analysis.
2.Cell trapping
Scientists can accurately distribute the nano-grade liquid on the substrate for surface modification.By modifying cell surface receptors or DNA on the surface of the substrate,cells can be captured in specific locations [24].As long ago as 1997,Chenet al.used modified substrates that contained extracellular matrix-coated adhesive islands [25].They also found that cell shape can govern whether individual cells grow or die,regardless of the type of matrix protein or antibody to integrin used to mediate adhesion.Therefore,it was essential to isolate single cells without causing noticeable damage or changing normal cell behavior.
2.1.Micro-trap
The most common method to isolate single cells with microfluidic chips was designing specific microstructures.As shown in Fig.2A,Wheeleret al.used a kind of T-shaped micro-trap microstructure to measure intracellular calcium ions concentration between individual cells in 2003 [26].Also,Li and Li used a Vshaped micro-trap microstructure to quantitatively analyze calcium ion of a single cardiac myocyte by fluorescence measurement in 2005 [27].In the past few decades,the capture structures did not change too much.As shown in Fig.2B,micro-trap microstructures were widely used in single-cell analysis [28–30].These microstructures were close in size to one single cell.Once one cell occupied the trap,the altered dynamic flow around it would minimize the chance of other cells joining it.In a way,the trap was selfregulating.
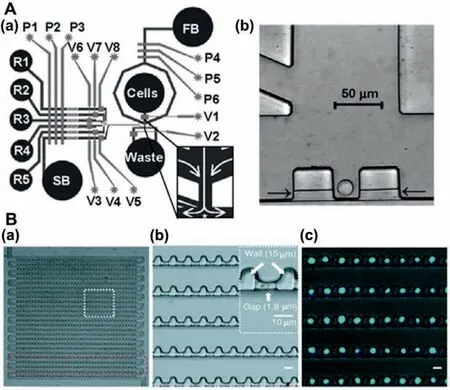
Fig.2.(A) The design of single-cell analysis device: (a) Schematic of the device contains fluidic channels (dark) and control channels (light),(b) CCD image of individual Jurkat T-cells trapped in cell dock.Reproduced with permission [26].Copyright 2003,American Chemical Society.(B) The design of single-cell trapping array:(a) Optical micrograph of the trap array,(b) design details of cell trap,(c) images of single cells in the array.Reproduced with permission [28].Copyright 2011,American Chemical Society.
High throughput,the advantage of microfluidic chips,was essential in single-cell analysis [31–33].To increase high throughput,newer approaches separate and grow single cells in individual wells or innovated trapping arrays.Many recent innovations in single-cell isolation by using microfluidic arrays [34],among these were the integrated microfluidic array plate and dynamic single-cell culture array.Yamamuraet al.fabricated a polystyrene single-cell microarray chip with over 30,000 microchambers [35],over 80% of the microchambers achieved single-cell status.The system was demonstrated suitable for high throughput analysis of intracellular calcium ion response at the single-cell level.Because polystyrene has poor mechanical properties,Liuet al.described a method of fabricating rounded bottom micro-trap arrays in poly(dimethylsiloxane) by molding a monolayer of ordered polystyrene microspheres [36].Both adherent and nonadherent cell types can be retained in the micro-traps with high efficiency.Therefore,Arrays of microstructures improved the efficiency of single-cell analysis in microfluidic chips.
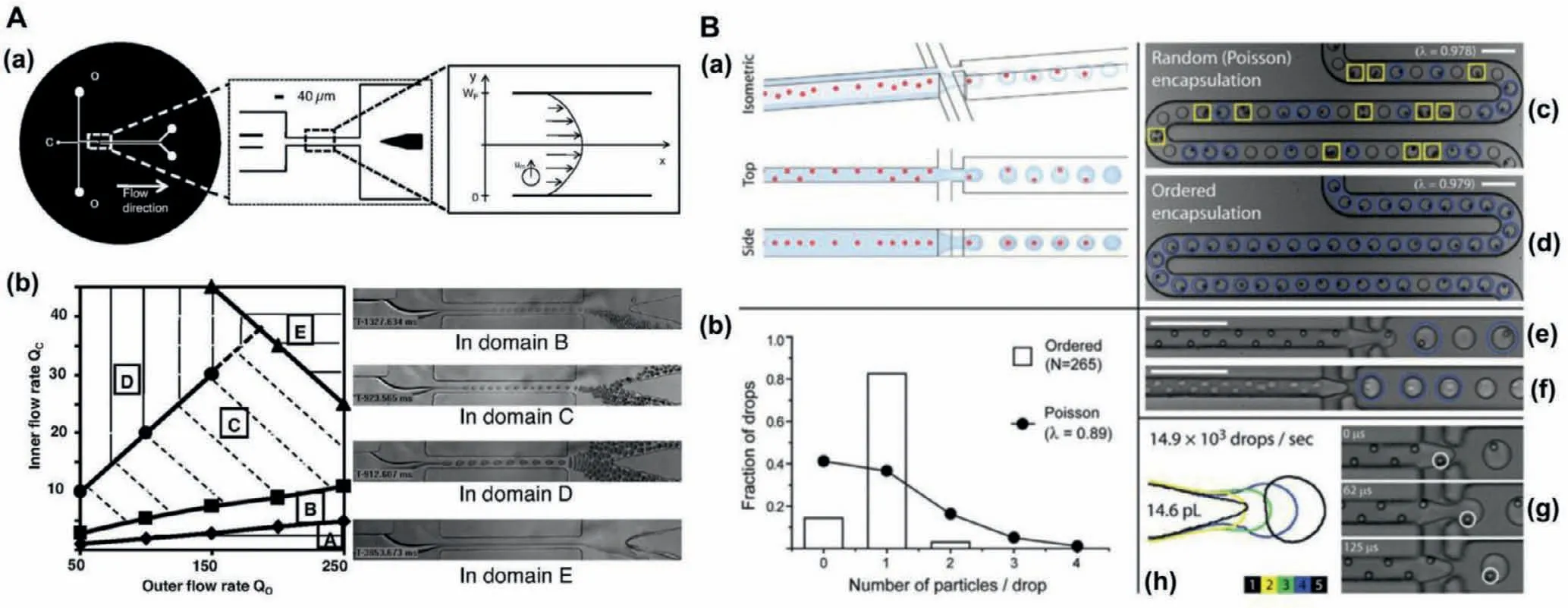
Fig.3.(A) The characterization of these experimental microfluidic devices: (a) Device geometry,(b) experimental phase diagram for droplet formation in the absence of cells.Reproduced with permission [42].Copyright 2008,the National Academy of Sciences.(B) The setup of ordered encapsulation,hydrodynamic interactions causes particles to self-organize or form diagonal/alternating patterns along one side of the microchannel.As depicted schematically in (a),hydrodynamic interactions cause particles to selforganize along one side of the microchannel or into a diagonal/alternating pattern.The uniform spacing in the direction of flow (see side view) leads to the formation of single-particle drops when the two lateral flows of oil pull drops from the aqueous stream (see isometric view) with the same (or slower) frequency that particles reach the microdrop generator (g).As the results for 0.89 beads per drop on average in (b) indicate,ordered encapsulation of beads (d,e) generates more single-particle drops (circles)and fewer empty (not marked) or multiple-particle drops (boxes) than would have been possible from (c) stochastic (Poisson) loading.With little or no loss in membrane integrity,cells also self-organize (f),where drops formed as in (h).Scale bars: 100 μm.Reproduced with permission [43].Copyright 2008,Royal Society of Chemistry.
