Progress in synthesis of highly crystalline covalent organic frameworks and their crystallinity enhancement strategies
Liping Guo,Jin Zhng,Qi Hung,Wei Zhou,Shngin Jin,∗
a School of Chemistry and Chemical Engineering,Qilu University of Technology (Shandong Academy of Sciences),Ji’nan 250353,China
b School of Chemical Engineering and Technology,Xi’an Jiaotong University,Xi’an 710049,China
c School of Chemistry and Chemical Engineering,Queen’s University Belfast,Belfast BT7 1NN,United Kingdom
Keywords:Covalent organic frameworks Crystallization mechanism Dynamic chemistry Layer stacking Linkage exchange Monomer exchange
ABSTRACT Covalent organic frameworks (COFs) have been attracting growing concerns since the first report in 2005.With the well-defined and ordered structures,COFs express big potential in mass transport,storage/separation and energy conversion applications.From the perspective of both theory and application,the construction of crystalline COFs with high quality and variety is highly worth to be devoted to.To give insight into the crystalline process of COFs and deeply understand the factors of COFs crystallization,this review was concentrated on the recent progress in construction of crystalline COFs.Accordingly,the types and crystallization process of COFs were summarized firstly.And then the factors on crystallinity and the measures for improving the crystallinity of COFs were classified and discussed in detail.Finally,the perspectives for the development of COFs in further was given at the end of this review.
1.Introduction
As an important branch of porous organic polymers (POPs),covalent organic frameworks (COFs) are made up of light weight elements (B,N,C,H,O and S) [1–3]and possess ordered structural networks extending out in two-or three-dimension through the connecting of covalent bond linkages [4–8].The typical characteristics of uniform ordered backbones and regular 1D column pores bring COFs outstanding performance in energy storage [9–11],heterogeneous catalysis [12,13],adsorption and separation [14,15],sensing [16–18],as well as photoelectric applications [19–21].Furthermore,the regular and well-defined porosity endows COFs excellent performance in mass transport [22]and energy deliver efficiently [23].In particularly,COFs could serve as the platforms for transport of protons [24,25],ions [26–28],charges [29,30]and even heat [31,32].Therefore,the design and construction of high crystalline COFs are deserved to be paid more attention to.
Conventionally,it had been widely considered that covalent linked crystalline polymer networks were hardly to be achieved because of the obstacles in microscopic reversibility in the solid state [33],and it was indeed that rare crystalline polymer networks through covalent bond connections had been reported.Until 2005,the seminal report of COFs reported by Yaghi and co-workers,subverted the traditional view and enriched the crystalline materials family [34].According to their work,the crystalline COFs can be achieved with the optimized reaction conditions (solvents,monomers,as well as reaction time).Afterward,lots of works have been conducted in increasing the diversity of COFs [35,36].The first 3D COFs were reported in 2009 through unremitting efforts[37].Another iconic progress is the achievement of a single crystal of 3D COF at the micron level in 2018 [38],indicating the artificial control of crystal growth at molecular level.These remarkable signs of progresses will continuously inspire researchers to devote more efforts to developing and expanding this type of crystalline material.
The distinctive periodic feature permits COFs better performance than those of amorphous counterparts,especially in photochemical conversion [39]and energy storage devices [40].Therefore,the purposeful design of COFs with high crystal quality are expected.The direction of the covalent bond in frameworks makes possible for forecasting the structure of targeting COFs [41].Moreover,according to the geometry symmetry hypothesis,the composition and topology and porosity of the resulting COFs can be further predicated legitimately.Nevertheless,not all aimed COFs could be successfully constructed because of the limitation of monomer geometric configuration,reactivity or other factors.Thus,understanding the crystallization process and influence factors are essential for COFs construction.
As the typical feature,the high crystalline structure of COFs would offer better performance in applications.Up to now,most reviews of COFs focused on the synthesis methods and applications[2,42–45],but only a small part of reviews contained the dynamic chemistry of COFs crystals [46]and strategies of COFs crystallization [36,47,48].Especially,the recent improvement of crystallography enables us to have a deeper and more intuitive understanding of the structure of COFs.Therefore,it is very necessary to give insight into the crystalline process and to comprehensively overview the development of COFs crystallization to date.With that in mind,this review listed the linkage of COFs firstly and then summarized the recent development of the crystalline forms of COFs.To deeply understand in the crystalline process of COFs,both the influences on the ordered structure and the strategies for improving crystallinity of COFs were discussed in detail.Afterward,the prospects were given at last.This review will provide the insightful guidance for construction of COFs with high quality both in crystallinity and application.
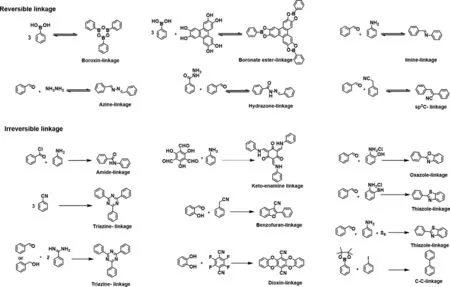
Fig.1.Summary of reversible and irreversible linkages in COFs.
2.Linkages of COFs
Dynamic covalent chemistry is the fundamental point when constructing typical COFs with high-quality crystal [49].Reversibility of the linkages permits defects self-correction during the generation and degeneration process,therefore the COFs would finally be obtained with the ordered crystalline structure and thermodynamic stability.Reversible boroxine and boronate ester linkages are the first reported COFs [34]and then followed by imine linkage[50],azine linkage [51]and hydrazone linkage [52]in line with the increased chemical stability (Fig.1) [53].Among these COFs,iminebased COFs have been explored most widely because of the diversity of aldehyde monomers and amino monomers [54,55].Subsequently,sp2C=C linkage,a newly emerged reversible linkage,had been developedviaknoevenagel condensation [56,57].Compared with C=N bond,the C=C bond is deemed to be more stable especially in strong acid and base environments.Moreover,the prepared sp2C=C COFs express enhancedπconjugation extending along with horizontal and vertical orientation,contribute to a promising further in photoelectronic application [58–60].
The self-correction effect of reversible bonds is indeed beneficial to improve the crystallinity,but exactly the reversibility of bonds would reduce the chemical stability of the frameworks in turn [61].So,less reversible linkages have been gradually explored to enable both stability and crystallinity of COFs.More obstacles should be overcome for the construction of crystalline structures with the formation of irreversible linkages.To date,several irreversible linkages have been already developed to synthesis COFs(Fig.1),including amide linkage,keto-emamine linkage,triazine linkage,benzofuran linkage,oxazole linkage,thiazole linkage,thiazole linkage,and dioxin linkage.In general,these irreversible linkages are formed by cascade reactions,which involve one reversible intermediate step.For example,keto-enamine linked COFs can be seen as the first example of ultrastable COFs achieved through revisable Schiff base reaction and irreversible tautomerism reaction[62].Dioxin linkage is also composed of reversible nucleophilic attack and irreversible ring closure [63].Amide linkage can be obtained through building blocks exchange from imine-based COFs,in which the formation of imine is reversible [64].Oxazole linkage and thiazole linkage are formed from the oxidative cyclization of the pre-generated reversible imine linkage [65,66].The formation of benzofuran linkage also contains two steps: reversible Knoevenagel condensation and the oxidative cyclization [67].For robust triazine linkage,Schiff base formation and Michael addition are needed [68].According to these cascade reactions,the first step reaction is reversible,which mainly contributes to the selfassembly of the starting monomers and error-correction to form ordered pre-skeleton,and then the second step reaction is carried out based on the ordered pre-skeleton.As a consequence,the whole formation process of these linkages is irreversible but still assembled into crystalline frameworks.In these systems,the rigid building blocks and directional covalent linkages also could prompt the periodic arrangement of skeletons [63].
Apart from the above-mentioned reversible and cascade reactions,irreversible direct reaction was further adopted for construction crystalline COFs.Because of the permanent bonding of monomers,higher requirements for reaction controllability are put forward for the structural reorganization through irreversible direct reaction.So interface reaction is a good candidate to achieve ordered frameworks of the desired structure [69].In 2019,Li and co-workers synthesized C–C linked COFsviaSuzuki polymerization through water/toluene interface reaction under argon protection,in which the formation of C–C linkage is irreversible [70].According to their work,crystalline COF freestanding film with lateral size of more than 10 μm was successfully obtained.The reason for the formation of crystalline structure was mainly due to the controlled reaction rate at the liquid/liquid interface.
