Effect of ligand initial conformation and counteranion on complexation behaviors of R-BTBP toward Pd(II) contained in highly active liquid waste
Lei Xu, Weny Ding, Anyun Zhng, Ziyng Liu
a Key Laboratory of Nuclear Agricultural Sciences of Ministry of Agriculture and Zhejiang Province, Institute of Nuclear Agricultural Sciences, Zhejiang University, Hangzhou 310058, China
b College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
c College of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China
ABSTRACT Soft N-donor bis-triazin bipyridines derives (R-BTBP) are a type of very promising extratant for extraction and complexation with long-lived trivalent minor actinides over lanthanides from highly active liquid waste (HLW).In addition to minor actinides, R-BTBP also holds very strong complexation ability toward fission palladium.However, few studies have been focused on the separation and complexation with the fission product Pd(II) by R-BTBP.Herein, the complexation behaviors of Pd(II) with four typical R-BTBP ligands were systematically studied by single crystal X-ray diffraction, 1H NMR titration and theoretical calculation.The effects of R-BTBP initial conformation and nitrate anions on the complexation behaviors of R-BTBP with Pd(II) were thoughtfully analyzed.Both the 1:1 and 2:1 binuclear complexes could be formed between Pd(II) and R-BTBP with initial II conformation in the presence of nitrate anions, while only one 1:1 type Pd(II) complex could be formed for those with initial OO conformation.Without nitrate anion, only one 1:1 type complex was formed in solution.The structure of the 1:1 Pd(II)/R-BTBP complex was firstly characterized by single crystal crystallography.DFT calculation results showed that a significant large rotational energy barrier (21.8~22.6 kcal/mol) must be overcome to form the II type 2:1 Pd(II) complex for those OO type R-BTBP ligands, however which would not prevent them from forming the 1:1 type complex.
Keywords:R-BTBP Fission product palladium Minor actinides Complexation Highly active liquid waste
Highly active liquid waste (HLW) containing the long-lived minor actinides (MAs such as Am and Cm) along with other longlived fission products (LLFPs) (like129I,93Zr,99Tc and107Pd) are generated from the PUREX (Plutonium and Uranium Refining by Extraction) process aiming at the recovery of U and Pu from spent nuclear fuel (SNF) using 30% tri-n-butyl phosphate (TBP)/kerosene as extractant [1–4].These LLFPs have potential and long-term radioactive hazards to environment and human health.Thus the effective partitioning and transmutation (P&T strategy) of LLFPs into stable or short-lived elements are very valuable [5–7].One key step of P&T is firstly needs to separate MAs from neutron poisons trivalent lanthanides by chemical method [8–13] and then transmute them into stable or short-lived elementsviabombardment of nuclei with neutrons.Due to the chemical similarity between trivalent MAs and lanthanides, the selective partitioning of MAs has always been one of the most challenging works that have not been solved [14–18].
Recently, it has been demonstrated that three classes ofN-heterocyclic bis-1,2,4-triazin ligands, 2,6-bis(1,2,4-triazin-3-yl)pyridine (BTPs) [6,19,20], 6,6′-bis(1,2,4-triazin-3-yl)bipyridine(BTBPs) [5,21–26] and phenanthroline-derived bis-triazine ligand(BTPhens) [27–29] have great potential in highly efficient separation of trivalent MAs over trivalent lanthanides from HLW.For example, one typical BTBPs type ligand, CyMe4-BTBP, has been selected as the current European relevant extratant for direct separation of MAs (Am(III) and Cm(III)) from genuine spend unclear fuel solution in SANEX (Selective Actinide Extraction) process [12].
In this process, it requires the extractant able to selectively extract MAs over all the other fission or corrosion products present in HLW.However, it has been found that in addition to MAs, these BTBPs ligands also have strong affinity for other fission products like Pd(II), Ag(I), and Ni(II)etc.in HNO3solution [30–33].Among these interfering elements, the most problematic element might be the107Pd and its isotopes.This is because that the content of107Pd and its isotopes in SNF is around 1~2 kg/ton and the portion of107Pd (with a half-life of 6.5×106year) is around 17%, which is much higher than that of other LLFPs [34,35].To solve this problem, one way was to use some hydrophilic masking agents, which are able to selectively complex with those problematic elements to form the water soluble complexes in aqueous phase to prevent them being co-extracted with MAs into organic phase when using BTBPs as extractant [36–38].However, the introduction of these masking reagents would unavoidably increase the secondary wastes, which was not desired in actual separation process.