Reports of these acquisition devices can be categorized into two categories by driving force.Cell sedimentation under the action of gravity was the one category [26,35,36].Meanwhile,the fluid drive was the other category [27,29].The cell suspension tended to flow through the pipe with a smaller flow resistance.When a single cell was captured at the microstructure,it blocked the side bypass pipe,thereby greatly increasing its flow resistance.So that the subsequent cell suspension would no longer pass through here.
2.2.Droplets
Monodisperse droplets of different sizes can be produced when a two-phase flow system was used in the specially designed micropipe structure [37,38].This technology has become an important branch of microfluidic technology [39,40].Because single cells can be made to reside within its picolitre-volume drop,isolated single cell from all other drops,cell-secreted molecules rapidly achieved detectable concentrations in the confined fluid surrounding the encapsulated cell [41].Encapsulation of cells within picolitre-size monodisperse drops provided new means to perform quantitative metabolite analysis on a single-cell basis for large cell populations.However,variabilities in the number of cells per drop due to stochastic cell loading were a major barrier.The number of cells contained in the formed drops was dictated by the probability that a given volume of the initial cell suspension contains a given number of cells,following a Poisson distribution.As shown in Fig.3A,a suspension containing one cell per three drops volume on average was generally used,resulting in about 22% rate of encapsulation for a 75% yield [42].As shown in Fig.3B,Eddet al.overcame this limitation by evenly spacing cells as they travel within a high aspectratio microchannel [43].Cells entered the drop generator with the frequency of drop formation.As a result,the number of suitable drops increased to about 80%.
Sorting after droplet encapsulation was also important.For example,circulating tumor cells had a very low concentration in blood (10 tumor cells in 200,000 white blood cells [44]).Therefore,discovering and recognizing cancer cells was essential in individualized molecular diagnosis.Chabert and Viovy presented a purely hydrodynamic method for the high-throughput encapsulation of single cells into pico-liter droplets [42],and spontaneous self-sorting of these droplets.Unlike previous systems,this system operated very satisfactorily with strongly heterogeneous cell suspensions,provided that the cells do not undergo strong cell-cell adhesion,and was able to accurately sort the droplets according to the size of the encapsulated cell.This property allowed them to encapsulate single cancerous T cells out of a whole blood suspension with an enrichment yield of more than 10,000.However,the droplet size also limited the long-term culture of single cells.Since the droplet was a relatively closed microchamber,it was difficult to achieve the renewal of nutrients and the discharge of metabolic waste.Recently,bioactive cargo delivery droplets have been reported [45,46].This method may be used for the long-term culture of single cells in the future.
3.Cell manipulation
In addition to the continuous improvement and optimization of microfluidic chip design and the exquisite control of single-cell position by pipelines,active control through external forces was also a crucial means.
3.1.Valves and pumps
Valves and pumps on microfluidic chips enable the precise delivery of nanoliter volumes of reagents to a single cell,which makes it possible to monitor the process of single-cell stress responses.Armbrechtet al.used pneumatic valves to precisely expose single cells to various drugs [47].And Thorsenet al.developed high-density microfluidic chips that contained plumbing networks with thousands of micromechanical valves and hundreds of individually addressable chambers in 2002 [48].These microfluidic devices were analogous to electronic integrated circuits fabricated using large-scale integration.In conclusion,these assays,and others,with valves and pumps,were achieved with significant improvements in reagent consumption,analysis time,and temporal resolution over macroscale alternatives.