Besides that,another irreversible pyrimidazole linkage was synthesized by Wang’s group [71].According to the reported work,pyrimidazole linked COFsviaGroebke-Blackburn-Bienaymé reaction of isocyanide,aminopyridine,and aldehyde monomers,and the imidazole rings in COFs ensured the chemical stability and functionality.This reaction could be a promising way to synthesize ultrastable and highly conjugated crystalline COFs,which is worth exploring in the future.
3.Crystallization mechanism of COFs
Oriented attachment and random attachment are typical ways of crystal growth [72].Through random attachment,the resulting crystals are inclined to form as polycrystals.Although there are several single crystals had been reported,most COFs still be polycrystalline.How to control the crystal nucleation and growth has always been explored by researchers.Thus it is extremely important to understand the crystallization mechanism of COFs,which can guide us to build high crystalline COFs [39].The crystallization mechanism of COFs remains a complex process,and more research in this area still needs to be further conducted.Nowadays,the common view of the crystallization starts from the nucleation of the polymer oligomers and then the crystal grew and extended to 2D or 3D spaces finally as the frameworks [73].The crystallization process is composed of linkage generation,decomposition,bond exchange and stacking through noncovalent interaction between 2D layers or interpenetration in 3D networks [46].It is widely accepted that crystallization is a dynamic process [47]and the study of the crystallization mechanic is still a big challenge especially by experiments.
Up to date,there have been some prospective studies by Dichtel’s group to give some insight into the crystallization process in boronate-linked COFs.In their studies,the nucleation process and growth process of COF-5 were firstly revealed through both computational simulation and experimental results [73].Kinetic Monte Carlo (KMC) model was adopted to uncover the microscopic process.As the results have shown,small oligomers were firstly formed and then stacked with each other in small size.At the same time,the small oligomers polymerized and grew to large ones and hereafter stacked with each other into bigger sizes (Fig.2a).Both of the domain size (lateral growth) and height (vertical growth) of oligomers increased with the time prolonging (Fig.2b).Moreover,the addition of H2O shifted the equilibrium away from oligomers growth in big size and thus adding H2O was in favour of the formation of COF crystals in big domain size,which provides a handy method in construction COFs in practice.Furthermore,the relationship of monomer concentration and the rates of nucleation and growth was further explored in COF-5 [74].They revealed that nucleation rate had a second-order relationship with monomer concentration and the growth rate only had a firstorder relationship.While the monomer concentration was lower than the threshold value,growth process could dominate over nucleation process.This conclusion could guide the development of crystallinity improvement in the lab experiments.
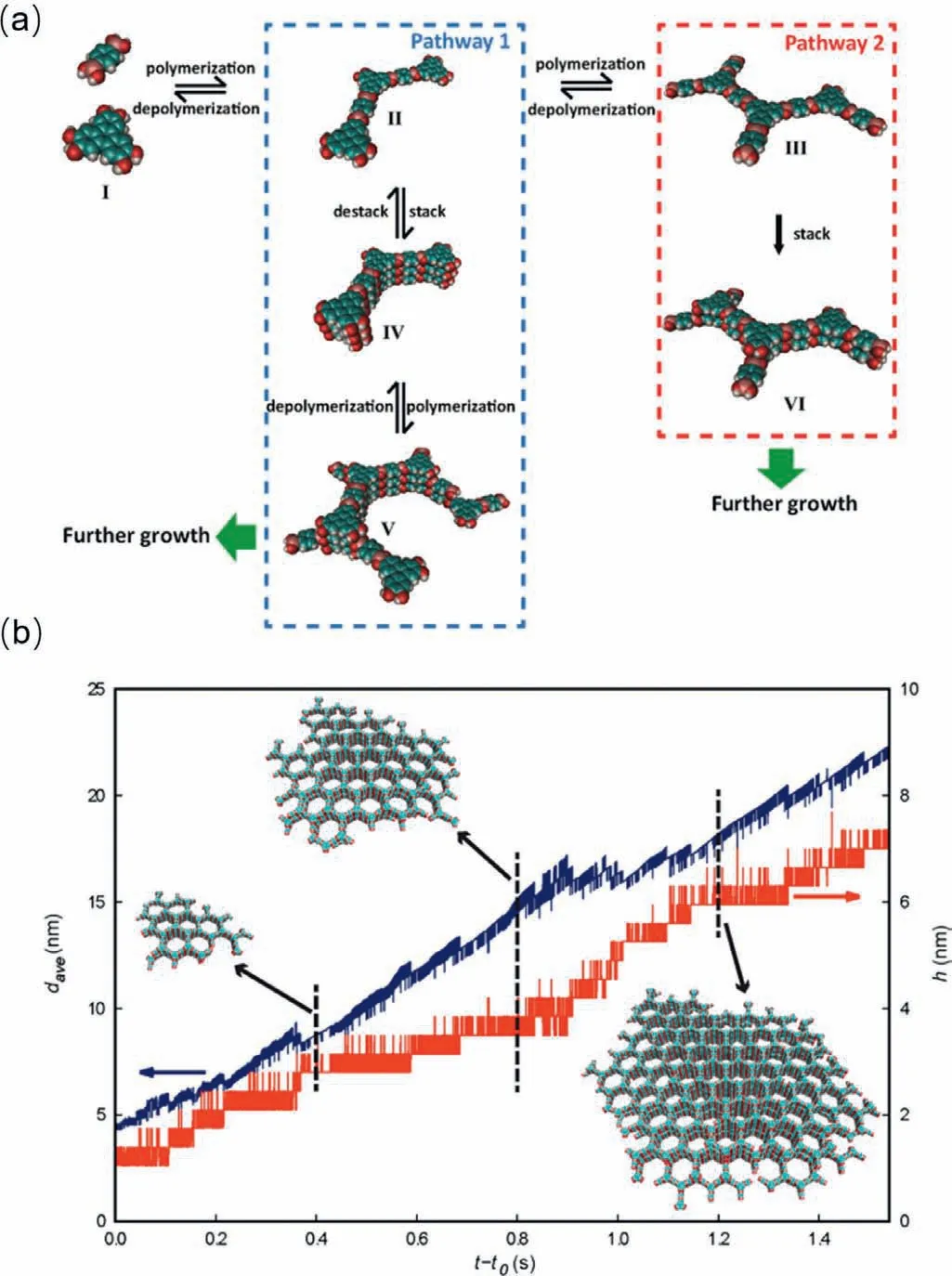
Fig.2.(a) Two nucleation pathways for COF-5.(b) Averaged size among layers and height of the COF-5 crystal during its growth.Reproduced with permission [73].Copyright 2017,American Chemical Society.
The crystallization process of imine-COFs was thought to be distinctive from boronate-linked COFs.Again,Dicthel and coworkers explored the crystalline process of imine-linked COFs.The results gave insights that the COFs formation started from the amorphous networks linked by imine bonds with low BET area.Thus,it is reckoned that sufficient reaction time for the imine exchange is needed to achieve a high crystalline structure [75].With the advanced characterization means,and the detect interval was minimized to 60 s,new founding was obtained that periodic COFs sheets were formed at the first 60 s in reaction and then stacked to more ordered structures over the course of several hours (Fig.3) [76].This finding differs from previous work because theinsituX-ray diffraction was adopted,which can record the data with smaller time intervals.
Although the crystallization processes of boronate and iminelinked COFs had been tentatively investigated,other COFs,particularly for the stable COFs that are derived from the cascade reaction process,still be remain unknown.Hence,the probe into these studies is indeed highly needed and highly worth to be done in the following work.
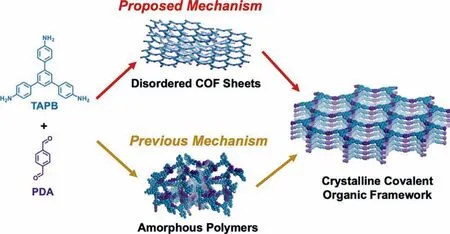
Fig.3.Comparison of proposed and previous COF formation mechanisms.Reproduced with permission [76].Copyright 2016,American Chemical Society.
