64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
Xueqian Chen, Wenchao Niu, Zhongying Du, Yong Zhang, Dongdong Su, Xueyun Gao
Department of Chemistry and Biology, Faculty of Environment and Life, Beijing University of Technology, Beijing 100124, China
ABSTRACT The prevalence of positron emission tomography (PET) imaging has advanced biomedical applications for its ultrahigh sensitivity, deep tissue penetration and quantitative visualization of diseases in vivo.64Cu with ideal half-life and decay characteristics has been designed as radioactive probes for disease diagnosis.The currently reported 64Cu-labeled nanomaterials have the advantages of long circulation time in serum, good biocompatibility and mature preparation methods, and have been used in vivo PET imaging,biodistribution and pharmacokinetic monitoring, and imaging guided therapy.At the same time, suitable carrier characteristics and radiolabeling strategies are particularly important in the 64Cu PET imaging process.In this review, we summarize different imaging probe designs and 64Cu radiolabeling strategies, as well as their eventual applications in biomedicine.The potential challenges and prospects of 64Cu labeled nanomaterials are also described, which provides broad prospects for radiolabeling strategies and further applications.
Keywords:64Cu Positron emission tomography Chelating agent-free Radiolabeling strategies Imaging guided therapy
1.Introduction
Cancer screening, early diagnosis and timely treatment are crucial aspects to reducing the risk of advanced tumors [1–3].Medical imaging technologies including positron emission tomography (PET), computed tomography (CT), photoacoustic tomography(PTOMS) and magnetic resonance imaging (MRI) are widely used to visualize physiological and pathological processesin vivo.Each imaging technology has its own advantages and can obtain extensive disease information [4–6].Among them, PET imaging is an important imaging technology in clinical diagnosis.It has the advantages of non-invasive, ultrahigh sensitivity, deep tissue penetration and quantitative visualization of diseases and biological processesin vivo,and the capacity to provide functional and metabolic information at the molecular level [7,8].Therefore, radioisotope-based PET probes with ideal imaging performance and easy preparation will significantly improve the accuracy of early cancer diagnosis,tumor staging and therapeutic evaluation.
Potential estimates of the use of radioisotopes in PET imaging are made by pertinently considering their half-lives and decay characteristics [9,10].Radioisotopes with short half-lives require rapid and high-yield radiosynthesis, which makes their applicationsin vivomore challenging and impedes their development as radioisotopes for PET imaging.At the same time, due to the radiological hazards, the use of radioisotopes with relatively long half-lives remains a concern [11,12].Therefore, it is necessary to select a radioisotope with relatively suitable half-life and an extremely simplified radiolabeling procedure for PET imaging.Cuprum-64 (64Cu) synchronously emitsβ+(0.653 MeV, 17.8%),β-(0.579 MeV, 38.4%), electron capture and gamma rays, witht1/2=12.7 h [13,14].These decay characteristics make it an excellent radioisotope, which has potential applications in the construction of radioactive imaging probes for clinical diagnosis and as a source of radiotherapy.Meanwhile, the half-life is not long enough to cause unnecessary radiation damage to the patient, improving the safety ofin vivoapplication.However, due to its rapid liver metabolism, it will not form effective aggregations in the lesion.At the same time, ions may cause strong toxicity.Therefore, using free64Cu2+ions as probe will not produce ideal imaging results[15].The electronic layer structure of Cu2+makes it easy to form relatively stable metal complexes with amines, imines and pyridine [16,17].Therefore, the use of polynitrogen ligands as chelating agents is the main strategy to stabilize Cu2+.At the same time,a series of64Cu imaging probes consisting of targeting moieties(small molecules, monoclonal antibodies, peptides or aptamers),linkers and chelating agents were developed for sensitive and specific disease diagnosis [18–22].With the development of nanotechnology, nanomaterials with mature preparation methods and good biocompatibility have become a reliable strategy forin vivoimaging [23–25].Through the effective combination of nanotechnology, targeting moieties and Cu2+organic chelating agents, such as 1,4,8,11-tetraazacyclododecane-1,4,8,11-tetraacetic acid (TETA) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), radiolabeled nanoprobes with high stability and long circulation time in serum can be successfully prepared, making it possible for the use of64Cu radiolabeled probes with controllable behaviorin vivofor qualitative and quantitative PET imaging [26–29].Nevertheless, despite extensive screening and optimization studies on the structure of the chelating agents, when applied to organisms, the chelating agent bound to64Cu remains susceptible to acidic environment and is easily replaced by other proteins in organisms to produce free64Cu [5,26].The false signal caused by free64Cu2+is still inevitable, so the stability and diagnostic accuracy of the radiolabeled probe cannot be guaranteed.
To avoid the limitations of using macrocyclic chelators, some nanoparticles themselves or their ligands contain groups with strong chelating properties with Cu2+, such as phenolic hydroxyl,carboxyl or sulfhydryl groups, which simplify the assembly of different functional moieties and show excellent labeling stability by a chelator-free method [30–33].In addition, thanks to the major advances in radiolabeling methods, the radiochemical doping of64Cu has become an important way to prepare stable and reliable64Cu PET imaging agents [10,34].In this type of method, radioisotopes are integrated into the particle structure of the nanomaterial, rather than simply being attached to its surface through a chelating agent [35].Since64Cu is used as a reactant in the probe preparation process and participates in the overall preparation of the doped nanoprobes [36,37], it is necessary to develop suitable methods that well match the half-life of64Cu.Currently,well-defined radiation-stabilized64Cu nanoparticles with good reproducibility can be prepared by one-pot method, chemical reduction, codeposition and coating methods [38–41].Therefore,64Cudoped nanoprobes have the stability of radiolabeling and the natural behavior of nanomaterialsin vivo, which can be used for high fidelity, highly sensitive and quantitative PET imaging [42].
PET imaging of64Cu radiolabeled nanomaterials is a great potential and fast-developing branch ofin vivoimaging, biodistribution and pharmacokinetic monitoring, and imaging-guided therapy.The ingenious construction of64Cu radiolabeled nanomaterials, through the rational design of nanocarriers and radiolabeling strategies, offers many possibilities for improving the accuracy of cancer diagnosis, which has great application potential in nuclear medicine imaging and further facilitate clinical transformation [10,29,34].In this review, we introduced in detail the design strategies and synthetic methodologies of various64Cu radiolabeled nanoprobes, mainly the latest developments and applications of chelating agent-free64Cu radiolabeled nanomaterials, and predicted their development trends (Fig.1).We believe that this review will provide a broad perspective for researchers in this field.