3.2.Dielectrophoresis
The dielectrophoresis method was the most popular way for single-cell manipulation due to the high specific dielectric properties among various types of cells.Under non-uniform electric fields,polarization effects would be activated,and the uncharged single cell-like particles would be moving laterally.It can be considered that in dielectric electrophoresis,a single cell without charge was polarized by a non-uniform electric field applied in space and then moved in direction with the distribution of electric field under the Coulomb force to achieve the purpose of being controlled.The concept of dielectrophoresis was first presented by Pohl in 1951 [49].He made important early tests using small plastic particles suspended in a liquid of insulating dielectric,and found that the particles could move in the presence of a nonuniform alternating or direct current electric field.Also,Khine and his group developed a desktop system in 2007 [50].Composed of a single control interface,combined with a one-time 96-hole microfluidic device,it can be operated individually,electroporated,and monitored in real-time for each cell in suspension.It was the first real-time feedback controlled single-cell array electroporation experiment.Furthermore,since it was difficult to manipulate a single-cell more precisely by simply changing the electric field distribution such as the electrode shape,position,or external forces,Padhyet al.proposed a method,which can to dynamically control the spatial profile of the dielectrophoresis forces by connecting the trap electrode with the resistor and the inductor to form a resonant resistance inductive capacitor circuit in 2021[51].Using dielectric trap droplets suspended in silicone oil,they demonstrated that the resonator amplitude,dissonance,and linearly can be continuously changed by changing the supply voltage,supply frequency,and circuit resistance to obtain the required depth,range,and stiffness of the trap.The results of such work significantly improved the flexibility and accuracy of dielectrophoresis manipulation methods.
3.3.Magnet
Magnet-based droplet manipulation technique was performed under the magnetic field to apply force.Compared with other manipulation methods,the magnetic drive had some unique advantages,such as no heat generation,easy operation,and no contact with the sample.Furthermore,environmental parameters of temperature,ion concentration,and pH were hardly affecting the magnetic field and magnetism [52,53].As shown in Fig.4,our research team firstly demonstrated the generation and deflection of magnetically functionalized droplets [54–56].Subsequently,Wanget al.reported the use of gradient magnetic fields to manipulate magnetic nanoparticles [57].Therefore,this work would be useful for research on responsive coatings,microfluidics and biomaterials.The magnetic digital microfluidic platform for manipulating full droplets has been developed by Guoet al.[58].Fast and reversible transport of liquids for sorting and mixing became to reality.This droplet manipulation device promoted the development of autonomous open channel fluid devices.Moreover,Banerjee and Sen reported the deformation and splitting of ferrofluid droplets under magnetic conditions [59].This method can be applied to open surface microfluidics.In addition,the manufacture of magnetic tubular microactuators [65],and magnetic liquids [60]for manipulating droplets have also been reported.
3.4.Optical tweezers
Optical tweezers technology,which exploited optical force generated by a tightly focused laser beam to trap small objects,has already emerged as a highly versatile and powerful tool in biological research [61,62].The technology can repel and attract microparticles from 100 nm to 10 μm in size to realize 3D trapping and manipulation,according to the different refractive index between particles and their surroundings [63,64].Integrated into microchips,optical tweezers produced distinct superiority in micromanipulation of individual cells [65–67],organelles [68],viruses [69]or biological macromolecule [70]for their non-invasive physical force,high force resolution accurate to pN and affinity to physiological microenvironment [71].
By combining dynamic fluids and dynamic light patterns,Wang.et al.recognized,captured,and moved target single cells precisely to the desired destination by optical tweezers to sort yeast cells and human embryonic stem cells with high recovery rate and purity [72].To enhance the capture efficiency of single cells,the optical tweezers system was located at the minimum cross section of a conical microchip to increase the probability of targets encountering traps [73].A cytometric tool based on refractive multiple optical tweezers integrated with microfluidics and optical microscopy was developed to immobilization contact-freely individual cells into a steerable high-density array of optical traps in a microfluidic chip and analyze single cells in a highly-parallel manner using fluorescence microscopy,so that the dynamic response of the cells upon glucose sensing could be probed,achieving singlecell high-throughput sorting and batch operation [74].Moreover,to reduce laser damage of cells,Linet al.developed a thermophoretic tweezers technology applying thermophoresis phenomenon to optical trapping,and succeed at the low-power trapping of suspended cells.That reached a tiny optical power 100–1000 times lower than original optical tweezers [75].Combination of optical tweezers and Raman spectroscopy,Raman optical tweezers (ROT)can manipulate and characterize where four different types of cells were separated at a rate of about 90% and the heterogeneity of the cancer cells,leukocyte subtype,and erythrocyte status was quantifiably characterized,respectively [76].By introducing single-cell RNA sequencing,ROT-chip was applied to investigate drug sensitivity successfully [77].To extend more applications in single-cell microchips,ROT can quantify microbes’swimming force and interaction with the environment by measuring their location distribution in the optical well or the minimum laser power required [78–80].It can also stretch and cut a single cell or biomacromolecule[70,81],having unlimited potential in single-cell analysis.
3.5.Acoustic tweezers
Acoustic tweezers use sound radiation forces to manipulate matter without contact.It can manipulate objects from the micrometer to the centimeter scale.Compared with optical tweezers,acoustic tweezers provide higher trapping forces per unit input power and enable the trapping of a wide range of sample materials in various media.In 2012,an acoustic tweezer was first demonstrated to trap and manipulate single microparticles,cells,and entire organisms.The power density required was significantly lower than optical counterparts (10,000,000 times less than optical tweezers and 100 times less than optoelectronic tweezers),which renders the technique more biocompatible and amenable to miniaturization.Cell-viability tests were also conducted to verify the compatibility of tweezers with biological objects [82].Liet al.combined this technology with the microfluidic chip in 2015[83].They utilized the wide resonance band of chirped interdigital transducers to achieve real-time control of a standing surface acoustic wave field,which enabled flexible manipulation of most known microparticles.Guoet al.also used this technology to quantitatively investigate the gap junctional intercellular communication in several homotypic and heterotypic populations by visualizing the transfer of fluorescent dye between cells [84].Both cell trapping and cell manipulation are about the control of cells.Table 1 summarizes and compared these methods.