4.Factors affecting crystallinity of COFs
4.1.Solvent effect
As the reaction medium,solvent plays an important role from the thermodynamically feasible viewpoint and thus affects the formation of covalent bond linkage.Appropriate solubility of monomers is necessary for the controllable thermodynamic reaction in synthesis of crystalline structure.Normally,the mixture composition of polar solvent and nonpolar solvent are optimized for solvothermal synthesis [77,78]and molten salt (such as ZnCl2) serves as the solvent and catalysis for ionothermal synthesis [79,80].Besides those,it should be noteworthy that interface reaction is derivative means of controlling the crystalline process by adjusting solvents.Xu and coworkers synthesized crystalline triazine-COFs (covalent triazine frameworks,CTFs) nanosheets in the interface between dichloromethane and trifluoromethanesulfonic acid (TFMS) [81].As emphasized in their work,the interface could permit the reaction reversibility under control and crystalline nanosheets were thus formed through van der Waals epitaxial effect.
Apart from conventional solvents,ionic liquid was first used for the synthesis of crystalline COFs in 2019.According to the work,with the presence of [BMim][NTf2]as solvent and catalyst,three 2D imine COFs were obtained at room temperature [82].Besides imine COFs,azine COF [83]and keto-amine COF [84]also could be successfully synthesized with the solvent of [BMim][NTf2].
Not only the reaction process,solvation also could influence the stacking model obviouslyviathe interaction with the skeletons.The interaction between adjacent COFs layers can be largely influenced by the presence of the solvent.During the synthesis of CTFs,a tandem transformation approach was developed to realize the stacking model conversion from AB stacking to AA stacking [85].According to the work,the reaction system was heated at 250°C for 12 h at first and the crystalline CTFs with AB stacking model was obtained;and then the product was further heated at 350°C under N2protection and the stacking model of resulting crystalline CTFs was changed as AA stacking.Here,the role of further high temperature (350°C) to remove the residual solvent TFMS and acceleration formation of the stacking in AA model at the same time.The transformation of the stacking layers was also observed in imine-COF [86].According to Zhao’s work,the AA stacking structure of imine-COF in the dried state would be changed to a quasi-AB stacking model after the solvated treatment.
4.2.Structural design of monomers
4.2.1.Monomer rigidity
Rigid building blocks are the first choice to construct crystalline structures because of the directional covalent bonding.Bein,Auras and co-workers constructed several COFs containing trior tetradentate central monomers through the molecular docking strategy [87].According to the results,the rigid and propellerlike 1,1,2,2-tetraphenylethene (4-PE) central unit acted as the selfrepeating docking sites and also as the bridge for connecting COFs layers.Therefore,the generated COFs nanosheets would be locked and the performance of stacking faults and dislocation could be further limited,resulting COFs with high crystallinity.Monomers with high rigidity,thus,are initially required for constructing longordered structures.
With the deepening of research,it is found that the rigid monomers are not the essential for fabricating crystalline COFs and COFs containing flexible monomers were also achieved successfully.Compared with rigid monomers,the free rotation feature of flexible units,such as 2,4,6-triaryloxy-1,3,5-triazines,make it more difficult for the construction of ordered structures in longrange [88].To address this issue,H-bond between the linkage and building block was introduced to lock the flexible unit,resulting in the decreased rotational freedom degree and therefore enhanced crystallinity of corresponding COFs [89].Because of more covalent bonds in 3D COFs,it is more difficult to synthesize 3D COFs.Very recently,Wang,Sun and co-workers had also achieved 3D COFs (FCOF-5) with flexible block 1,2,4,5-tetrakis(bromomethyl)-benzene (TFMB) [90].Interestingly,with the presence the flexible C-O,FCOF-5 backbones showed breathing feature and exhibited expansion and contraction during gas adsorption and desorption,respectively.This new type of soft COFs presents enormous potential in the application of adsorption and separation.
4.2.2.Monomer geometry structure
The topology diagram can predict the direction of covalent bond and provide the possible crystalline net of structure [91].No matter acting as knot or linker,the monomers must be directionally connected with each other in a manner of the predesigned topology diagram strictly,and only in this way can the integrity of the periodic unit be ensured [92].Therefore,the geometry and bonding angles of monomer are especially important for the construction of final product with a regular arrangement structure.For example,planar monomers are required when constructing of 2D COF,but tetrahedron monomers are usually needed for 3D COF fabrication.Mismatching angles would cause large angle strain and are enthalpically not favored to produce thermodynamically stable ordered frameworks.Compared with the design of two components,multicomponent design would encounter more difficulties to ensure directionally connecting and structural integrity in the way of following the rules of topology diagram [36].Thus the geometry and bonding angel of monomers,especially as knot,are the primary factor when constructing crystalline structure.
Also,the geometry of monomers could largely influence the layer stacking performance and thus affect the final crystallinity of COFs [93].Different torsion angel out of plane of monomers could cause varied COFs in surface area and crystallinity [94].But the mechanic of planarity of monomer affecting the crystallinity structure is still not clear enough and needs further research.
Planar building block is expected for the construction of 2D COFs.While for 3D COFs,building block with extending into three spatial directions is needed,such as 2,3,6,7,14,15-hexakis(4-formylphenyl)triptycene [95],tetra(p-aminophenyl)methane[96]and tetrahedral 1,3,5,7-tetraaminoadamantane [97].In addition to the inherent tetrahedral configuration of units,the presence of side groups also could change the geometry of the monomer.According to the reported work,the planar tetra (p-aminophenyl)-biphenyl (BPTA) was adopted to construct 2D COF firstly.After the introduction of methyl groups into BPTA,the resulting building block tetra(p-aminophenyl)-bimesitylene (BMTA) becomes a twist tetrahedral geometry with the biphenyl dihedral angle of 60°,and consequently the 3D COF was obtained successfully (Fig.4) [98].
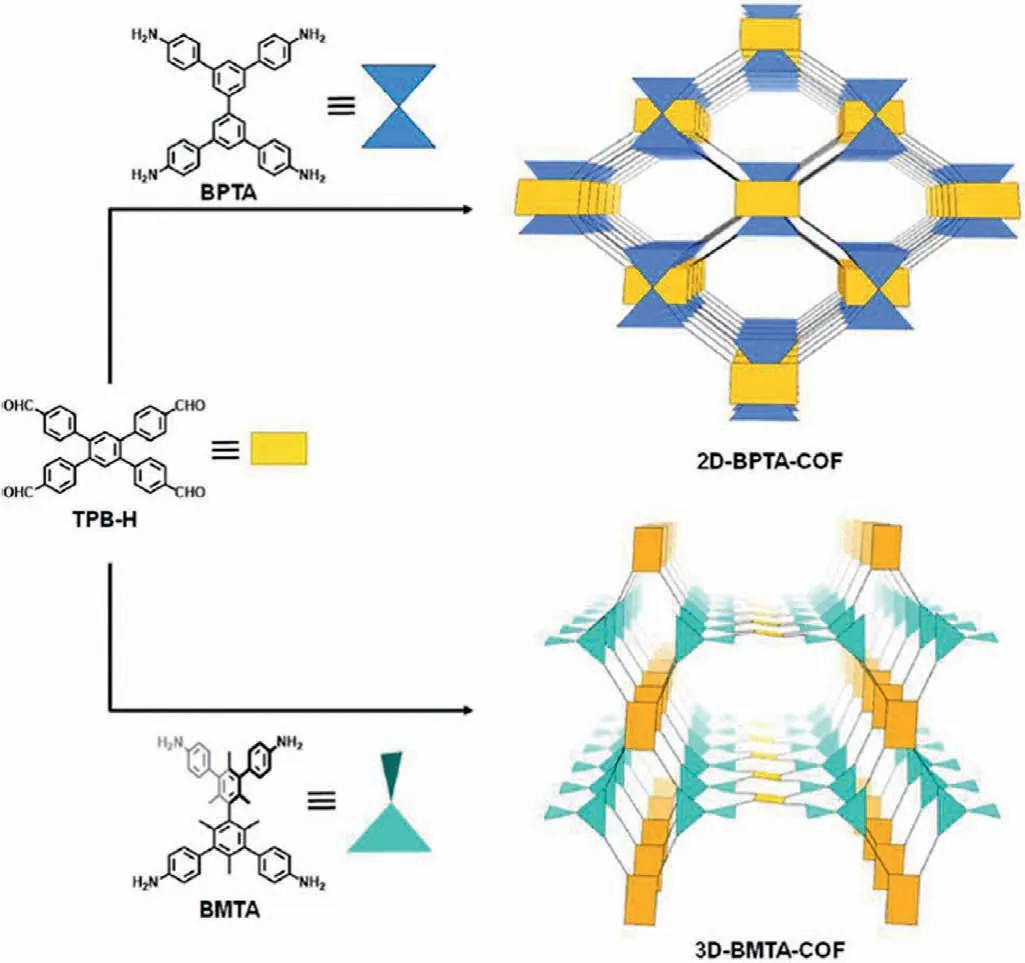
Fig.4.Schematic of the synthesis of 2D-BPTA-COF and 3D-BMTA-COF with different monomer geometry.Reproduced with permission [98].Copyright 2020,American Chemical Society.