Considering these bis-triazine ligands have strong complexation ability both for MAs and Pd(II), we thus attempt to simultaneously separate MAs and Pd(II) using these R-BTBP ligands.Recently, in our group it has been found that the simultaneous partitioning of MAs and Pd(II) from HNO3media could be achieved through extraction chromatography method by some silica-based material functionalized by BTPs/BTBPs chelating ligands [39–42].In this separation process, Pd(II) and MAs are firstly simultaneously absorbed by BTPs/BTBPs functionalized silica-based materials and then they can be further separated by using 0.2 mol/L thiourea/0.1 mol/L HNO3(for Pd(II)) and 0.01 mol/L HNO3/1.0 mol/L NaNO3(for MAs) as eluents, respectively, while many other coexistent fission products exhibited almost no adverse effects.It provides a feasible approach for direct separation of MAs and Pd(II)from HLW.Although much progress has been made on the separation and complexation of minor actinides over lanthanides using triazinylpyridine ligands, far less progress has been made on their separation and complexation with fission product Pd(II) [30,32].In this work, we focus on revealing the complexation behaviors of Pd(II) with four typical BTBPs ligands (Fig.1) by single crystal crystallography,1H NMR titrations and theoretical calculation methods.The effect of ligand initial conformation and counteranion on the complexation behaviors of R-BTBP toward Pd(II) were mainly discussed.
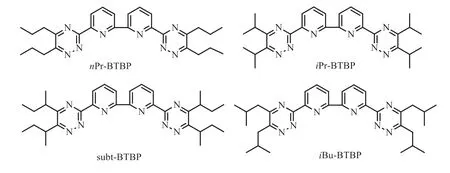
Fig.1.Structures of four R-BTBP ligands studied in this work.
Two completely different conformations were found in the single crystal structures ofnPr-BTBP, subt-BTBP,iPr-BTBP andibu-BTBP.The conformations of BTBPs were defined based on the relative positions of two adjacent nitrogen atoms bearing on the triazine and pyridine rings.As shown in Fig.2, if two adjacent nitrogen atoms are on the same side of pyridine nitrogen, it is called the inward-inward (II) conformation; however, if two adjacent nitrogen atoms are on the other side of pyridine nitrogen, it is defined as an outward-outward (OO) conformation.Therefore,nPr-BTBP and subt-BTBP have an II conformation, whileiPr-BTBP andibu-BTBP hold the OO conformation.These two kinds of conformations were also found in single crystal structures of C7-BTBP,C2-BTBP and CyMe4-BTBP ligands [43,44].These two different conformations might result from the steric effects of different substituents as well as the hydrogen bond interactions present in their solid state structures (Figs.S9–S12 in Supporting information).
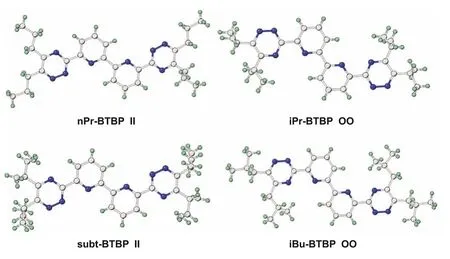
Fig.2.Single crystal structures of four typical R-BTBP ligands with two different conformations.
NMR spectroscopic titrations of organic ligands and diamagnetic metal ions have been accepted as an effective method to identify the speciation and structure of metal-ligand complexes in solution[28,30,44,45].Thus,1H NMR titration of palladium salts with four typical BTBPs ligands were performed to identify the Pd(II) complexes species in solution.
Initially, the Pd(NO3)2was used as the titrant to explore the complexation behaviors of Pd(II) with C3-BTBP which has an initial inward-inward conformation.As presented in Fig.3a, upon addition of Pd(NO3)2(0<M/L ≤1.0), the resonance peaks of pyridine protons H1and H3shifted upfield from 8.80 ppm to 8.57 ppm to 8.52 and 8.48 ppm, respectively, on the contrary, the signal of H2shifted downfield from 8.17 ppm to 8.66 ppm, indicating the formation of one new species in solution.These changes suggested that the ligand initially formed 1:1 type complex (marked with black triangles) with palladium since the transformation of ligand to a new species was complete after addition of 1.0 equiv.of Pd(NO3)2.What is more, the peaks of H4and H4′ displaying two independent triplets in free ligand shifted downfield and became overlapped with concentrations of Pd(II) increasing, suggesting that the chemical environments of protons H4and H4′ became more similar after complexing with metal ions.These changes indicate that Pd-N bond were formed between Pd(II) and nitrogen atoms of triazine and pyridine rings [30,43,46].