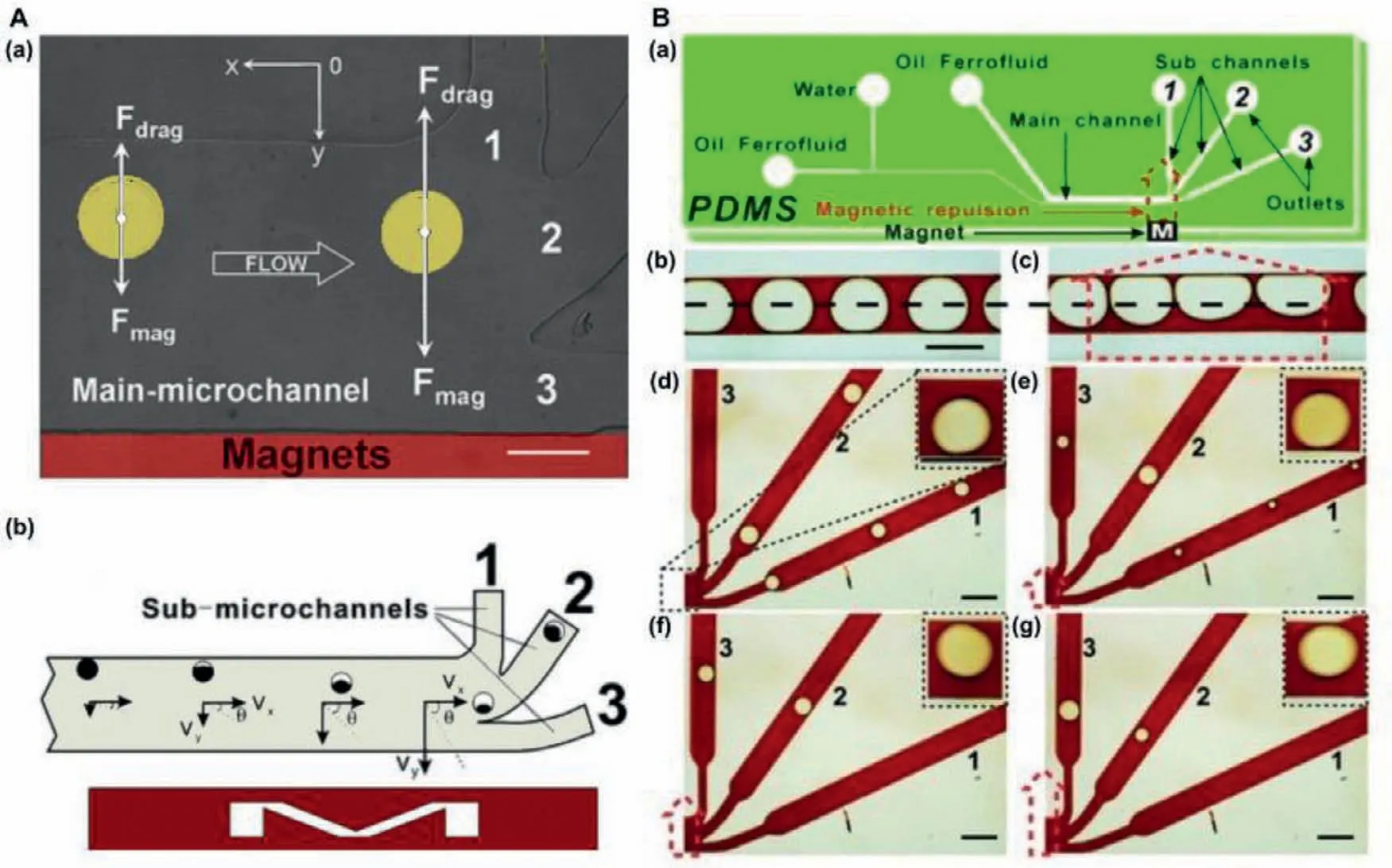
Fig.4.(A) The design of main-microchannel: (a) A microscope picture of superparamagnetic droplet deflection in the main microchannel,(b) diagram of droplet velocity component.Reproduced with permission [54].Copyright 2009,Royal Society of Chemistry.(B) The design of microfluidic chip: (a) Microfluidic chip including the position and orientation of applied magnetic repulsion,(b) symmetrical and (c) unsymmetrical droplets,(d–g) the variation of splitting of big droplets under an increasing magnetic repulsion.Reproduced with permission [55].Copyright 2011,Royal Society of Chemistry.
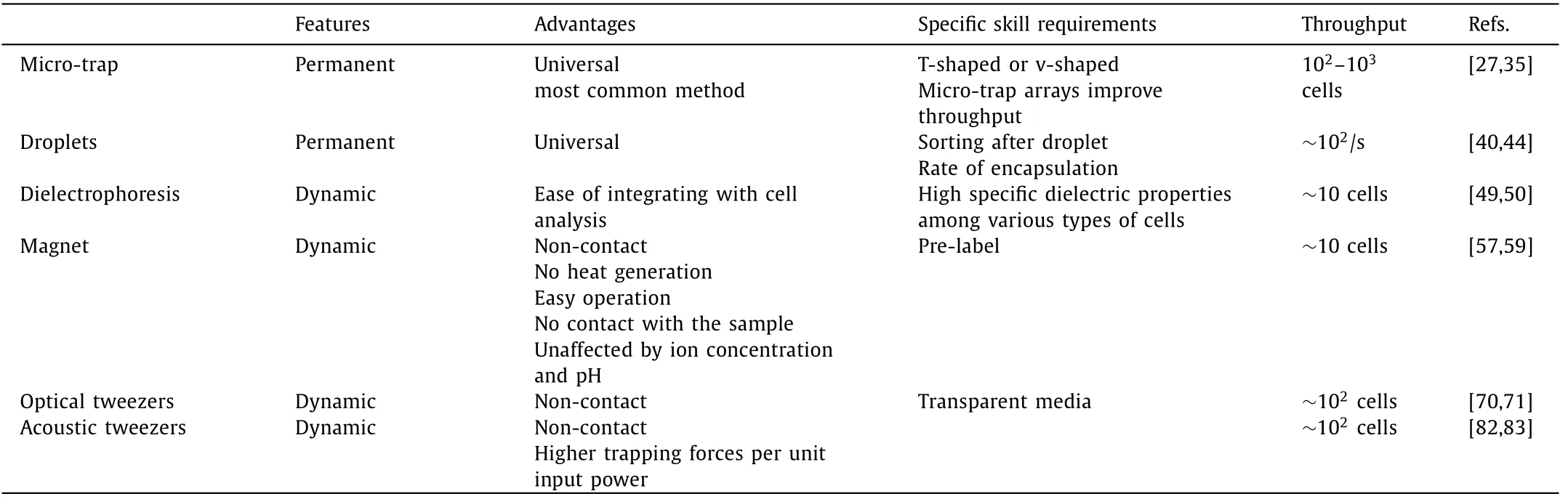
Table 1 Cell trapping and cell manipulation on microfluidic devices.