When monomer bearing sidechains,it should be noted that there is a certain angle between skeleton and side chain,and thus the close packing of networks can be hindered to a certain extent.Quaterthiophene contained COFs with asymmetric monomer was prepared [99].Base on the design,the steric hindrance could be prevented through the asymmetry alkyl chains and the COFs layers can be stacked closely,contributing to the high crystalline structure and therefore enhanced conductivity.
Not only the crystallinity,but also monomer symmetry can affect the topology of corresponding COFs.For instance,when dihydroxyterephthalaldehyde (DHTA) was used as the building block,the resulting COFs posed two pore structures: hexagon and triangle pore.In contrast,when ethyl orn-butyl substituents were introduced into DHTA monomer,the corresponding COFs only bore a single pore [100].Therefore,carefully choosing monomers are needed when designing and constructing COFs.
4.3.Type of catalyst
Catalyst is an important assistor to guarantee the successful occurrence of the reaction and also affect the formation of dynamic bonds formation.Through effecting the reaction kinetics,catalysts could affect the process of error correction.Therefore,the type of catalyst is one of important factors that cannot be ignored when constructing of crystalline COFs.Because of the diversity of building blocks and variety of linkage formation,the selected catalysts are varied based on different reaction conditions.Acetic acid (AcOH) was the most widely used to construct COFs based on Schiff base reaction [36].Amine group react with aldehyde through nucleophilic addition with the presence of acidic environment firstly,and then the C=N bond is formed through dehydration and deprotonation [47].The whole process is reversible and therefore the reaction kinetics can be largely affected by the catalyst concentration [101].This principle is suitable for the formation of imine linkage,hydrazine linkage and azine linkage.Besides those above mentioned,Lewis acid,such as metal triflates and zinc chloride,was also reported as catalyst to permit the COFs formation.According to Dichtel’s work,with the presence of scandium(III) trifluoromethanesulfonate (Sc(oTf)3),the synthesis of imine-COFs was accelerated and the crystallinity and surface area of COFs were also increased largely [102].
For the less revisable linkage,the catalyst also acts as an important role in crystalline structure assembly.Zinc chloride was the common catalyst in ionothermal reactions,which acted as both the solvent and catalyst [79].For example,Lotsch and coworkers prepared highly crystalline imide-COFs at 300°C for about 48 h with the help of ZnCl2[80].According to the work,the reversibility of imide formation was enhanced by the presence of ZnCl2and thus the final product showed high crystallinity.For triazine linkage formation,besides ZnCl2,the usage of trifluoromethanesulfonic acid or P2O5also achieve triazine-based COFs (CTFs) [103].
Basic catalyst is another main class of catalyst family in the synthesis of COFs,such as Cs2CO3for CTFs [39],KOH or dimethylamine for sp2C=C carbon-COFs [57,104]and trimethylamine for dioxin-COFs [63].In our previous work,base catalysts with different alkaline strengths (pKa) were compared in the synthesis of CTFs and the results showed that potassium tert-butoxide(tBuOk) with the highest pKa (17.5) expressed the best performance in crystallinity enchantment of CTFs [105].Likewise,1,8-diazabicyclo[5,4,0]-7-undecene (DBU) was the selected catalysts among base catalysts when constructed porphyrin-based sp2C=C carbon-COFs [60].
Additionally,a new type catalyst,transition-metal nitrates,was emerged as a catalyst to assist the generation of COFs [106].According to the report,Fe(NO3)3·9H2O was the optimized catalyst and the resulting COFs could be obtained with the presence of oxygen within 20 min to 2 h,in which the reaction time was dependent on the building blocks.Moreover,this low cost type of catalyst showed good universality,contributing to the successful synthesis of 2D imine COF and 3D imine COF as well as azine COFs.
According to the reported works,even though varied catalysts were selected for the synthesis COFs and increasing ordered arrangement of skeletons,solid evidences are further needed to disclose the mechanism of catalyst in crystallinity of COFs in the following work.
5.Strategies for high crystallinity COFs
5.1.Layer stacking regulation
5.1.1.Layer interaction modulation
Layer interactions are usually considered as the main driving force for stacking and therefore play an important role in COFs crystallization [107,108].The interactions between layers are closely related to the groups in building blocks.Recently,a new strategy was developed for ultrafast construction COF within 30 min in gram scale.In this work,the bond rotation was firstly restricted by the hydrogen bondviaintra-and inter-layer hydrogen bonding and thus the planarity of the molecular was further enhanced (Fig.5).Furthermore,dipole-dipole interaction between interlayers could prompt layers in antiparallel stacking.As a result,the crystalline process was shorted effectively and targeting COFs was well-prepared in a large scale [109].
Suitable interaction can promote crystallization.But too strong interaction is unfavorable for the crystalline processes,such as the charge effect in ionic COFs.To decrease the charge repulsion,guanidine-based ionic COFs were developed with high crystallinity[110].According to the work,three aldehyde monomers with differ planarity,tris(4-formylphenyl)-1,3,5-triazine (TFPT),tris(4-formylphenyl) benzene (TFPB) and tris(4-formylphenyl) amine (TFPA),were used as knot-linker to tune the layer interaction.The results showed that both the diluted charge interaction and the enhancedπ-πstacking interaction were finally boosting the crystalline COFs.
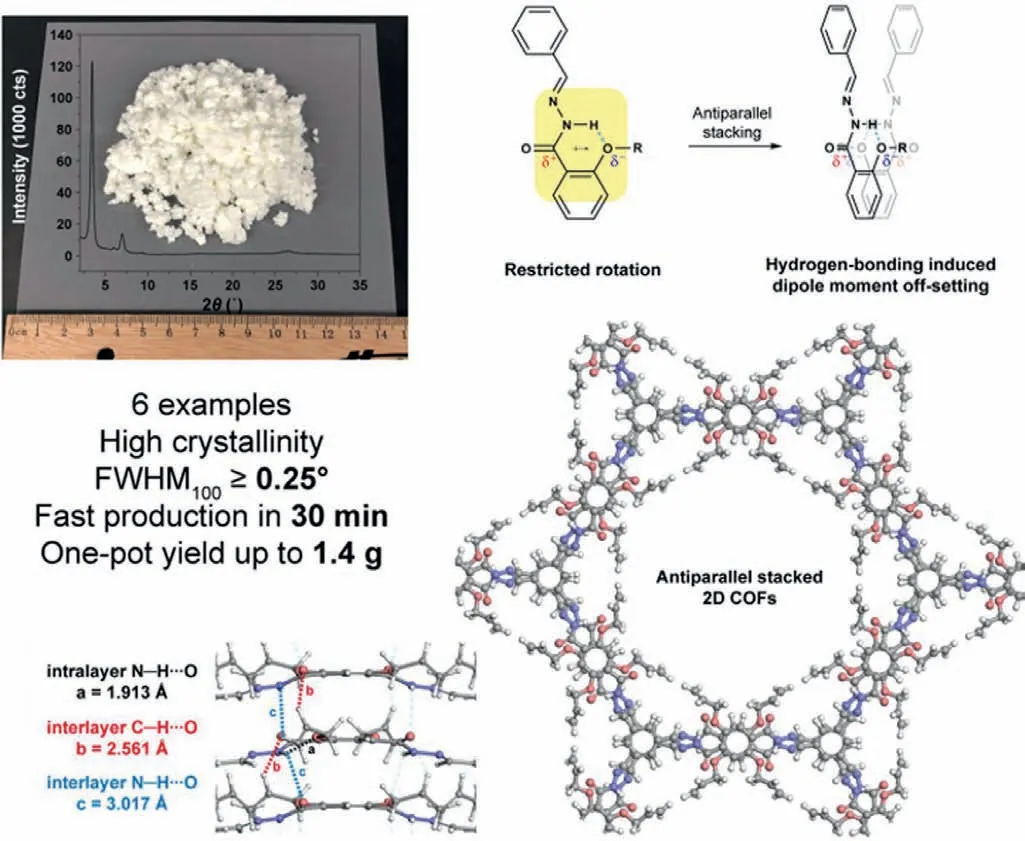
Fig.5.Illustration of construction COFs via dipole-induced antiparallel stacking.Reproduced with permission [109].Copyright 2020,American Chemical Society.