Subsequent formation of another new minor species of 2:1 Pd(II)/R-BTBP complex at high M/L ratios (M/L>1.0) was deduced base on the appearance of three new groups of NMR peaks(marked with black circles) locating at 8.65 ppm, 8.50 ppm and 8.05 ppm, respectively.It means that a binuclear complex of Pd(II)with C3-BTBP was formed in solution.Furthermore, considering the geometry feature of Pd(II) complex, NO3-was thought to be participated in formation of the 2:1 Pd(II)/C3-BTBP complex.This hypothesis was further validated by the titration of C3-BTBP with Pd(BF4)2in the absence of nitrate anions.As presented in Fig.3b,when M/L ≤1.0, only one new group of NMR signals (marked with black triangles) locating at 8.65 ppm, 8.45 ppm and 3.10 ppm were observed, indicative of the formation the sole 1:1 Pd(II)/C3-BTBP complex in solution.The above mentioned 2:1 Pd(II)/C3-BTBP complex however disappeared in this case.
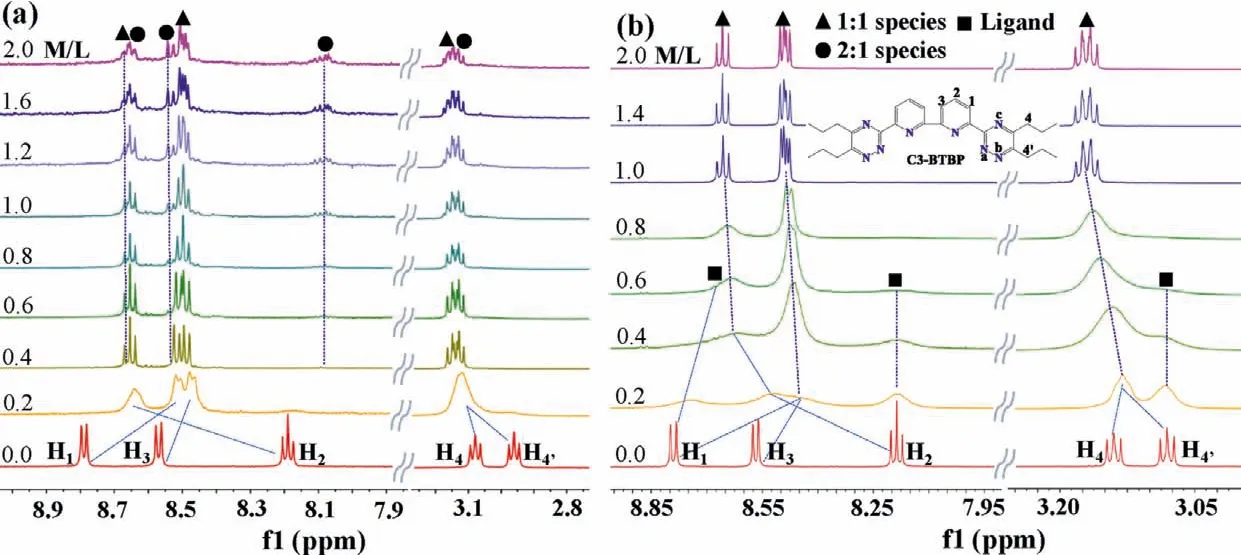
Fig.3.(a) Stacked 1H NMR spectra of C3-BTBP (10 mmol/L) titrated with Pd(NO3)2; (b) titrated with Pd(BF4)2 in CD3CN at 295 K.