4.Integrated with detection
4.1.Optical detection
Microchip electrophoresis with laser-induced fluorescence detection is a powerful tool to detect metabolites at the single-cell level.The key advantages of fluorescence detection of intracellular metabolites include high sensitivity,nondestructive nature,and high throughput [85].Fluorometric assays are generally based on the presence of fluorescent tags or probes.Only very few metabolites can be analyzed directly in single cells by autofluorescence.Thus,most metabolites need to be pre-labeled to achieve detection[86].McClainet al.reported a microfluidic device integrated cell handling [87],rapid cell lysis,and electrophoretic separation and detection of fluorescent cytosolic dyes in 2003.The device function was demonstrated using Jurkat cells that were loaded with the fluorogenic dyes-carboxy fluorescein diacetate,Oregon green carboxylic acid diacetate,or Calcein AM.Also,Gaoet al.integrated single-cell injection,cell analysis,separation and detection of intracellular constituents on a microfluidic chip [88].Mettoet al.also integrated cell transport,analysis,injection,electrophoretic separation,and fluorescence detection into a single device [89].They used a new channel manifold design to significantly improve the reliability and robustness of the cell lysis and lysate injection.Recently,dielectrophoretic technology,developed from electrophoresis technology,has become one of the popular technologies for particle separation,capture,and manipulation [90,91].In 2016,Khamenehfaret al.demonstrated a dielectrophoretic-microfluidic chip integrated fluorescence detection enabled single-cell measurements for multidrug resistance in heterogeneous acute myeloid leukemia patient samples [92].
Due to spectral overlap,single-cell fluorescence imaging was difficult to image multiple metabolites at the same time.Liet al.described a multicolor fluorescence detection-based microfluidic device for single-cell metabolomics research [93].They simultaneously analyzed hydrogen peroxide,glutathione,and cysteine in single-cell analysis.Similarly,with fluorescence and chemiluminescence detections also need pre-label.Zhaoet al.described the first application of microchip electrophoresis with chemiluminescence detection in single-cell analysis in 2009 [94].Intracellular glutathione was firstly labeled by incubating cells with diazo-luminol,and then individual cells were injected,in-line analyzed,and separated.Generally speaking,most metabolites need to be pre-labeled to achieve detection,which limited the application of fluorescence imaging.In addition,labeling had a certain impact on the physical and chemical properties of metabolite molecules,especially small molecule metabolism.Thus,the label-free detective method is essential to single-cell metabolomics.
4.2.NMR detection
NMR is a powerful tool for the structural characterization of organic compounds,including metabolites.There were many reports about NMR used in metabolite analysis [95–97].Owing to relatively low sensitivity,NMR has found only scarce applications in single-cell metabolite analysis.Many works were dedicated to designing a more sensitive NMR probe for metabolite studies [96–101].But the sensitivity was still in the 100 pmol range which is much larger than single-cell metabolite concentration.Therefore,further efforts need to be made to render NMR applicable to the metabolite analysis of single biological cells.Overcoming the sensitivity issues,NMR would be a powerful tool for single-cell metabolomics analysis in the future.
4.3.Electrochemical detection
Electrochemical methods can perform real-time and quantitative detection of metabolites in single cells.In the 1970s,Sternsonet al.have used electrochemical detection to study adrenergic neurotransmitters and related compounds [102].Now,the application of electrochemical methods in single-cell analysis was no longer limited to neurotransmitters.The combination of microfluidic devices and electrical signals has become one of the methods to detect the complex cell metabolomics and interrelationship of diverse metabolites,which was helpful for rapid,highthroughput analysis of single-cell metabolome.Caiet al.fabricated a microfluidic chip integrated microelectrodes for the lactate content of single heart cells in 2002 [103].Also,Parket al.tested the oxygen metabolism and degradation of algae cell photosynthesis by mounting Au and Ag/AgCl electrodes on a micro-sieve PDMS microfluidic device [104].In the further development of singlecell analysis and metabolomics,the detection of metabolomics has become a hot and difficult research topic.Song and Bai used two micro-electrode arrays to study the physiological changes of a single cell by measuring the electrochemical gradient of solute molecules secreted by a single cell on a microfluidic chip [105].To further analyze the interaction between different cells through the detection of cell metabolites,Townsendet al.designed and prepared gold-pillar electrode arrays and integrated microfluidic devices to stimulate endothelial cells (EC) to release nitric oxide (NO)by detecting adenosine triphosphate (ATP) derived from red blood cells (RBCs) [106].The interaction between RBCs and EC was explored by the use of pharmacologic tests with the absence and presence of glybenclamide in RBCs,which determined the relationship between the release of ATP and the release of NO from endothelial cells.
Electrochemical methods were also difficult to achieve simultaneous detection of multiple components.A combined electrochemical detection method with other detected methods can improve this matter to some extent.Chenget al.used three microelectrodes as an electrochemical lactate microbiosensor to measure the amounts of lactate produced by the heart cell [107].The device also allowed simultaneousin-situmicroscopy,enabling optical measurements of cell contractility and fluorescence measurements of extracellular pH and cellular Ca2+.However,due to the variety of cell metabolites,diverse structures,and large dynamic range,it was difficult to detect all kinds of metabolomics comprehensively[108],how to conduct a more full-scale detection and analysis of single-cell metabolome and explore its role in cells communication requires further research.