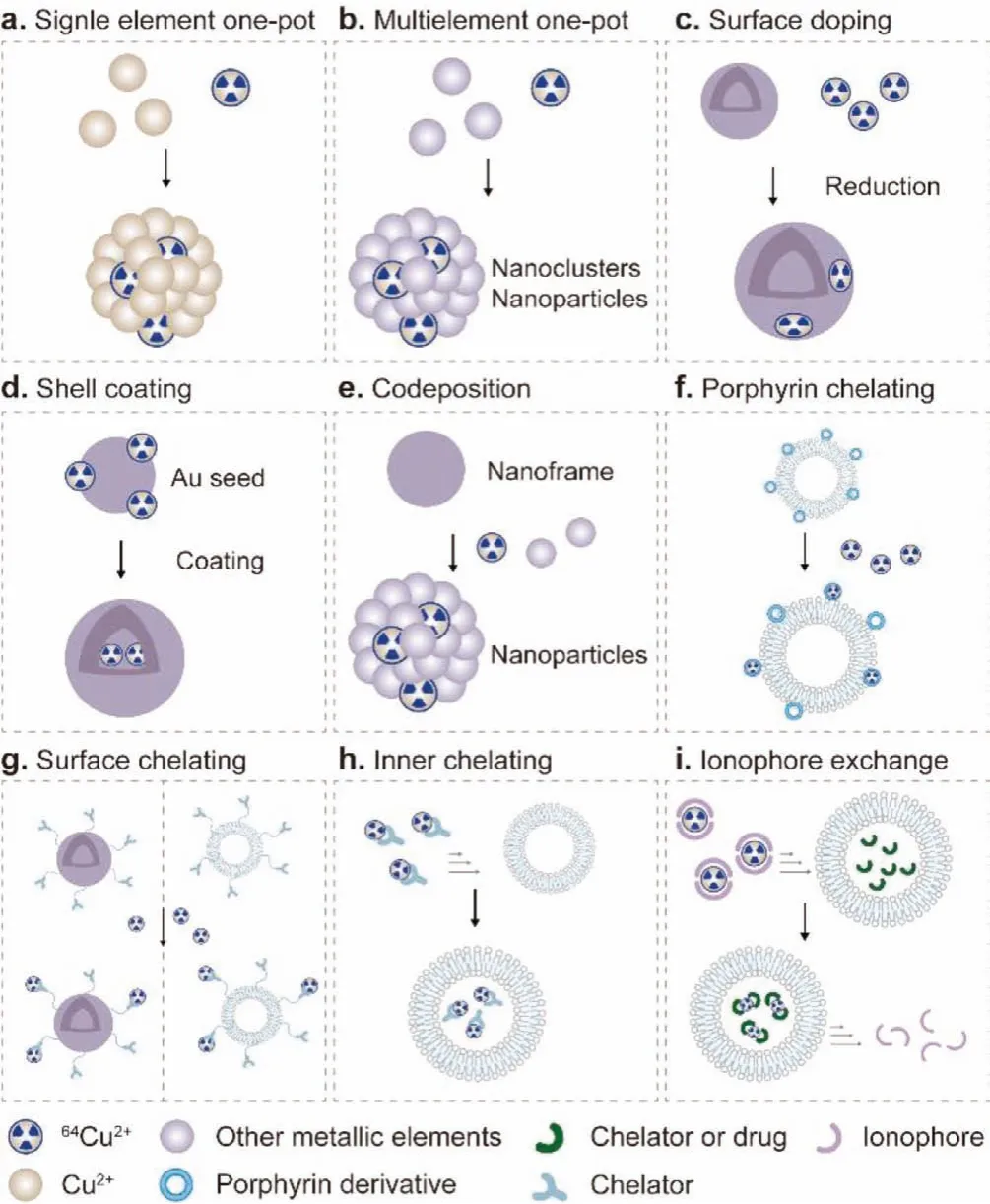
Fig.1.Schematic of 64Cu radiolabeling strategies.
2.PET imaging of radioactive 64Cu-labeled nanomaterials
The use of radioactive64Cu as a nuclear tracer to investigate the effects of PET imagingin vivorequires appropriate radiolabeling strategies and matched nanocarriers.In recent years, nanomaterials have provided suitable carriers for64Cu due to their specific targeting, excellent loading capacity and easy functional modification [14,43,44].At the same time, the stable radiolabeling strategy of64Cu is another important factor that must be considered.Currently, there are two main strategies: chelating agent-based or chelating agent-free radiolabeling [10].Combining nanocarriers and radiolabeling strategies, we mainly introduce Cu and Cu-doped metal clusters or nanoparticles, as well as nanoparticles or liposomes containing Cu chelating agent-like agents (porphyrin or similar structures) and their64Cu PET imaging applications.Table 1 summarizes the design strategies and techniques used to prepare radiolabeled [64Cu]Cu nanoparticles and their applicationsin-vivoimaging [35,38-40,42,45-75].