Obviously,the interaction between adjacent layers can certainly be tuned through the introduction of varied monomers.Two imine-COFs were synthesized with different aldehyde monomers:benzenetricarbaldehyde (BTCA) and 2,4,6-triformylphloroglucinol(TP).The different stacking ways of these two COFs were caused by the different interaction of the adjacent layers with the different monomers [111].
5.1.2.Pore maintenance
Ordered layer stacking often brings columnar pore structure,which is the virtue of the crystalline COFs.For the short-ordered or low crystalline structure,pore shrinkage or collapse after isolation is one of the main reasons for poor crystallinity.As we all know,the theoretical specific surface area can be obtained precisely based on structural mode [112].The higher crystallinity of resulted COFs,the smaller distinctions between the theoretical and experimental BET surface areas.But for most COFs,the experimental BET surface areas are smaller than theoretical values.Besides the poor crystallinity of the COFs [113],the partial structural collapse during activation is another possible reason for distinctions between the theoretical and experimental surface areas [114].Therefore,how to maintain the generated pore and to keep the ordered structure in long range is great meaningful for synthesis of crystalline materials.
One of the pore-maintenance strategy is steric hindrance engineering,through which well-defined 3D imine COFs with noninterpenetrated diamondoid (dia) topology was successfully obtained [115].The methoxy and methyl groups grafting on the building blocks enhanced the steric hindrance and then improved the rigidity of the networks,leading to the effective decrease of structural interpenetration and pore collapse in final 3D COFs.Besides the presence of groups,foreign molecules also can be introduced for the maintenance of the ordered channel of COFs.Very recently,Guo and his co-workers added polyethylene glycol-20000(PEG-20 K) chains into the columnar channel of BT-COFs to suppress the interlayered dislocation,leading to the high charge transfer efficiency and long term stability during photocatalytic hydrogen generation process [116].
Another method to maintain the columnar ordered pore inside COFs is the conservation of the layer stacking out of plane.The COFs without substituted groups in building blocks or enlargedπ-systems often suffer from exfoliation by solvents or layer correlation destroyed under the external stimulate.For the fragile COFs,supercritical carbon dioxide (scCO2) was optimized as the extraction way to switch the interlayer correlations of 2D imine-COFs.After scCO2activation,both fragile COFs and robust COFs showed high long ordered structure deduced from the sharp peaks in XRD patterns [117].It should be noted that vacuum activation can eclipse the already-formed ordered structure of COFs,resulting amorphous materials with the pore collapse.To avoid the loss of crystallinity with vacuum treatment,scCO2activation and nitrogen-flow activation method were adopted during COF isolation (Fig.6) and the results showed that the crystallinity of COFs can be well maintained [118].Therefore,post-treatment measure should be carefully implemented after the polymerization to obtain the targeting COFs.
5.1.3.Layer planarization
For 2D COFs,the layer stacking was often largely influenced by the torsion of building blocks or linkages.Therefore,suppressing the torsion of units and increasing the planarization of layer are effective to improve the crystallinity of COFs.H-bonding interaction between hydroxyl group in monomer and imine linkage is the most commonly used to lock the sheet in planar conformation,resulting in higher crystallinity of COFs with fewer defects [119].According to Jiang’s report,the content of H-bonding can be tuned through altering molar ratios of dihydroxyterephthalaldehyde (DHTA) and terephthaladehyde (TA) when constructing imine-COFs [120].The result showed that the corresponding H2P-Ph polymer was almost amorphous with low BET surface area of 20 m2/g when no H-bonding interaction.With the H-bonding content increase,both the XRD peak intensity and surface area of corresponding COFs were improved obviously.When the system was full of H-bonding,the corresponding H2PDHPh COFs exhibited the highest crystallinity and BET surface area was up to 916 m2/g.Besides that,the H-bonding content also can be controlled through changing the number of hydroxyl groups in monomer [121].The increased hydroxyl group number can fix conformation more firmly,and thus more ordered structure can be obtained easily.
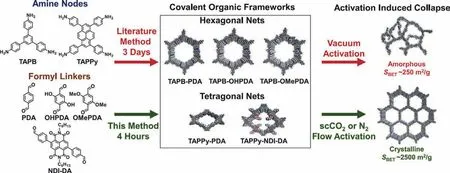
Fig.6.Pore maintenance in COFs via scCO2 activation or nitrogen-flow activation.Reproduced with permission [118].Copyright 2020,the John Wily and Sons.
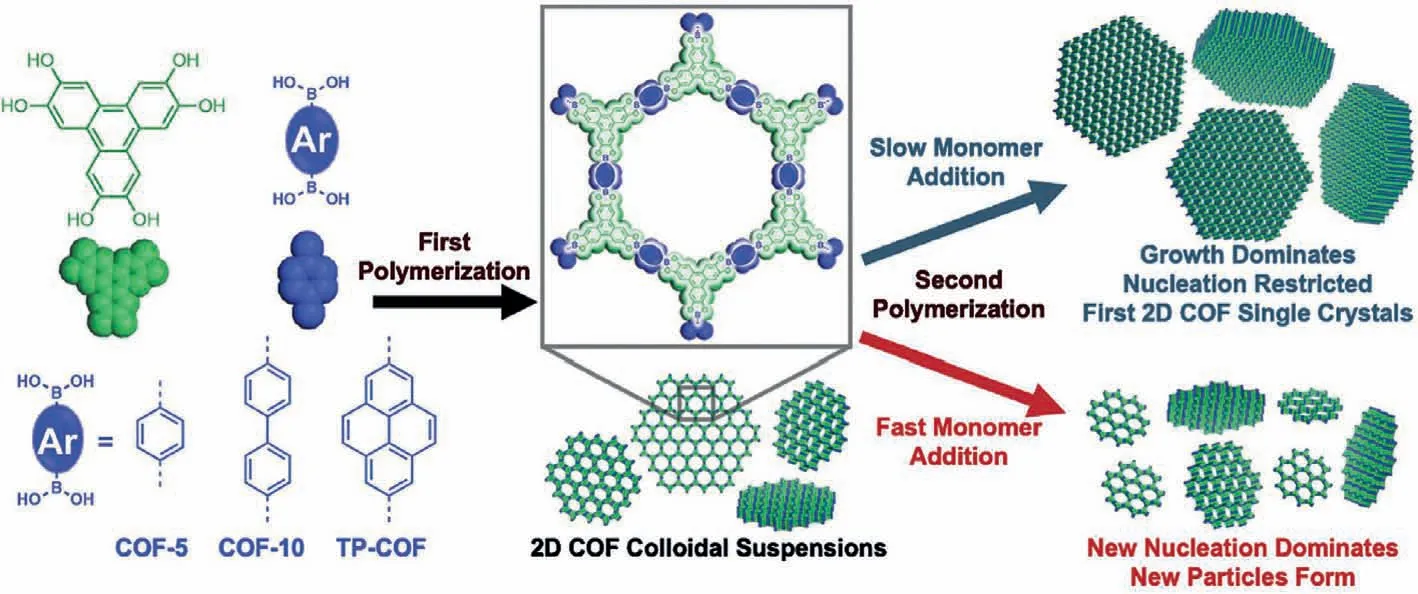
Fig.7.Schematic of controlled 2D COF with different monomer adding rate.Reproduced with permission [122].Copyright 2018,Science.