Similar results were also found in the titrations of subt-BTBP with Pd(NO3)2and Pd(BF4)2, respectively, indicating that C3-BTBP and Subt-BTBP with same initial conformation hold similar complexation modes with Pd(II).As presented in Fig.S13a (Supporting information), when using Pd(NO3)2as titrant, one new group of signals relating to the protons H1, H2and H3increased to the maximum at M/L=1.0 and then decreased gradually, which was assigned to the 1:1 species.When M/L value was beyond 1.0, two new groups of tiny broaden peaks belong to protons H2/H1and H3appeared at 8.65 and 8.05 ppm, respectively, indicative of the formation of the 2:1 Pd(II)/Subt-BTBP complex.However, when using Pd(BF4)2as titrant (Fig.S13b in Supporting information) only one type signals of the 1:1 species was observed.Again, it demonstrates that the presence of nitrate anions is one of the preconditions for the formation of binuclear Pd(II)/R-BTBP complexes.
As shown in Fig.4a, the results of1H NMR spectra ofiPr-BTBP titrated with Pd(NO3)3suggested that only one 1:1 Pd(II)/iPr-BTBP species was formed in solution, since the conversion of NMR signals aroused from protons H1, H2, and H3locating at 8.85 ppm,8.60 ppm and 8.20 ppm to a new species was completed after addition of 1.0 palladium equivalent.Further increasing the Pd(NO3)3equivalents, these signals kept constant.Similar results were also found in the titration ofibu-BTBP with Pd(NO3)3(Fig.4b).Based on these results, it is concluded that the bimetallic complex of Pd(II) cannot be formed for those R-BTBP with initial OO conformation.