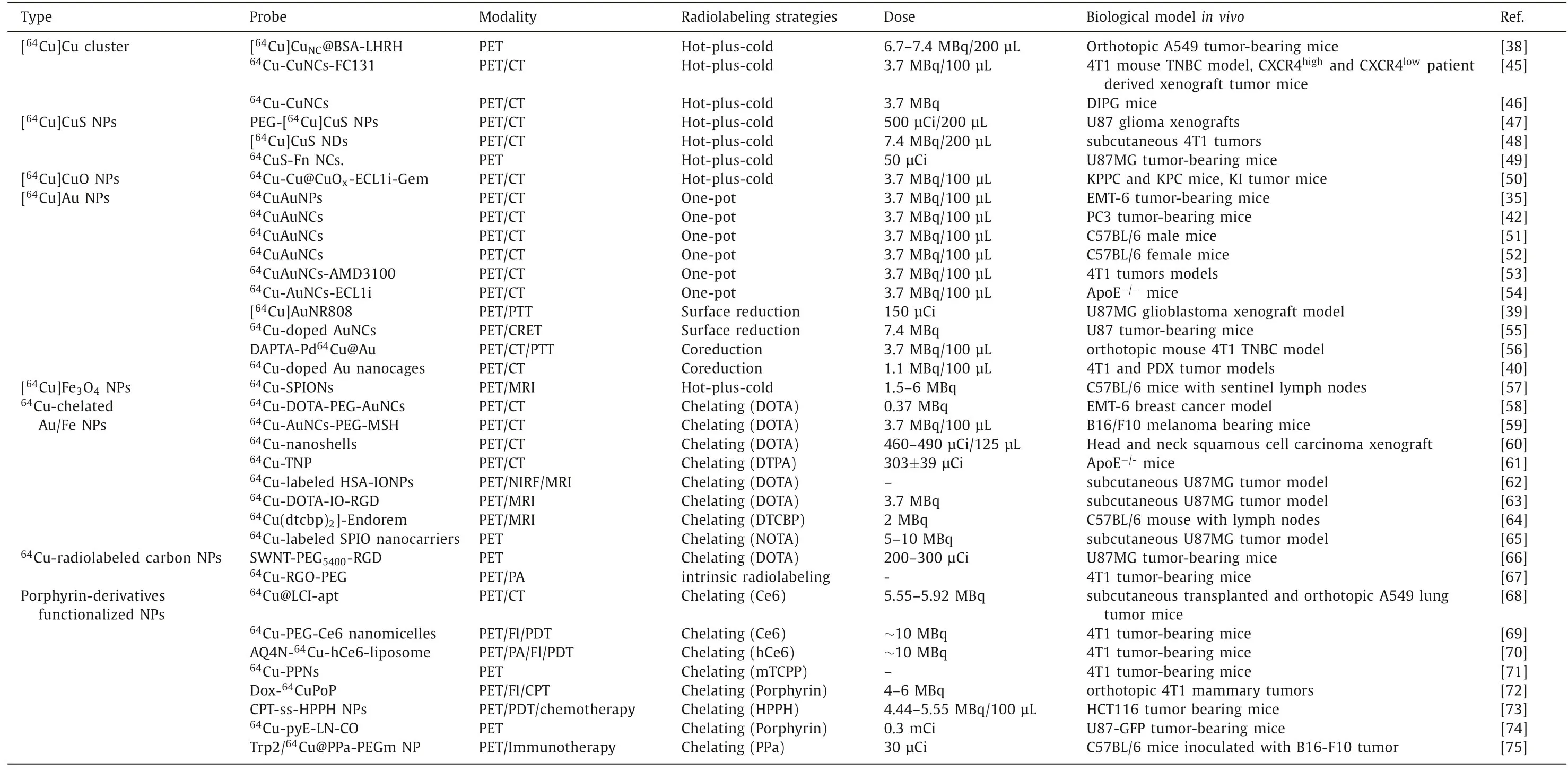
Table 1 The summaries of design strategies, synthetic methodologies and biological applications of representative radiolabeled [64Cu]Cu nanoparticles.
2.1.PET imaging of [64Cu]Cu clusters, 64Cu-doped nanoparticles and 64Cu-radiolabeled nanoparticles
2.1.1.[64Cu]Cu clusters for PET imaging
Metal clusters have attracted worldwide attention due to their ultrasmall size, robust preparation and good biocompatibility.As an emerging functional material, metal clusters have great potential in the field of biomedicine [36].The introduction of the radionuclide64Cu into Cu clusters can be used for sensitive and deep-penetrating PET imaging.In the preparation strategy of this type of radioactive imaging agent, the radionuclide64Cu can be directly incorporated into the core of the clusters without the need of an external chelating agent, thereby achieving stronger radiochemical stability [76,77].At the same time, its reduced preparation process makes it more straightforward and less timeconsuming.Most importantly, by avoiding the use of chelating agent molecules that may affect behaviorsin vivo, the integrity and stability of nanomaterials are greatly improved [26,78].In this section, we summarize the design strategies of64Cu labeled Cu clusters and their applications in tumor imaging (Fig.2A).
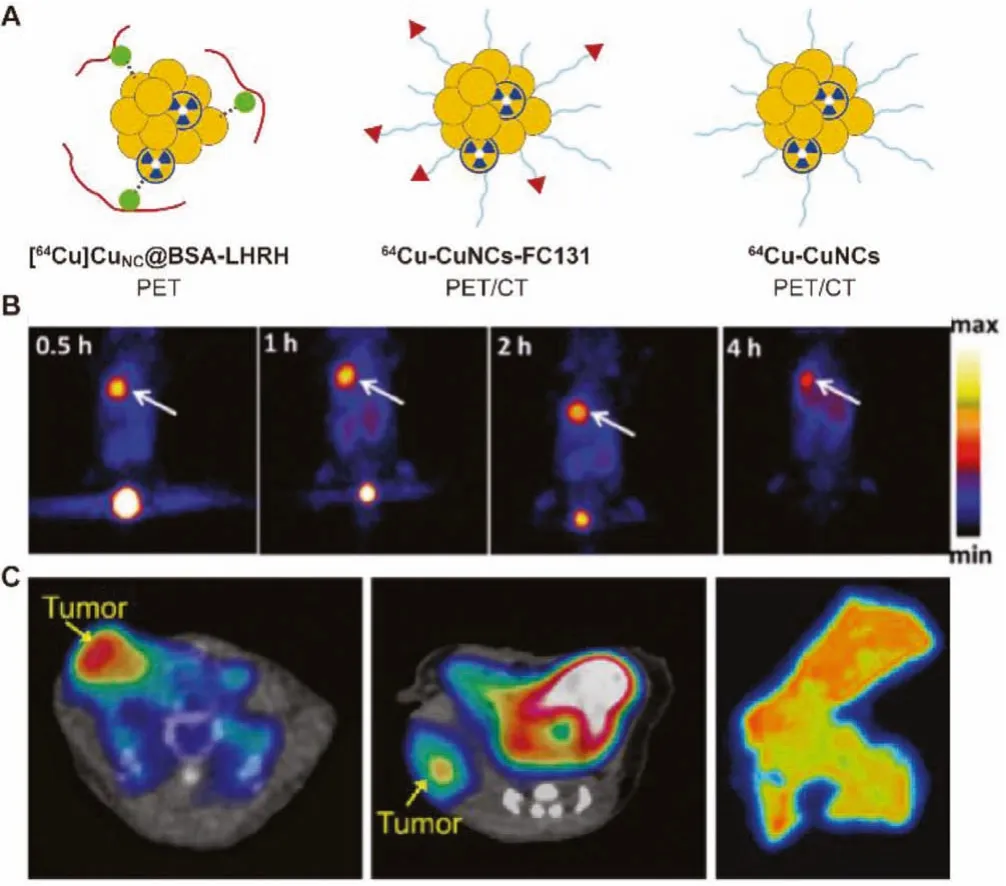
Fig.2.(A) Schematic diagram of 64Cu radiolabeled Cu clusters.(B) In vivo PET imaging of [64Cu]CuNC@BSA-LHRH on orthotopic A549 lung tumor bearing mice.Reproduced with permission [38].Copyright 2015, American Chemical Society.(C) In vivo PET imaging of 64Cu-CuNCs-FC131 in a 4T1 mouse TNBC model and CXCR4high PDX model and autoradiography of human TNBC tissues.Reproduced with permission[45].Copyright 2019, American Chemical Society.