5.2.Dynamic control
The formation of COFs is the dynamic process composing of arrangement of polymer chains as well as the generation and degeneration of linkage.Hence,dynamic control is taken as a simple but effective method to enhance the crystallinity of COFs.As above mentioned,the mixed solvents could tune the solubility of monomers and therefore control the formation process of COFs[77,78].Apart from the solvent control,the following methods were also implemented to control the dynamic process of COFs.
5.2.1.Monomer concentration control
Based on the reaction equilibrium kinetics,monomer concentration is highly related to covalent bond making and breaking,and thus further to the whole crystallization process.In general,slowing the rate of nucleation and growth could strive for more time for “error checking” and “proofreading”,which is beneficial for crystal growth.Therefore,the rate of nucleation and growth could be tuned through the controlled monomer concentration.The strategy of slowing the monomer addition was first proposed in 2018 by Dichtel and co-workers [122].According to their work,the newly added monomers would further grow on the already seeds,instead of as the new nucleus,which was prone to the formation of single crystal with domain size of 1.5 μm at lateral dimensions (Fig.7).The experimental results showed that the size of COF-5 with low addition rate (0.10 equiv./h) was 400 nm,much bigger than that of conventional COF-5 with the size of 40 nm.Latter,using the same strategy,the size of imine-COFs (TAPB-PDA COF)was up to 690 nm with the slow rate of 1 equiv./h [123].
Unlike boronate ester linkage and imine linkage,the triazine linkage is much less reversible and thus construction of triazinebased COFs (CTFs) with high crystallinity are more challenging.With the same principle,controlling the aldehyde monomer feeding rate can tune the concentration of monomer and thus tune the nucleation rate [124].The results showed that when the feeding rate of aldehyde monomer to 30 μL/min,the resulting CTFs expressed the best crystalline structure,which was confirmed by the XRD pattern results.In addition to the external measurement to maintain the low monomer concentration,the internal controllable monomer amount in reaction system also can tune the crystalline process.According to the reported work,slowing the monomer concentration had already been realized throughin-situgeneration.Based on the aldehyde and amidine polycondensation method,the nucleation rate was slowed down by controlling the aldehyde monomer concentration throughin-situgeneration from alcohol monomers [39].This strategy was effective and convenient,and three alcohol monomers were employed to construct crystalline CTFs successfully.
5.2.2.Modulator induction
Modulator induction was first used in synthesis of boronate ester COF (COF-5) [125].According to the work,monoboronic acids were adopted as modulators and small amount (5%) of modulator can largely increase both crystallinity,crystal domain size and the surface area of COF-5.The presence of modulator could decrease the formation of COF through attachment and detachment.Moreover,the modulator could saturate the defects and decrease strain in crystal,making the resulting crystal more stable.Besides that,the COFs domains were terminated by the modulator and further increased modulator would decrease the domain size a little.
In 2018,Yaghi and Wang’s group developed a general strategy of construction single crystal imine COFs with crystal size in the micrometer scale by adding aniline as modulator and four single crystals of 3D imine COFs were successfully achieved: COF-300,COF-303,LZU-79 and LZU-111 [38].According to their work,aniline containing mono-functional group would change the crystallization process by inhibiting nucleation and thus imine exchange occurred with the enhanced reversibility of imine generation and degeneration (Fig.8).Here the modulator should be matched with the reactivity of the amine building blocks so that permitting the imine exchange.Additionally,the crystal size of resulting 3D COFs can be controlled by altering the amount of aniline in system.Moreover,aniline was also used as a modulator in fabrication of 2D COFs [121].With the presence of aniline,the nucleation was slowed down and thus the corresponding COFs exhibited more ordered structures and much higher BET surface area.
Recently,the benzaldehyde was further verdict to be another modulator for increasing crystallinity of imine-COFs by Verduzco and co-workers [126].According to their work,both the BET surface areas and crystallinity of targeting 2D imine COFs were improved effectively with the addition of 3-4 equiv.benzaldehyde.With the similar strategy,4-tert-butylcatechol (TCAT) was used as a modulator and nucleation suppressor to prompt crystal growth of 2D boronate ester-COFs [127].Compared with the approach of slow addition monomers into system,this chemical control strategy of suppressed nucleation with the presence of mono-functional molecular simplified the operation and expressed big potential application in more complex 2D COF systems.
5.3.Template induction
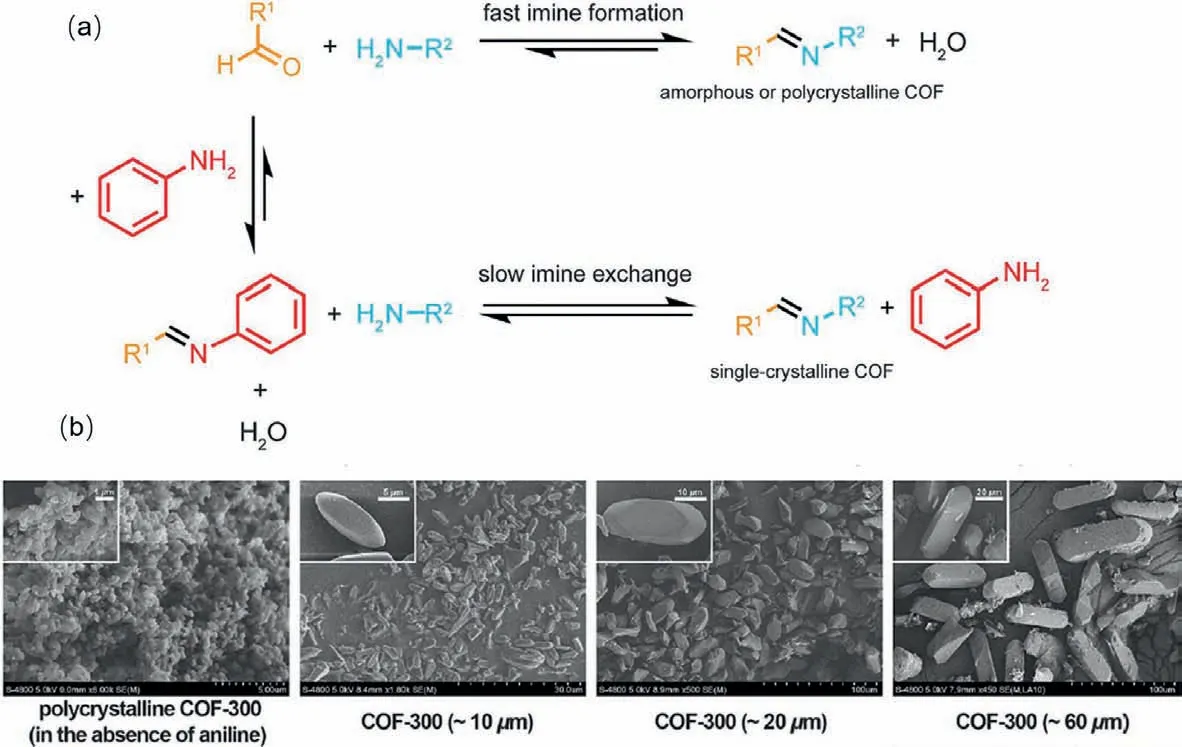
Fig.8.(a) Crystal growth of COFs with or without aniline.(b) SEM images of COFs-300 with controllable size.Reproduced with permission [38].Copyright 2018,Science.
As we all know,template is usually used for materials construction with specific morphology.In the same way,the ordered skeletons of polymers were firstly formed and then the post-reactions occurred,synthesizing the targeting COFs.In this part,the specific crystal plane of COF and the already crystalline COF and heterogeneous agent were adopted as templates,respectively.Based on these templates,the desired COFs were constructed with target crystalline structure after induction or post-treatment.