4.4.MS detection
Due to the chemical diversity of metabolomics,MS and other label-free bioanalytical techniques are the preferred analytical methods [109–113].It has been reported that HPLC-MS/MS,CE-ESIMS,laser ablation electrospray ionization MS,matrix-assisted laser desorption ionization MS (MALDI-MS),and other technologies have been used widely,therefore,these techniques can be combined with microfluidic chips for single-cell metabolite analysis [114].
MALDI-MS has enough sensitivity for single-cell analysis,and MALDI-MS has many advantages,such as good salt tolerance,simple sample preparation,and low sample consumption [115].Therefore,MALDI-MS was considered to be one of the ideal methods for single-cell metabolomics analysis [116].MALDI-MS has been used in metabolomics and single-cell analysis [117–120].As shown in Fig.5A,Huet al.demonstrated a chip integrated with isotopic labeling of intracellular metabolites,microbial cell culture,and MS imaging with single-cell resolution [121].This strategy may be further extended to the metabolism research of fungi and other species with the simple organization of cells by MALDI-MS.However,the use of isotope labeling in this study made it hard to achieve metabolomics research.As shown in Fig.5B,Guillaume Gentilet al.reported a method combined with the nondestructive and quantitative extraction of intracellular fluid and subpicoliter resolution,fluidic force microscopy was used,and then MALDIMS was used [122].The developed method can detect and identify 20 metabolites recovered from the cytoplasm of individual HeLa cells.Different from methods previously reported for singlecell metabolism researches,this method can retrieve and analyze intracellular metabolites without removing cells from their environments and damaging cell viability,which provided a new method for single-cell metabolite analysis on chip.MALDI-MS was widely used in single-cell analysis,however,it should be noted that MALDI matrix interference would greatly limit the observation of metabolites.
ESI-MS had the advantages of high sensitivity and low background interference,thus it is an ideal tool for single-cell metabolism analysis on chip [123–126].Onjikoet al.demonstrated that single-cell CE-ESI-MS can detect whether the differential expression of the genome was transformed into the metabolite domain between a single embryo cell [127].As shown in Fig.5C,this work measured the small molecule activity of a single embryonic cell by CE-μESI-MS,which can capture a snapshot of the small molecule activity of single cells in the early cleavage stage of the 16-cell Xenopus embryo.Onjikoet al.indicated single-cell MS including CE-μESI has new possibilities of cell and developmental biology.Therefore,micromanipulations had great benefits,making single-cell MS adapt to a wider range of applications.As shown in Fig.5D,Zhanget al.demonstrated an integrated droplet-based microfluidic system for on-line ESI-MS analysis of living single-cell lipids,with the minimum sample consumption and negligible cross contamination [128].The system can compare the heterogeneity between normal cells and cancer cells,and can reflect the heterogeneity changes of the same cells before and after drug treatments.This work provided a new method for single-cell metabolites on a microfluidic chip.Weiet al.developed pulsed-dc-ESIMS as a method for systematic analysis of components in small volume samples [129].This method had been successfully applied to single-cell metabolomics analysis and already obtained a twodimensional profile of metabolites (including exact MS and MS/MS data) from a single plant and mammalian cell.The pulsed-dc-ESIMS would be a widely used friendly method without additional modification to a commercial MS spectrometer.Table 2 summarizes the characteristics of these different methods integrated with microfluidic devices.
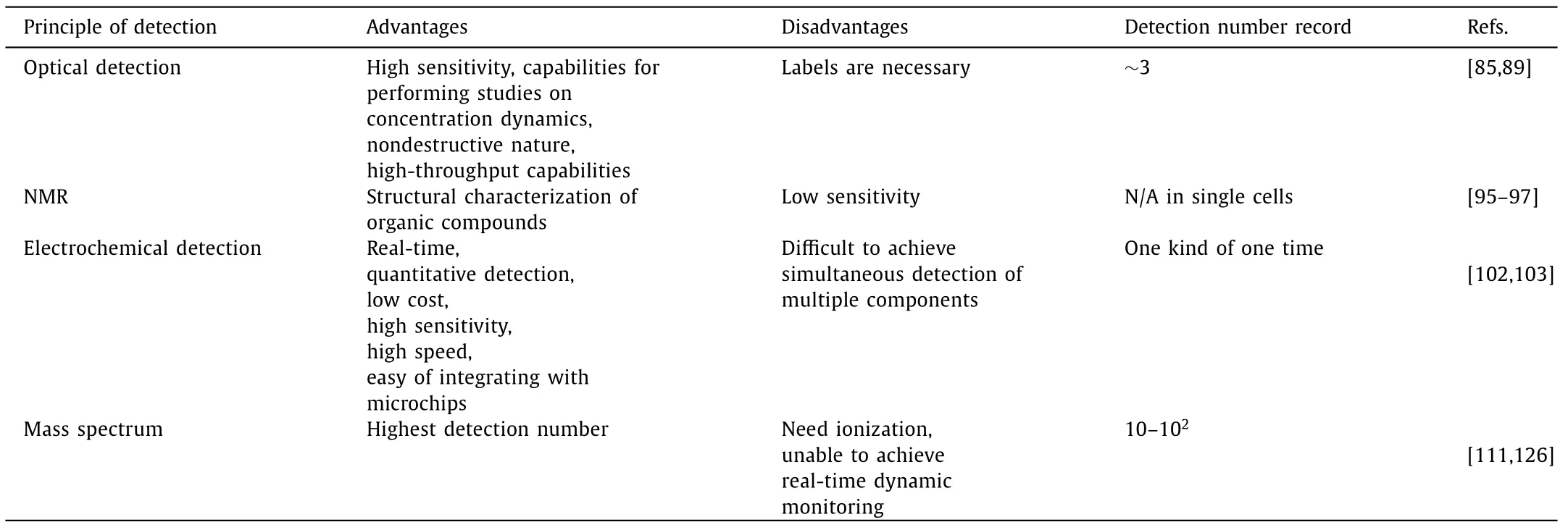
Table 2 Different methods integrated with microfluidic devices for single-cell metabolite analysis.