In 2015, Gaoet al.pioneered the design of radioactive Cu clusters ([64Cu]CuNC@BSA) using bovine serum albumin (BSA) as a scaffold [38].[64Cu]CuNC@BSA adopts a hot-plus-cold precursor strategy, which does not require additional chelating agents,and greatly improves the stability of radiolabeling.By pre-coupling a luteinizing hormone releasing hormone (LHRH) peptide with BSA molecule, a lung cancer-targeted radioactive [64Cu]CuNC@BSALHRH was prepared for PET imaging of orthotopic lung cancer.Half an hour after the tail vein injection, the orthotopic A549 tumorwas clearly delineated (10% ID/g) at the left lung of model mice with very little background signal at right lung and other organs(Fig.2B).The example shows the superiority of this radioactive Cu label in PET imaging applications.
In 2019, Liuet al.employed the clinically used chemokine receptor CXCR4 specific binding peptide FC131 (cyclo[2-Nal-Gly-DTyr-NMe-D-Orn-Arg]) as a ligand to prepare radioactive Cu clusters through an intrinsic radiolabeling strategy [45].64Cu was directly integrated into Cu clusters to obtain the imaging agent64Cu-CuNCs-FC131 with high specific activity and excellent stability.The imaging experiments showed that in three triple negative breast cancer (TNBC) models, PET imaging required only a small dose of contrast agent to evaluate the application potential of Cu clusters(Fig.2C).In addition, the use of a de-identified human TNBC organization to evaluate the CXCR4 binding ability of64Cu-CuNCs-FC131 emphasizes the potential of future clinical applications.In 2020, the same group employed focused ultrasound (FUS) to effectively open the blood-brain barrier (BBB), and then delivered ultrasmall, biodegradable and intrinsically radiolabeled CuNCs for PET imaging and quantification of BBB opening efficiency in the mouse diffuse intrinsic pontine glioma (DIPG) model [46].Both PET imaging and quantitative analysis of mouse brain showed high signal intensity of brain tumors (2.73% ± 0.18% ID/g), which confirmed that FUS-mediated64Cu-CuNCs can acceleratein situdrug delivery after entering tumor in the future, making it a potential early diagnosis and therapy means for DIPG.The radiolabeled Cu clusters prepared by this chelating agent-free strategy provides an effective tool for PET imaging in the biomedical field and has great potential for successful clinical transformation in the future.
2.1.2.[64Cu]CuS and [64Cu]CuO nanoparticles for PET imaging
Cu clusters can achieve sensitive PET imaging of tumors,but they lack inherent therapeutic properties.Composite copper nanoparticles, such as CuS and CuO nanoparticles, not only possess inherent radiolabeled Cu elements, but also have effective drugcarrying capabilities and drug delivery functions [47,79].Among them, CuS nanoparticles with strong near-infrared (NIR) absorption characteristics are novel and promising photothermal coupling agents, and CuS-based nanomaterials have received widespread attention in the field of diagnosis and treatment [80,81].In this section, we summarize the advantages and design strategies of64Cu labeled CuS and CuO nanoparticles and their applications of tumor imaging and photothermal therapy (PTT) (Fig.3A).
In 2010, Liet al.pioneered the design of chelating agentfree and PEG-coated [64Cu]CuS NPs for PET imaging [47].It has great radiolabel stability and favorable pharmacokinetic characteristics (7.6% ± 1.4% ID/g) in human U87 glioblastoma model.The PET/CT images in U87 tumor-bearing mice showed that the average tumor-to-muscle (T/M) ratio was 6.55:1 at 24 h.In addition, unlike Cu clusters, the strong NIR absorption of CuS NPs enables them to be used as efficient PTT agents for photothermal ablation (PTA)therapy.In 2015, Liet al.reported a novel multifunctional, PETvisible and polyvinylpyrrolidone-coated CuS nanodots ([64Cu]CuS NDs) [48].The ultrasmall size of 5.6 nm ensures the escape of nonspecific uptake in reticuloendoreticuloendothelial system (RES) organs, and fast renal clearance up to 95% of [64Cu]CuS NDs within 24 h.The CuS NDs still have potential for improvement, such as the introduction of receptor-specific targeting parts, to achieve a wide range of clinical significance.
Subsequently, in view of the excellent performance of [64Cu]CuS NPs in PET imaging and PTT, Pellegrinoet al.reported active targeting and radiolabeled photothermal probe64Cu:CuS nanocrystals bearing biotin molecules, which makes it more easier for radiotracers to be transferred to clinical medicine [82].Similarly, Chenet al.used ferritin as a biological template to design64CuS-Fn nanocages (NCs) through biomineralization for photoacoustic/PET bimodal imaging and PTT [49].The tumor uptake of64CuS-Fn NCs in U87MG tumor-bearing mice reached maximum (~10% ID/g) at 8 h postinjection, and the tumor accumulation in PET imaging was consistent with the PA signal (Fig.3B).The integration of PA and PET provides high sensitivity, spatial resolution and unique recommendations for PTT, and has broad prospects in clinically transformable cancer therapy.