5.3.1.Topology template induction
The already formed crystalline structure of COFs can be served as the template to prompt the new COFs formation.Recently,the facets (001) of imine COFs were adopted by Jiang and co-workers as seeds and facilitated the generation of C=C bond [128].π-Interaction between building block and (001) plane of imine-COFs was the driving force for drawing and thus confining the new bond formation on the surface of the (001) plane of COF template.According to their work,three sp2C=C COFs were obtained with tetragonal,hexagonal and kagome topologies,respectively.This strategy provides a general and facile method for producing new COFs with more diversity.
5.3.2.Linkage transformation
The stability,physical and chemical properties and crystallinity of COFs are largely determined by linkages.Moreover,COFs are classified by type of linkages.Thus,the linkage is vital for COFs.Imine-COFs were the type COFs that be widely explored in recent years because of the formation reversibility of C=N bond and diversity of the building blocks for imine COFs,but the stability of imine bond is still insufficient,especially in some harsh condition.Therefore,fabrication of stable COFs through linkage transformation of reported COF is facile and feasible.
The first type of linkage transformation could be obtained through the further reaction between the pre-linkage with groups embedded in the monomers.Through changing monomers from triformylbenzene to triformylphloroglucinol,the corresponding COFs were also changed asβ-ketoenamine COFs from imine COF,in which the chemical stability was enhanced [129].
Another type is linkage post-treatment through chemical reactions,by which new linkage can be achieved but cannot be obtained byde novosynthesis.Liu and co-workers reported a new robust quinoline COF from imine COF with post-modification [130].Through the aza-Diels Alder cycloaddition reaction (Fig.9),the imine can react with arylalkynes and thus the imine COF was changed as quinoline COF,through which the stability was enhanced largely and the crystallinity of the new formed COF could be maintained well.Moreover,as mentioned in Section 2,thiazole linkage can be formed through oxidative annulation from imine linkage [65,66]even with different components.
Besides the cycloaddition reactions,the redox reactions are the most widely used chemical means for new linkage formation.Based on the direct oxidation of sodium chlorite,the imine COFs of tris(4-aminophenyl)benzene-terephthalaldehyde-COF (TPB-TP-COF)and 1,1,2,2-tetrakis(4-aminophenyl)ethene-terephthalaldehyde-COF(4PE-1P-COF) were converted to amide COFs 1’ and amide COF 2’,respectively.After conversions,the crystallinity and permanent porosity of amide COFs were maintained well [131].Besides that,imine COFs also can be transformed into other types of COF linkages.For example,COF-300-after reduction (COF-300-AR) and COF-366-after reduction (COF-366-AR) were successfully prepared from the imine COFs (COF-300 and COF-366-metal) reduction with NaBH4,which cannot be obtained from the direct reaction from monomers [132].Moreover,based on the imine COF as the starting material,carbamate-based COF was finally obtained by Yaghi and co-workers through three-step solid state transformations [133].Not only imine linkage,sp2C=C linkage also can be changed with post-modification.According to Cui and co-authors’work,C–C linked COFs was well prepared by direct reduction of C=C bond in the C=C linked COFs using NaBH4[134].
5.3.3.Building block exchange
Building block exchange is another strategy for transformation from amorphous porous covalent organic polymers to crystalline structures [135].According to the reported work,dynamic imine exchange was mostly used to obtain new crystalline structures[136].Four COFs had already been constructed through dynamic imine bond formation along with four derivatives of terephthalaldehyde replacing terephthalaldehyde in polymer chains [137].Similarly,Zeng’s team synthesized the other four imine COFs with the same strategy by replacing the building blocks [138].
Also,more complicated COFs could be obtained from simple ones through building blocks exchange [139].As we all know,amino group can react with aldehyde to form an imine bond,thus amino group is hardly preserved completely in imine-based COFs.Even so,amino-functional imine-based COFs were still reported through building blocks exchange [140].Firstly,two kinds of COFs were synthesized as mother COFs (COF-PTPA and COFPTBD)viacondensation of 1,3,5-tris(4-formylphenyl)triazine (PT)with 1,4-phenelynediamine (PA) and benzidine (BD),respectively.Then,amino-functional monomer,BD-NH2and PA-NH2were employed to replace PA in COF-PTPA and COF-PTBD,respectively (Fig.10).Under higher building blocks concentration and suitable reaction time and temperature,the targeting COFs contained amino groups were finally obtained with high crystallinity.
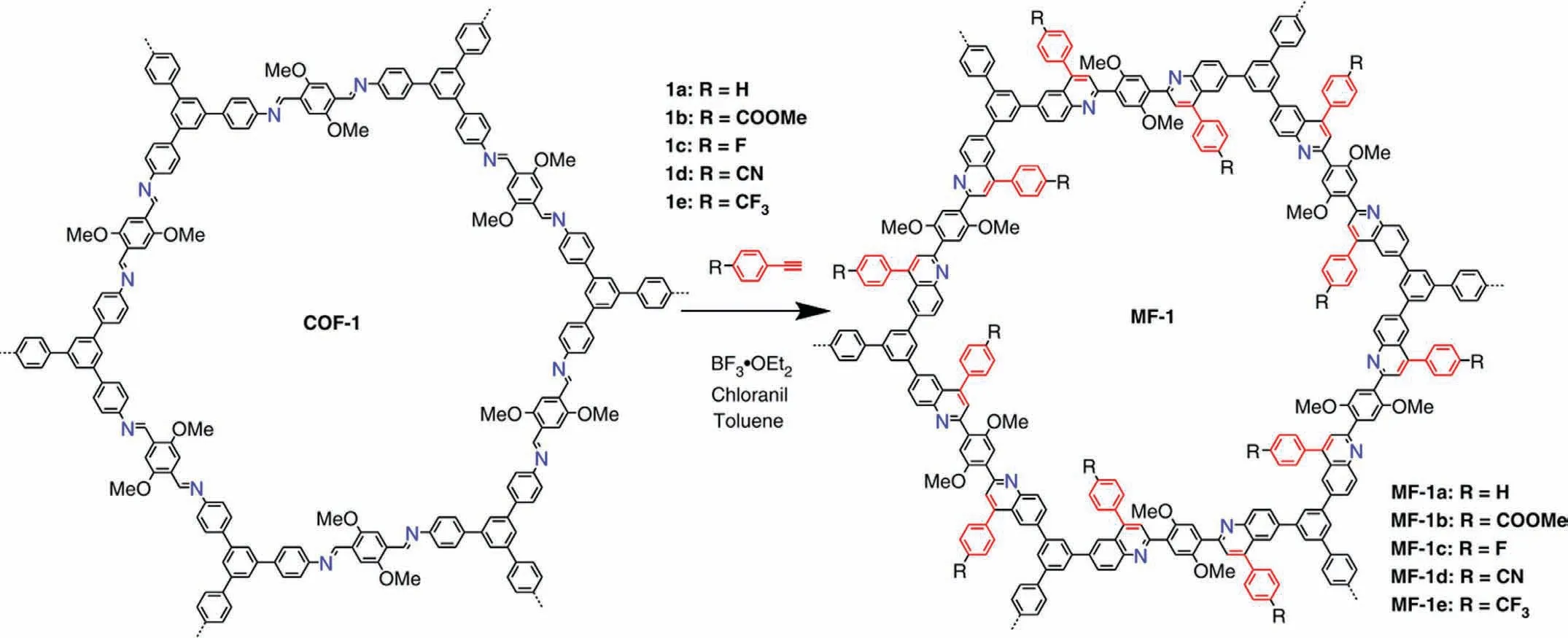
Fig.9.Synthetic route of the linkage transformation through aza-DA cycloaddition.Reproduced with permission [130].Copyright 2018,Nature.