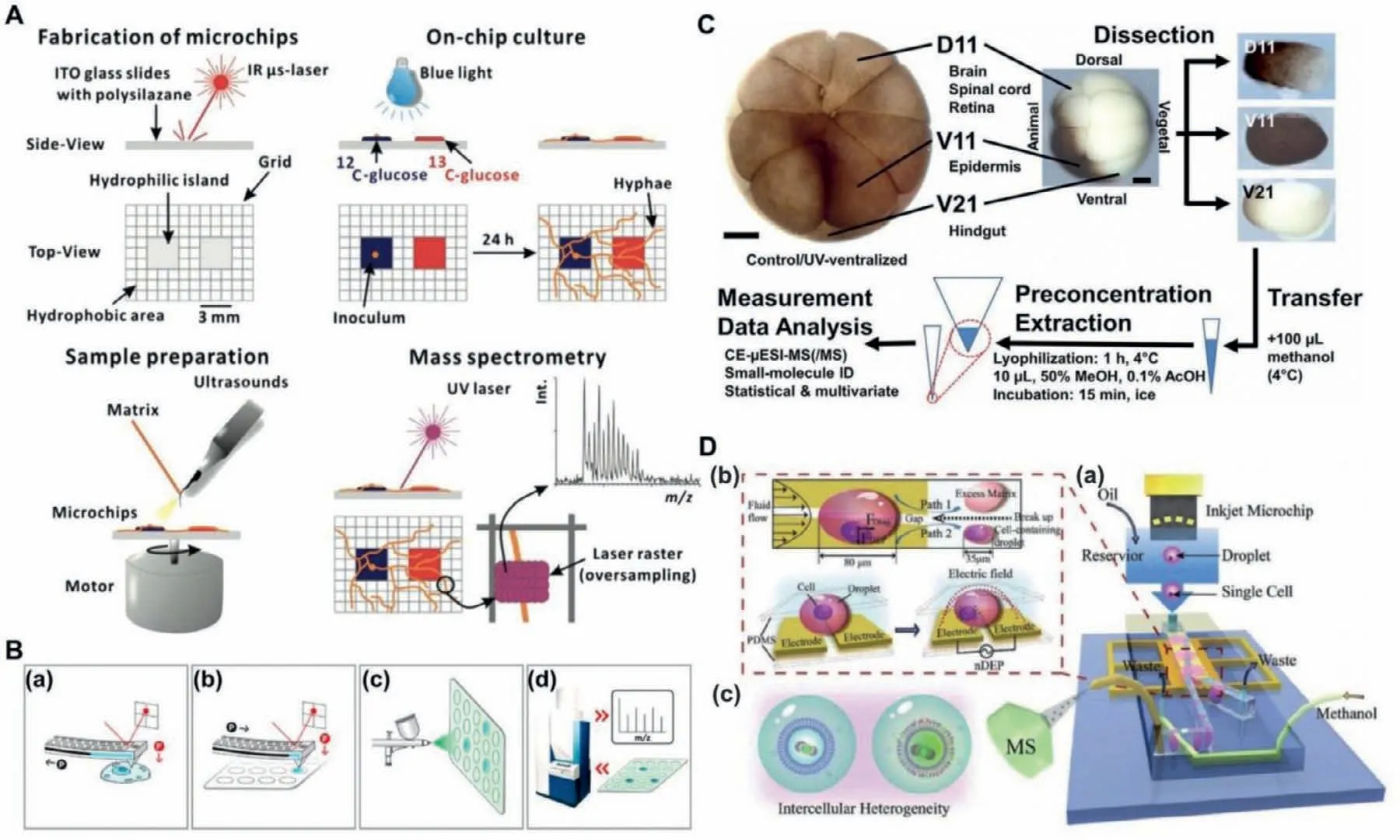
Fig.5.(A) Development and application of the open microchip.Reproduced with permission [121].Copyright 2012,American Chemical Society.(B) The flow charts of the method for single-cell metabolic analysis: (a–d) Sampling,dispensing cytoplasmic extract,spraying matrix,and acquisition of MS spectra.Reproduced with permission[122].Copyright 2017,American Chemical Society.(C) The workflow to discover small molecular activities during early-stage embryo development,metabolomes extracted and measured by a custom-designed single-cell CE-μESI-MS.Reproduced with permission [127].Copyright 2015,the National Academy of Sciences.(D) The scheme of an integrated microfluidic system for online live single-cell lipid profiling and analyzing: (a) Schematic description,(b) illustration of single-cell manipulation and matrix removal,(c) the application of this system.Reproduced with permission [128].Copyright 2019,WILEY-VCH.
5.Applications
5.1.Identifying metabolic pathways
Researching metabolites in biological processes is the basic application of single-cell metabolomics analysis.Zhanget al.applied integrated droplet-based microfluidics with MS to study the change of single-cell metabolites in the biological process of dysfunctional oxidative phosphorylation [130].Their method could not only realize matrix-free,selective,and sensitive detection of metabolites in single cells,but also have the capability for reliable and high-throughput single-cell analysis.Hartmannet al.developed an approach to characterize the metabolic regulome of single cells together with their phenotypic identity [131].They applied the approach to clinical samples and identified tissuerestricted,metabolically repressed cytotoxic T cells in human colorectal carcinoma.This approach enables the robust approximation of metabolic and functional states in individual cells.But all these studies remain at the level of proof-of-principle.At this stage,there is still a big challenge to deduce useful metabolic pathways.