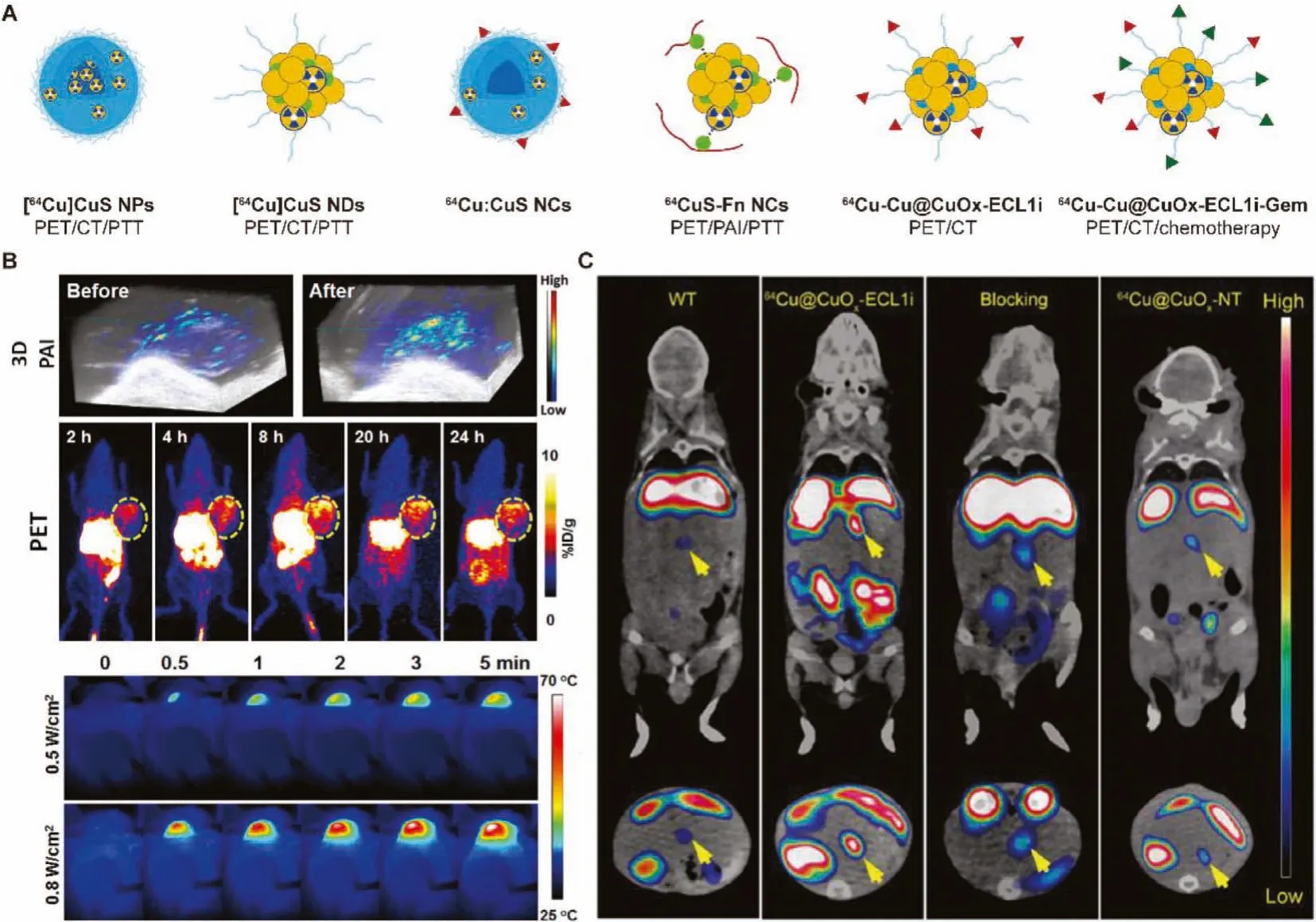
Fig.3.(A) Schematic of 64Cu radiolabeled CuS and CuO nanomaterials.(B) In vivo PET/PA imaging and PTT of 64CuS-Fn NCs.Reproduced with permission [49].Copyright 2016, American Chemical Society.(C) In vivo PET imaging and biodistribution of 64Cu-Cu@CuOx-ECL1i in wild-type littermate, 7–9 weeks old KPPC mice.Reproduced with permission [50].Copyright 2021, American Chemical Society.
In 2021, Liuet al.engineered the ultrasmall CuO nanoparticles64Cu-Cu@CuOx-ECL1i-Gem with extracellular loop 1 inverso(ECL1i)-modified and loaded gemcitabinevia64Cu intrinsic radiolabeling for PET-guided drug delivery into the pancreatic ductal adenocarcinoma (PDAC) tumors [50].ECL1i peptide with targeting specificity for chemokine receptor 2 improves tumor accumulation and unessential nonspecific retention.PET images of KPPC mice and KPC mice distinctly demonstrated increased retention of64Cu-Cu@CuOx-ECL1i at the tumor region (Fig.3C).Quantitation showed that the tumor accumulation rates of64Cu-Cu@CuOx-ECL1i in KPPC and KPC model mice were 11.16% ± 1.22% ID/g and 5.47% ± 0.75% ID/g, respectively.In addition, the T/M ratio imaged in KPPC mice (17.03±3.43) was 2 times higher than that of KPC mice (8.71±1.94).Taking advantages of selectively and sensitivity of actively targeted PET imaging, CCR2-targeted therapy of Cu@CuOx-ECL1i-Gem has shown admirable therapeutic efficacy in a syngeneic xenograft mouse model, demonstrating a promising image-guided therapy.
2.1.3.64Cu-doped Au nanoparticles for PET imaging
Au nanoparticles with particle sizes less than 100 nanometers have the advantages of good stability, good biocompatibility and easy modification [83].In biological applications, the sizedependent metabolic properties of Au nanoparticles have always been a hot research topic [23,58].In particular, PET imaging based on Au nanoparticles has ultrahigh sensitivity andin vivoquantitative ability of biological processes, making it an ideal choice for intuitive analysis of pharmacokinetics [5,84].This section summarizes the different techniques for radiolabeling of Cu-doped Au nanoparticles for pharmacokinetic analysis andin vivoPET imaging(Fig.4).