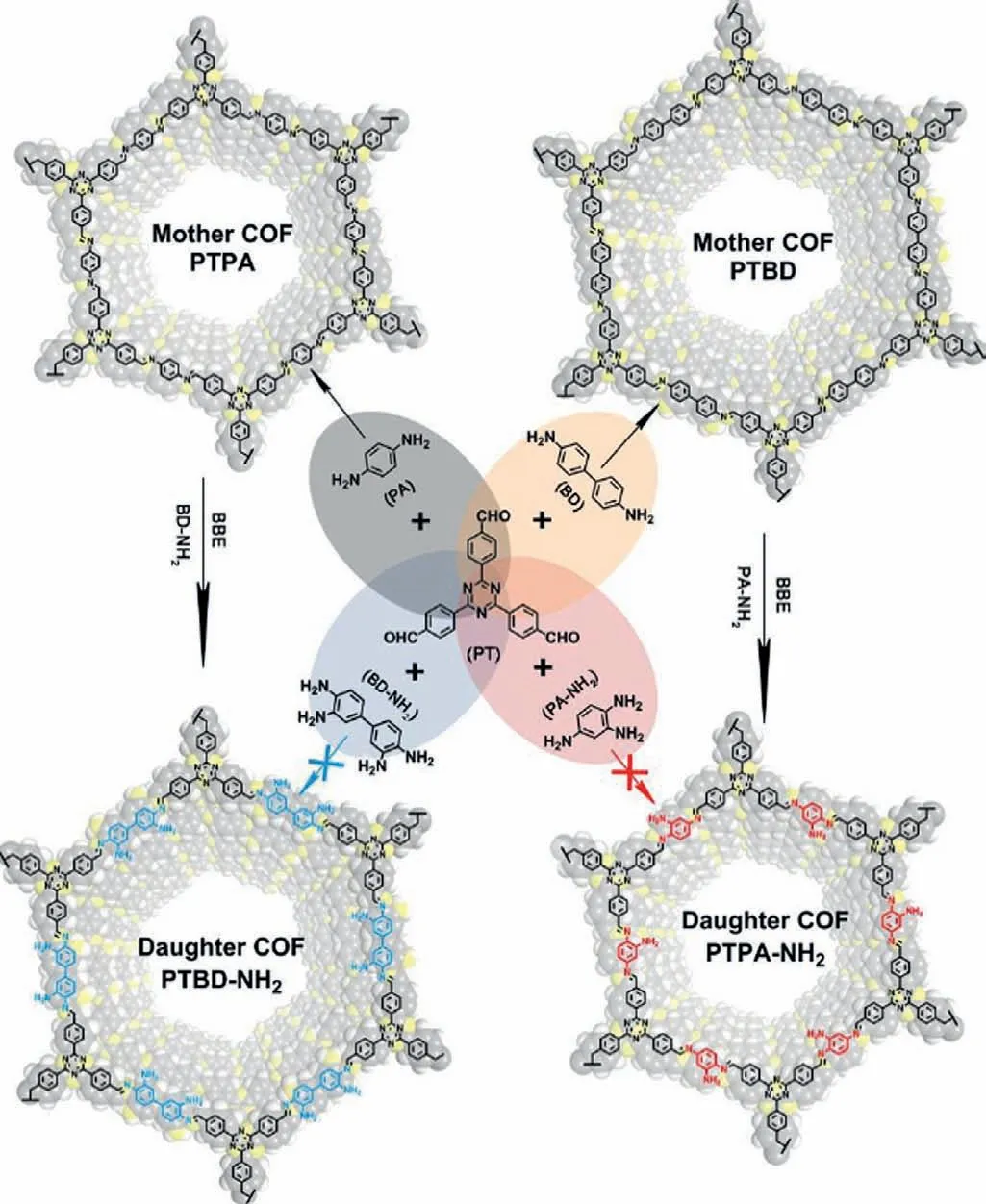
Fig.10.Illustration of synthesis of amino-functional COFs with building block exchange.Reproduced with permission [140].Copyright 2017,Royal Society of Chemistry.
In addition,the third component without reactive group was also utilized to organize the arrangement of polymer chains and increase the crystallinity.According to Banerjee’s work [141],12 COFs with high crystallinity were successfully obtained in seconds with the assistance ofp-toluenesulfonic acid (PTSA).During polymerization,the PTSA-amine salts were firstly formed through Hbonding interaction,which acted as the template for COFs backbones.And then,the added 1,3,5-triformylphloroglucinol (TP) could replace the PTSA through the formation of covalent bond with amine with grinding.In this way,the COFs with a surface area of 3109 m2/g were obtained within 60 s,which recorded the value among reported 2D COFs.In addition,the COFs could also be shaped in sheet,hollow structure or other forms.But up to now,this method still remains to be expanded into other types of COFs,which may be related to the react activity of aldehyde monomers.
5.3.4.Heterogeneous nucleation
Generally,it is unfavorable to the growth of crystalline materials when the product is precipitated fast from reaction solution.To prevent the precipitation of amorphous structures,aminofunctionalized SiO2(NH2-f-SiO2) was adopted as the heterogeneous nuclei to prompt the crystallinity of targeting COFs [142].Here,NH2-f-SiO2acted as seeds and the amino groups on the surface of SiO2could direct COFs growth,resulting the enhanced crystallinity and high surface area of COFs.Recently,our group developed a general strategy to prompt the crystallinity of CTFs using NaCl as heterogeneous agent [143].The crystalline CTFs were prone to be induced by the NaCl lattice and then grow on the surface of NaCl.Furthermore,the formation process of CTFs were slowed with the hindrance of the physical separator.Therefore,high crystalline CTFs were successfully achieved finally with wide monomer scope.
6.Conclusions and outlook
As the dynamic process,manipulation of crystallization is indeed a huge challenge.With the outstanding advantages brought from well-defined and ordered structure,the pursuit for COFs of high crystallinity is always a target with continuing enthusiasm.The emerging new types COFs,such as 3D Cage COFs [144]and foam COFs [145]and aerogel COFs [146],provide more chance for practical applications with promising futures.To meet the needs of varied applications,the development of COFs with high-quality crystal should be accelerated.Despite the progress that had been achieved,there are still some issues to be addressed from the perspective of long-term development.
As we all know,the crystalline process of COFs is still a black box and only several works have been done to give insight into the crystalline process.According to Dichtel and co-workers work in Section 3,the combination of simulation and experiment is of great value to understand the crystallization process,which is worth to be further deeply implemented in the future research.The computer-aided simulation could be carried out under ideal conditions,such as in homogenous system.Although it is largely departed from the actual experimental condition,the conclusions still are instructive.From the experimental perspective,the microscopic changes cannot be monitored with macro means.Therefore,simulation conclusions should be corrected by the experimental conditions and the final optimal simulation results would give effective guidance for crystallization improvement.
Up to date,most of crystalline structures are speculated from the agreement degree of XRD results between experiment and simulation.Thus,future studies will be directed toward developing more advanced and fined analysis technologies to give more reliable information about crystal growth and crystal structural parsing [147].It had been already proved that the crystalline process was recorded with smaller time interval and the crystallization process was overthrown with the assistance ofin-situXRD measure [76].Therefore,it is promising and desired to adopt otherinsitucharacterizations for probing the chemical and crystal structure more clearly and precisely,such asin-situTransmission Electron Microscope andin-situNuclear Magnetic Resonance.Advanced and fined measurements would definitely promote the development of high COFs with high crystalline quality field.
Crystallization is a dynamic process and thus dynamic control is an effective strategy for COF crystal growth.As proven by Yaghi and Wang,the presence of aniline could change the crystallization process and enhance the reversibility of imine generation and degeneration as modulator,resulting in a large single crystal of 3D imine COF with size of tens of microns [38].Besides the assistance of modulator,monomer concentration is another reported dynamic control strategy to enhance the crystalline COF.As above mentioned,the amount of monomer was controlled through physical or chemical method to slow the crystalline process of CTFs,and finally the less revisable triazine based COFs (CTFs) with high crystallinity were achieved [39,124].Hence,the strategy of dynamic control in crystalline process is highly deserved to do.
In summary,crystalline COFs have already successfully achieved vigorous development in synthesis and applications over the past few years.Based on the current research,a promising future of the crystalline COFs can be expected with great efforts to explore crystallization mechanism and extend application in the coming years.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by Natural Science Foundation of Shandong Province,China (No.ZR2021QB070).
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