5.2.Discovering cancer cells
One of the important applications on single-cell metabonomics is to discover cancer cells.Cancer cells with high metabolic rates were detected between normal cells and cancer cells,such as circulating tumor cells (CTCs).CTCs are exfoliated cells of the primary tumor,circulating in the blood.However,their main functions are not completely clear until now.The concentration of CTCs in peripheral blood of patients with solid tumors is very low,which is meaningful to study CTCs in single-cell analysis.Based on the characteristics of single-cell metabolism,Del Benet al.developed a droplet method to detect CTCs [44].This method can detect 10 tumor cells in 200,000 white blood cells,and provided proof of concept that CTCs can be detected according to the metabolism of cancer cells.Therefore,the development of single-cell metabolomics on microfluidic chips is not only significant for drug therapy,but also has great potential for individualized molecular diagnosis of cells with very low concentrations in blood (such as CTCs).
5.3.Screening drugs
The high-throughput characteristic of microfluidic chips makes screen drugs easily [92].Armbrechtet al.used pneumatic valves to precisely expose single cells to various drugs [47].With this chip,they successfully analyzed the time-dependent effect of the anticancer peptides melittin,aurein 1.2 and aurein 2.2 on MCF-7 cell populations at the single-cell level.Zhanget al.demonstrated an integrated droplet-based microfluidic system for online ESI-MS analysis of living single-cell lipids,with the minimum sample consumption and negligible cross-contamination [128].The system can compare the heterogeneity between normal cells and cancer cells,and can reflect the heterogeneity changes of the same cells before and after drug treatments.This work provided a new method for single-cell metabolites on microfluidic chips.
5.4.Recognizing heterogeneity
Reasons behind heterogeneity include genetic diversity,the existence of multiple and redundant metabolic pathways,altered micro-environmental conditions,and so on.The metabolic activity of cancer cells is a complex,heterogeneous,and nuanced process that may be the key to successful treatment.Recognizing heterogeneity may hold the key to appropriate cancer diagnoses and treatments in the future.Xiaoet al.characterized metabolic heterogeneity at a single-cell resolution using single-cell expression data and defined principles of the tumor microenvironment in 2019[132].There are genetic determinants of cancer metabolic biology,but it is possible that multiple genetic pathways contribute to an abnormal metabolic phenotype.Further,oversimplifying the metabolic activity of cancerous cells is not the most important,rather cells follow multiple metabolic programs and utilize multiple fuels.A greater understanding of these processes will shape how we treat one of the greatest epidemics of our time [133].
6.Conclusions and future prospective
In this review,we summarized the recent developments in single-cell metabolite analysis on microfluidic chips.Microfluidic chips play a growing and ever-critical part in the analysis of singlecell,which is a frontier field of contemporary sciences.First,microfluidic chips can isolate single cells by designing specific microstructures and droplets.Second,the single-cell position can be exquisitely controlled on a microfluidic chip by pipelines and external forces.These external forces including valves and pumps,dielectrophoresis,magnet,optical tweezers,and acoustic tweezers are reviewed in this review.Third,many kinds of detections have been integrated with microfluidic chips.Both label detective methods and label-free detective methods are mentioned.Label methods have a certain impact on the physical and chemical properties of metabolite molecules,especially small molecule metabolism.Therefore,the label-free detective method is more effective in single-cell metabolomics.NMR is a powerful tool for structural characterization but it has sensitivity issues.Electrochemical detection can perform real-time and quantitative detection of metabolites in single cells but it is difficult to achieve simultaneous detection of multiple components.MS has enough sensitivity and it can achieve simultaneous detection of multiple components.Also,MS combined with microfluidic chips for singlecell metabolite analysis has been widely discussed.
Though great efforts have been made in the development of single-cell metabolite analysis on microfluidic chips and advances of the integrated detection tools,some critical issues we think still need to be considered and resolved to further broaden the application of single-cell metabolites or metabolomics.These critical issues may be attracted great attention as the future developing directions in this field.
(i) There are two main hurdles for single-cell analysis,and both relate to the inherently a small sample size.The first challenge is improving the ability of the instrument to distinguish between closely related molecules,for example,the resolution.Since single-cell analysis deals with a small sample size (picoliters to nanoliters),conventional separation techniques are illequipped to deal with such low sample volume without diluting the sample excessively or causing significant sample loss.The second challenge is increasing the ability to detect lower and lower concentrations reliably like the sensitivity.As sensitivity increases,the viability of the instrument itself to perform analysis on the single-cell scale increases.What is more,the sampling phase is the most time-consuming aspect of singlecell analysis.Further development is needed in the areas of sample preparation and interfacing.Implementation of new interfaces which could provide efficient transfer of single-cell lysates to an MS,and more high-throughput analysis will open up the possibility to perform single-cell metabolomics on large populations of cells.The high-throughput analysis will be indispensable to resolve metabolic heterogeneity in populations and compartmentalization of metabolites.
(ii) Despite the impressive progress of microtechnology in recent years,many microfluidic studies or devices remain at the level of proof-of-principle and have been seldom applied to the real world of metabolomic analysis.To further advance the practical application of single-cell metabolomics analysis on a microfluidic chip,it is necessary to infer useful metabolic pathways.Targeted MS which can measure substrates and isotopes at consecutive time points would be a possible way.Through targeted MS to measure substrate and isotope isotopes at consecutive time points,metabolic flux and pathway kinetics can be determined.After that,metabolomics can provide a "snapshot"of metabolites at a particular moment,and13C/15N tag tracking (or fluxomics) can be regarded as a "snapshot".In terms of single-cell resolution,the use of this analysis is still in its infancy.
Overall,as a new tool for single-cell metabolite analysis,many great advances and remarkable progress have been reported in these years.But there is still plenty of room for the promotion and development of single-cell metabolomics analysis on microfluidic chips.The method for comprehensively drawing the metabolic panorama at single-cell resolution is still relatively lacking,and it is in the early stages of development.It can address questions of biological significance in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by 1226 Engineering Health Major Project (Nos.BWS17J028 and AWS16J018).
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