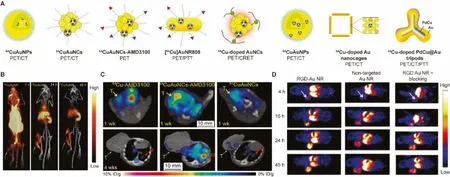
Fig.4.(A) Schematic of 64Cu radiolabeled Au nanomaterials.(B) Representative PET/CT images in tumor-bearing mice of 64CuAuNPs.Reproduced with permission [35].Copyright 2013, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(C) In vivo PET/CT imaging of 64CuAuNCs-AMD3100 in 4T1 tumor-bearing mice at 1 and 4 weeks after tumor implant, T: tumor, M: metastasis.Reproduced with permission [53].Copyright 2016, American Chemical Society.(D) In vivo PET imaging and biodistribution of RGD-[64Cu]AuNR808 in U87MG tumor-bearing mice, white arrows: tumor.Reproduced with permission [39].Copyright 2014, American Chemical Society.
The one-pot method can directly synthesize radiolabeled nanoparticles.64Cu is distributed in the lattice of the nanoparticles, which significantly improves the stability of the radiolabel,especially can avoid the change in the behaviors of the nanoparticlesin vivocaused by the surface modification of chelating agents [35].Liu’s group first reported64Cu doped Au nanoparticles (64CuAuNPs) with monodisperse distribution (27±3.2 nm)by directly synthesizing radioactive nanoparticles [35].64CuAuNPs enhanced vascular permeability and retention (EPR) effect in tumor tissues and had good biocompatibility.It was successfully applied in accurate and high-contrast PET/CT imaging in breast cancer mouse models (Fig.4B).Since the EPR effect,64CuAuNPs were continuously accumulated within tumor region over time, and tumor uptake reached 16.8% ± 0.98% ID/g with a high T/M ratio at 48 h post injection.However, the accumulation of nanoparticles in the non-specific mononuclear phagocyte system (MPS) is a problem faced by relatively large nanoparticlesin vivo, which may cause potential long-term toxicity and affect the contrast ofin vivoimaging.To overcome the above-mentioned challenge, Liu’s group subsequently reported the PEG stabilized and64Cu doped Au nanoclusters64CuAuNCs (2.5±0.8 nm), using the similar radiolabel strategy to evaluate thein vivobehavior and the effect of PET imaging [42].In addition to favorable stable radiolabeling, the prepared64CuAuNCs showed distinctin vivoperformance compared to the larger particles: rapid systematic clearance involved higher renal and hepatobiliary clearance, low MPS accumulation and low non-specific retention.All of these advances demonstrate the great potential of such radiative nanoclusters in monitoring and detecting tumorsin vivo.To expand the application of the prepared radioactive nanoclusters,64CuAuNCs were further used to quantify focused ultrasound combined with microbubble (FUSM)-mediated uptake of pons in DIPG based on PET imaging [51].After disruption of the fully functional BBB of DIPG and administration of64CuAuNCs, the synergistic quantification by PET imaging and inductively coupled plasma-mass spectrometry (ICP-MS) showed the accuracy of this strategy and its potential for further application in DIPG therapeutic drug delivery.The radioactive nanoclusters were then used for monitoring the delivery of FUSM-mediated intranasal administration [52].Due to their ultrasmall size,64Cu labeled Au clusters can largely escape the accumulation of MPS, thereby significantly reducing non-specific signal in PET imaging, which is an effective strategy to improve their practical potential.
In addition to passive targeting, active targeting strategies based on specific receptor proteins of cells and tissues of interest are very effective ideas for improving tumor detection [85,86].For the accurate diagnosis of breast cancer (BC) and its metastasis in the early stage, Liu’s group prepared radiolabeled nanoclusters(64CuAuNCs-AMD3100) targeting BC up-expressed chemokine receptor CXCR4 [53].The ultrasmall size (1.7±0.3 nm) of64CuAuNCs-AMD3100 provides favorablein vivodistribution and renal and fecal clearance within 2 days.Compared with64Cu labeled AMD3100 and non-targeted64CuAuNCs, the CXCR4 targeting probe shows excellent sensitivity in detecting overexpressed CXCR4 in xenograft tumor and early BC premetastatic lesions in the lung, which shows great potential in accurately diagnosis of tumor lesions to guide treatment.Based on a similar strategy (Fig.4C).Liu’s group recently reported radiolabeled nanoclusters (64Cu-AuNCs-ECL1i), which was prepared through a one-step reaction with a atherosclerosis-related CCR2 peptide, which can accurately diagnose the initiation and progression of atherosclerosis [54].The active targeting strategy offers a unique accumulation of radioactive probe molecules at the region of interest, which greatly improves the high SBR signal and accuracy of imaging.
Mild radiolabeling technique such as chemical reduction can be used to effectively radiolabel complicated and functionalized nanomaterials while maintaining their multifunctionality [39].Chen’s group reported64Cu doped Au nanorods [64Cu]AuNR808 for tumor diagnosis and PTT [39].64Cu can be directly radiolabeled on functionalized Au nanoparticles by chemical reduction without affecting other loaded functional groups.At the same time, the prepared radioactive nanoprobe can be directly applied, which reduces the complicated processing after radiolabeling.Large particle size and conjugated RGD endow it with long blood circulation and tumor targeting ability, which significantly improved the tumor accumulation of [64Cu]AuNR808 compared with preblocking and non-targeting probe group (Fig.4D).Compared with chelate-labeled nanomaterials, [64Cu]AuNR808 has RGD targeting and higher radiochemical stability, which significantly improves the accuracy of localization and quantification in PET imagingin vivo.Subsequently, Chen’s group used the same method to prepare64Cu doped Au nanoclusters64CuAuNCs, with spontaneous selfilluminating/PET dual-modein vivoimaging in a U87MG glioblastoma tumor-bearing mice [55].PET imaging results showed that significant tumor uptake (14.9% ID/g) was observed 18 h post injection.More importantly, the frequency of the blue region of Cerenkov radiation emitted by64Cu matches well with the innate absorption of the prepared64CuAuNCs.Even in the absence of external excitation, the strong NIR fluorescence caused by Cerenkov resonance energy transfer (CRET) can achieve highly sensitive fluorescence imagingin vivo.64CuAuNCs with self-illuminating/PET dual-mode and high tumor uptake show great potential in accurately diagnosing cancer.In general, the chemical reduction method for radiolabeling is highly efficient and mild, and can be used for the radiolabeling of probes that are sensitive to extreme conditions, especially complex multifunctional probes.