For the solid Pd(II)/R-BTBP complexes, only one 1:1 Pd(II)/iPr-BTBP complex has been isolated and structurally characterized.This is the first time that the solid 1:1 Pd(II) complex with any R-BTBP type ligands has been successfully characterized by single X-ray crystallography, although many attempts have been tried by other researchers using CyMe4-BTBP ortBu-CyMe4-BTBP as chalets[32,47].As presented in Figs.5a and b, the geometry of Pd(II) exhibits a distorted square-planar with oneiPr-BTBP molecular binding as a quadridentate ligand with central Pd(II).The bond distances from Pd to N11 and N15 are 2.032(6)and 2.028(6),respectively, which are longer than those to the pyridine nitrogen atoms ranging from 1.918(5)to 1.930(7).The almost identical bond lengths of Pd-N13 to Pd-N12, and Pd-N11 with that of Pd-N15, are in line with the symmetry feature of the 1:1 Pd(II)/iPr-BTBP complex as identified in its1H NMR spectra (Fig.4a).Similar bond distances of Pd-N have also been found in Pd(II)/terpy[48] and Pd(II)/BTP [49] complexes.Additionally, two nitrate anions were found in the second coordination player to keep the charge balanceviathe formation of hydrogen-bonding interactions with water molecules and ligands (Fig.5c).
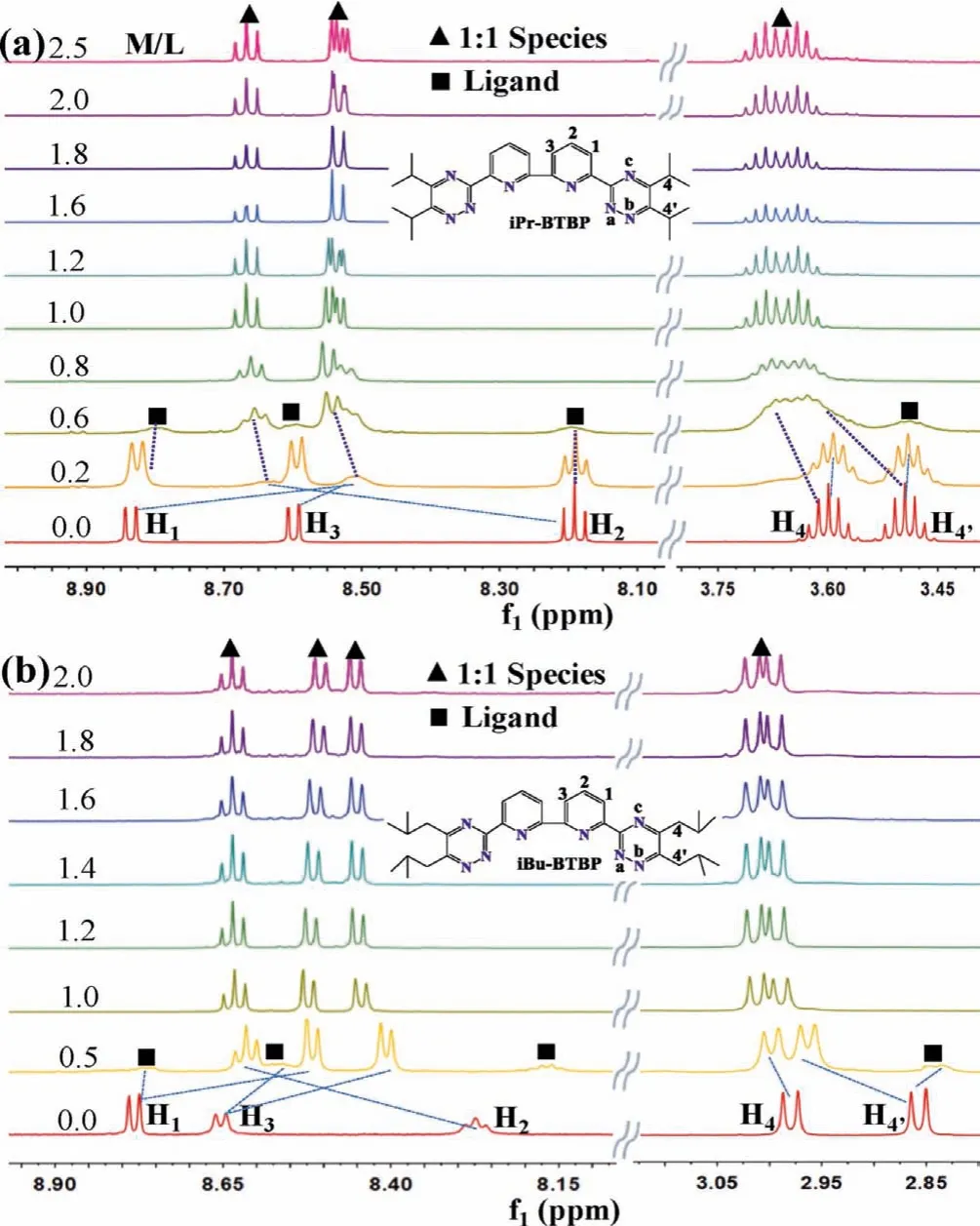
Fig.4.Stacked 1H NMR spectra of (a) iPr-BTBP (10.0 mmol/L) and (b) iBu-BTBP(10.0 mmol/L) titrated with Pd(NO3)2 in CD3CN at 295 K.
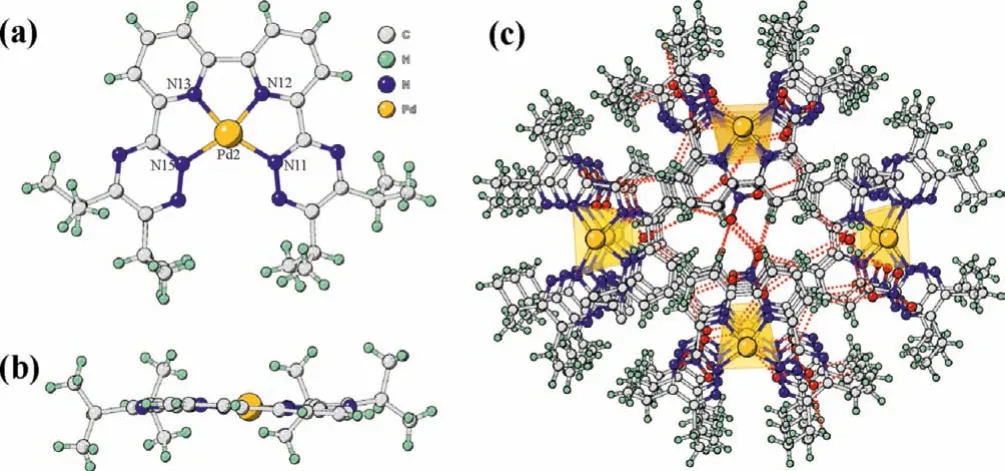
Fig.5.Structure of the Pd(iPr-BTBP)2+ moiety in the single crystal of [Pd(iPr-BTBP)]2(NO3)4(H2O)2(CH3CN).(a) A front view, (b) a top view, (c) hydrogen bonding interactions in the structure.Selected bond lengths (): Pd2-N11 2.032(6), Pd2-N12 1.918(5), Pd2-N13 1.930(5), Pd2-N15 2.028(6).