Surface coating is an alternative strategy for obtaining radioactive nanoparticles.Andresen’s group reported an example of using this chelating agent-free radiolabeling method [41].First,64Cu2+nonspecifically binds to the surface of freshly prepared Au seeds,additional Au shell by reducing the additional HAuCl4·4H2O coats and astricts the64Cu within the particles to obtain64Cu embedded AuNP (20–30 nm), where the radiolabeling efficiency is 53% ± 6%.Using the chelating agent-free strategy,64Cu embedded AuNPs with different coatings were prepared, and the radioactive nanoparticles showed favorable radiostability.Plasma half-life and tumor accumulation results showed that PEG coated64Cu embedded AuNP with had the best pharmacokinetic characteristics,which plasma half-life is about 9 h, and the tumor uptake is 3.89%± 0.1% ID/g 24 h post injection.
Codeposition with other cold metal ions can directly incorporated64Cu into crystal lattice of nanoparticles [56].Xia’s group reported a radiolabeled core-shell tripod structure of PdCu@Au that was coreduced by Na2PdCl4and CuCl2[56].64Cu is embedded in the crystal lattice and the Au coating improves the stability of the radiolabel.In addition, an additional TNBC-targeted D-Ala1-peptide T-amide combined to the radioactive tripods greatly improves the accuracy of PET imaging and image-guided PTT.After systematic photothermal treatment, an obvious reduction of tumor metabolic activity was monitored by18F-labeled fluorodeoxyglucose PET/CT imaging.Based on the same strategy, Liu’s group reported an easy preparation of64Cu doped Au nanocages by codeposition of HAuCl4, CuCl2and64CuCl2on the basis of Au nanocage[40].The intergration of64Cu into the lattice of Au nanocages was improved, and the radioactive doped nanocages showed good tumor accumulation after PEGylation, thus showing great potential in PET image-guided cancer therapy.The above examples using different strategies to achieve64Cu radiolabeling, optimized the radiostability of PET probes, and made advancesin vivoPET imaging, biodistribution and pharmacokinetic monitoring, and imaging guided therapy.
2.1.4.64Cu-doped iron oxide nanoparticles for PET imaging
PET imaging can provide ultrahigh sensitivity and quantitative molecular images, but its relatively low spatial resolution and lack of anatomical details of lesions hinder the in-depth development of clinical research [26,87].Utilizing the advantages of64Cu PET imaging, it can synergistically work with other imaging modalities.Iron oxide nanoparticles (IONP) are effective T2contrast agents for MRI, providing high-resolution two-dimensional or three-dimensional morphological and functional images with good soft tissue contrast [88].Compared with PET imaging alone,64Cudoped iron oxide nanoparticles have significant advantages in multifunctional imaging applications.
Louieet al.synthesized64Cu-doped iron oxide nanoparticles(DIO/64Cu) with dextran coating by microwave-assisted synthesis technology [89].Due to the controllable time, this synthesis method can not only synthesize nanoparticles (DIO/64Cu) of different sizes to obtain the best blood retention time, but also effectively shorten the synthesis time, thus reducing the radioactive loss.Nanoparticles are potential candidates for MRI/PET bimodal imaging in future.In 2018, Strandet al.developed a hybrid PET-MRI probe based on superparamagnetic iron oxide nanoparticles (SPIONs), which was labeled with64Cu through a chelating agent-free method [57].SPIONs have the advantages of high labeling efficiency (97%) and excellent chemical stability (>95% at 24 h).In a tiny lymph node (LN) (1.7–2.5 mm), PET imaging can not only clearly describe LNs, but also distinctly prove the existence of64Cu-SPIONs in the large intestine, suggesting the final elimination path of the probe.Combining the high sensitivity of PET and the high spatial resolution of MRI, the hybrid64Cu-SPIONs probe provides more comprehensive information of diseases, and provides sensitive indications and accurate anatomical location for sentinel lymph nodes (SLN) lesions.
2.1.5.64Cu-chelated metal (Au, Fe) nanoparticles for PET imaging
The combination of64Cu2+organic chelating agents such as TETA or DOTA with high stability and long cycle nanomaterials to form radiolabeled nanoprobes has made it possible to use64Cu2+labeled PET probes with controllable behaviorin vivofor qualitative and quantitative applications [26–29].In this section, we summarize recently reported64Cu2+chelating agent-labeled Au and iron oxide nanoparticles and their applications of tumor imaging(Figs.5 and 6).
Au nanocages have great potential in tumor therapy due to their high photothermal efficiency and strong drug delivery capacity, but the tumor accumulation and pharmacokinetics remain unclear [90,91].Xia’s group prepared two nanocages of different sizes(30 nm and 55 nm), which was conjugated with DOTA-NHS-ester and radiolabeled with64Cu to obtain the radioactive nanocages(64Cu-DOTA-PEG-AuNCs) [58].Results showed that compared with the 55 nm nanocages, the 30 nm radiolabeled nanocages have significant improvements in biodistribution, including a longer blood retention time and obviously reduced uptake by the RES.More importantly, the 30 nm nanocages showed a very favorable tumor uptake (~8% ID/g), T/M ratio (~23-fold) and favorable intratumoral diffusion at 24 h post injection in EMT-6 breast cancer mouse model (Fig.5B).Later, Liu’s group prepared a64Cu radiolabeled Au nanocages (64Cu-AuNCs-PEG-MSH) based on the similar strategy,except that the nanocages surface was modified with an additionalα-melanocyte-stimulating hormone (α-MSH) peptide for targeting enhanced melanocortin 1 receptor upregulated B16/F10 melanoma in a mouse model [59].A higher tumor accumulation was observed on the melanoma target probe, providing good imaging results(Fig.5C).Xieet al.studied the biodistribution of intratumor injection of Au nanoshells (64Cu-nanoshells) based on same strategy[60].Reducing the accumulation in MPS system and improving the efficiency of target localization are the goals of studying biodistribution profiles and visualizing biological processesin vivoin future clinical applications.