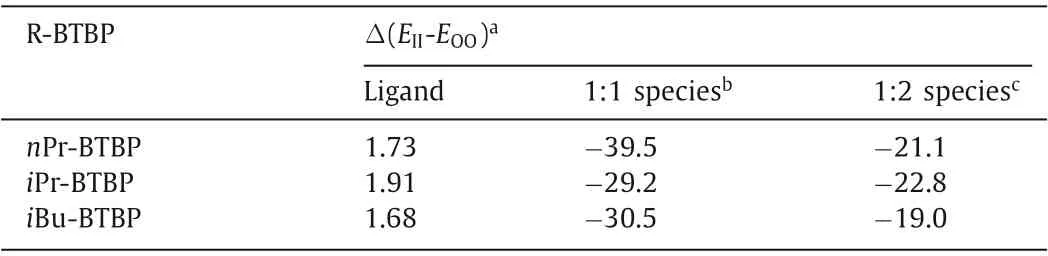
Table 1 Calculated energy differences ΔE (kcal/mol) of R-BTBP ligands and their complexes with Pd(II).
Further, the light yellow powders of the 1:1 and 2:1 Pd(II)/subt-BTBP complexes (Fig.S14 in Supporting information) were prepared and characterized by elemental analysis and1H NMR spectrum.The measured elemental contents in the 2:1 Pd(II)/subt-BTBP complex are 39.10, 4.29 and 17.10 for C, H amd N elements,which can be assigned to a chemical formula of [Pd2(NO3)4·(subt-BTBP)]·H2O (theoretical contents are 38.75, 4.21 and 16.82 for C, H and N, respectively).For 1:1 Pd(II)/subt-BTBP complex, the measured elemental contents are 41.57, 4.87 and 17.91 for C, H amd N elements, which could be assigned to a chemical formula of [Pd(NO3)2(subt-BTBP)]·2HNO3·2H2O (theoretical contents are 41.27, 18.05 and 5.16 for C, H and N, respectively).These results demonstrate that both 1:1 and 2:1 Pd(II)/R-BTBP species can be present in solid state.Also the1H NMR spetra of the solid 2:1 and 1:1 Pd(II)/subt-BTBP complexes in CD3CN was shown in Fig.S15b (Supporting information), for 1:1 species, two new signals(labeled with ) found at 8.50 ppm and 8.65 ppm were ascribed to H1/3and H2(Fig.S15a in Supporting information) based on their peak area and patterns.In addition, two new multiple-peaks at around 3.40 ppm relating to H4and H4′ become overlapped in the 1:1 Pd(II)/subt-BTBP complex.These results agree well with the above1H NMR spectral features of the 1:1 Pd(II)/R-BTBP species (Fig.S12a in Supporting information).Notably, another two new peaks (labeled withFig.S15c in Supporting information)appearing at 8.45 ppm and 8.05 ppm are assigned to protons of 2:1 Pd(II)/subt-BTBP complex.Additionally, the coexistence of signals corresponding to the protons of 1:1 species (labeled with , Fig.S15c) indicated that the 2:1 species is less stable than the 1:1 species and it can transform to 1:1 species in solution.
To further understand the effect of ligand initial conformation on the complexation behaviors of R-BTBP with Pd(II), the structures ofnPr-BTBP,iPr-BTBP,iBu-BTBP as well as their 1:1 and 2:1 Pd(II) complexes with II and OO conformation were optimized at B3LYP lever in gas phase.Based on the structures of 1:1 Pd(II)/iPr-BTBP (Fig.5) and previously reported 2:1 Pd(II)/R-BTBP binuclear complexes [30,43], the optimized structures of three ligands and their 1:1 and 2:1 complexes with Pd(II) were shown in Figs.S16 and S17 (Supporting information).The results showed thatnPr-BTBP,iPr-BTBP andiBu-BTBP with OO conformation are all more stable than their corresponding II conformers by 1.70–2.0 kcal/mol(Table 1), indicating that R-BTBP type ligands are inclined to be exist as OO conformation in gas phase and similar results have also been found for R-BTP type ligands [49].But once these R-BTBP/RBTP ligands interacted with solvent molecules like water or acetonitrile, they would change their conformations to obtain a more stable state.In this case,nPr-BTBP and subt-BTBP adopt the initial II conformation, whileiPr-BTBP andiBu-BTBP hold the OO conformation as confirmed by single X-ray crystallography.
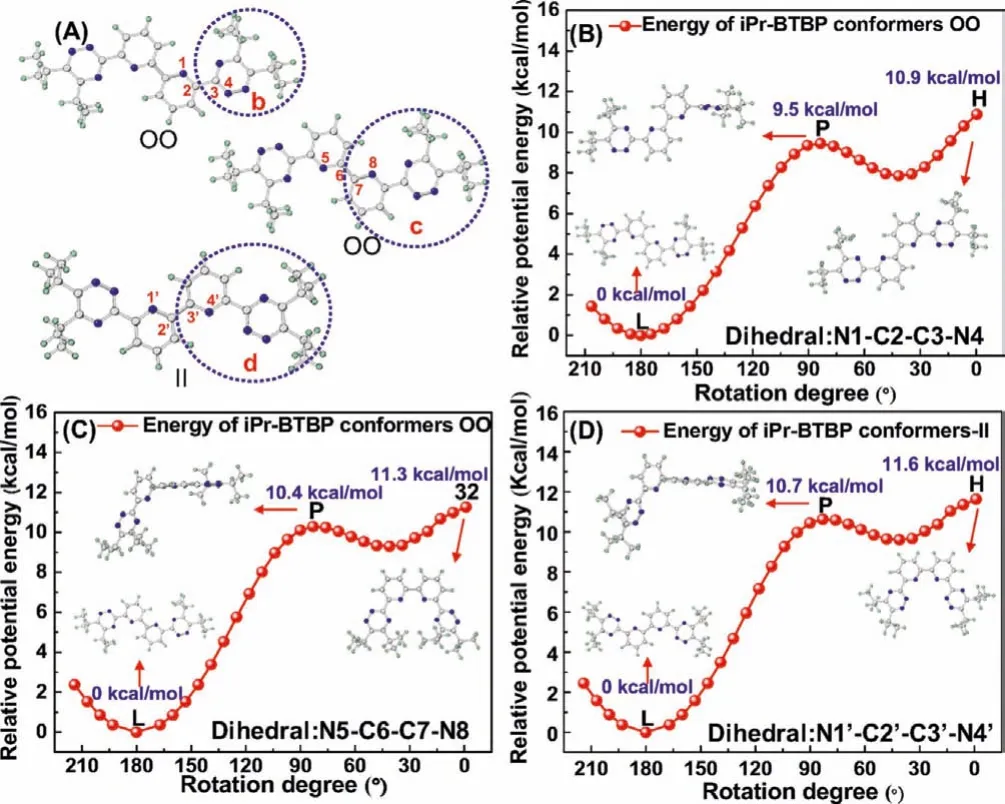
Fig.6.(A) Three dihedral angles (b) N1-C2-C3-N4, (c) N5-C6-C7-N8 and (d) N1′-C2′-C3′-N4′ defined for the rotational energy calculations.Relative potential energy curves of iPr-BTBP (B) from initial OO to the final IO conformation, (C) from initial OO to the final coordinated OO conformation, (D) from initial II to the final coordinated II conformation.
Nevertheless, the binding energy calculations (Table 1) showed that all the 2:1 and 1:1 Pd(II)/R-BTBP complexes with II conformation are much more stable than their corresponding OO conformation by 20–40 kcal/mol.The noteworthy average shorter bond distances of Pd-N bonds in II conformation than those with OO conformation (Table S3 in Supporting information) found in both 1:1 and 2:1 Pd(II)/R-BTBP complexes could also support this conclusion.These results demonstrated that all R-BTBP ligands in Pd(II)/R-BTBP complexes have the II conformation, which agrees well with the results of single crystal X-ray diffraction study.It means that the coordinatediPr-BTBP andiBu-BTBP molecules in their Pd(II) complexes hold different conformations with their free ligands.In other words, these two ligands have an initial OO conformation, which is unfavorable to form the 2:1 Pd(II)/R-BTBP binuclear complexes and thus the conformational changes of free RBTBP ligands from OO to II type is necessary prior to coordinating with Pd(II).
To reveal the complexation mechanism of Pd(II) with R-BTBP as confirmed in1H NMR studies, the rotational energies of free R-BTBPs transformation from initial OO or II conformation to the final coordinated conformation were calculated.To make the calculation easier,iPr-BTBP with relatively simple structure was selected as a model molecule for R-BTBP.As discussed above, theiPr-BTBP molecule with initial OO configuration, can only form the 1:1 II type complex with Pd(II).To simulate its conformation exchange from OO to II type to complexation with Pd(II), two steps of rotation of C-C bonds that link the pyridine and triazine rings as well as two pyridine rings are needed.Therefore, two dihedral angles N1-C2-C3-N4 (Fig.6A, part b) and N5-C6-C7-N8 (Fig.6A,part c) varying from 0° to 206° with an interval of 7° were set to calculate these two steps of rotational energy barriers ofiPr-BTBP transformation from initial OO to the final coordinated II type conformation, respectively.The calculation results showed that the largest rotational energy barrier for one triazine ring ofiPr-BTBP was around 10.9 kcal/mol (Fig.6B) and for one pyridine ring and triazine ring as a whole was around 11.3 kcal/mol (Fig.6C).Therefore, it could be predicted that the largest rotational energy barrier for whole molecule was up to 21.8–22.6 kcal/mol, which are much lower than the energy difference (29.2 kcal/mol, Table 1) between the II and OO conformers for 1:1 Pd(II)/iPr-BTBP complex and are close to that (22.8 kcal/mol, Table 1) of the 2:1 Pd(II)/iPr-BTBP complex.
On the other hand, compared with two steps rotation of OO typeiBu-BTBP, one step of the rotation of C-C bond that links the two pyridine rings (Fig.6A, part d) is also needed to form the 1:1 complexes fornPr-BTBP and subt-BTBP with initial II conformation.By rotation of the dihedral angle N1′-C2′-C3′-N4′ (Fig.6A,part d) from 0° to 206° with an interval of 7°, the largest rotational energy barrier was calculated to be 11.6 kcal/mol.This energy barrier is significantly lower than that of the energy difference between the II and OO conformers of the 1:1 Pd(II)/iPr-BTBP complex (29.2 kcal/mol, Table 1).This energy barrier could not prevent those OO type R-BTBP ligands to form the 1:1 Pd(II)/R-BTBP complex.Notably, thenPr-BTBP and subt-BTBP type ligands with initial II type conformations are very facilitated to form the 2:1 Pd(II)/R-BTBP complex (Fig.6A) without needing any conformational change.However, for those R-BTBP with initial OO conformation, they must overcome a significant rotational energy barrier of 21.8 kcal/mol to achieve a II conformation that is need to form the 2:1 Pd(II)/R-BTBP complex.Such a high energy barrier must have restricted the iPr-BTBP oriBu-BTBP ligands to form the 2:1 species.
In summary, R-BTBP type ligands were found to have two different initial conformations, which exhibit significant effect on their complexation behaviors with Pd(II).It was found that both the 1:1 and 2:1 binuclear complexes could be formed between Pd(II) and R-BTBP with initial II conformation in the presence of nitrate anions, while without nitrate anions only one 1:1 species could be formed.For those R-BTBP with initial OO conformation, only the 1:1 type Pd(II) complexes were formed.The solid structure of the 1:1iPr-BTBP/Pd(II) complex has been firstly characterized by X-ray crystallography and it has the typical 4-coordinate square-planar geometry.The results of DFT calculation studies demonstrated that the all R-BTBP molecules in these Pd(II) complexes adopt the stable II conformation.Therefore, a significant large rotational energy barrier of 21.8–22.6 kcal/mol has restricted them to form the II type 2:1 Pd(II) complex for those OO type ligands.For those R-BTBP with initial II type conformations, they are easy to form the 2:1 Pd(II)/R-BTBP binuclear complex without needing conformational change.The results obtained in this work provide some new insights into the complexation behaviors of fission palladium with R-BTBP ligands, which can help us to better understand the physicochemical changes occurred in the macroscopic actinides separation process using R-BTBP type ligands and improve the separation efficiency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Nos.91126021 and 21376210), Natural Science Foundation of Zhejiang Province (Nos.LY22B070003 and 2016R401088).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.074.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