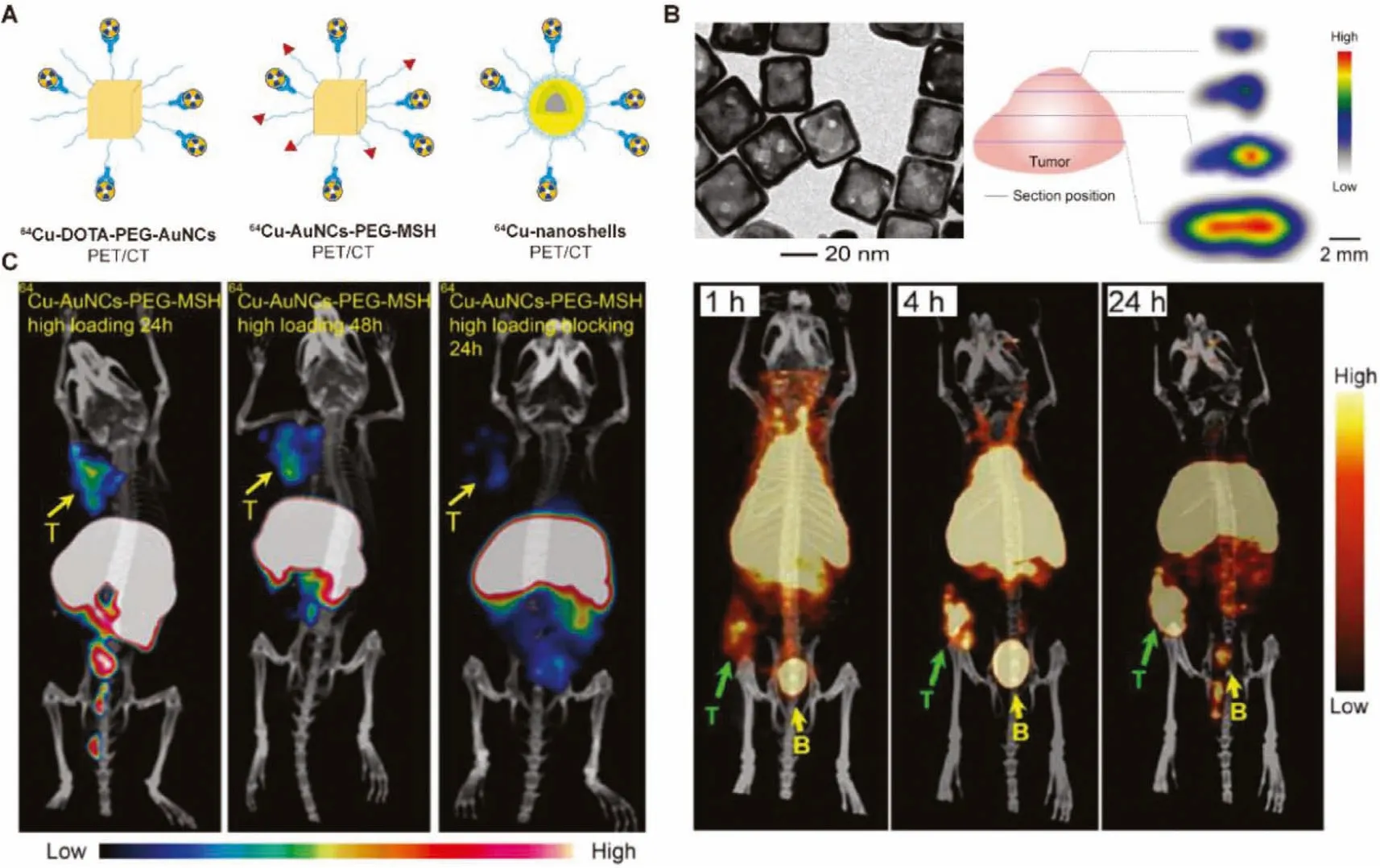
Fig.5.(A) Schematic representation of 64Cu-chelator radiolabeled Au nanomaterials.(B) TEM image, in vivo PET/CT imaging and autoradiography about intratumoral accumulation of 30 nm-sized 64Cu-DOTA-PEG-AuNCs in EMT-6 breast cancer mouse model.Reproduced with permission [58].Copyright 2012, SAGE publications.(C) PET/CT images of 64Cu-AuNCs-PEG-MSH in melanocortin 1 receptor upregulated B16/F10 melanoma tumor-bearing mice.Reproduced with permission [59].Copyright 2018, SAGE Publications.
In terms of excellent internal circulation and MRI imaging characteristics, many multifunctional water-soluble SPIONs are coated with different ligands and have been reported for targeted drug delivery and multimodal tumor imaging (Fig.6A).To label the effective tracer64Cu, researchers have employed different chelating agents (DOTA, NOTA, BP and DTPA) to functionalize SPIONs for PET imaging [61-65,92].In 2010, Chenet al.designed a HSA coated IONP (HSA-IONPs) functionalized with64Cu-DOTA and Cy5.5 forin vivoPET/NIRF/MRI trimodality imaging in a subcutaneous U87MG tumor-bearing mice [62].Multimodal integrated imaging found that particles have better tumor accumulation in the lesion (Fig.6B).Chenet al.developed a PASP-coated IONPs conjugated with the targeted cRGD and64Cu-DOTA to further detect tumors with high accuracy and effectiveness [63].In addition to tumor diagnosis, Gonget al.firstly reported that SPIONs labeled with64Cu and cRGD showed excellent uptake (5.6%±1.7% ID/g at 6 h post injection) and accumulation in tumors, and then functionalized them with a pH-sensitive hydrazone bond to achieve specific drug release in the tumor microenvironment (Fig.6C) [65].The highresolution of MRI and the quantitative monitoring of64Cu PET can accurately monitor the anti-cancer effect of nanocarriersin vivo, so that PET/MRI imaging can guide tumor-targeted drug delivery and further realize cancer therapy.More interestingly,Sutcliffeet al.evaluated64Cu radiolabeled Fe3O4nanocarriers to noninvasively track and quantify the accumulation of NPs in lettuce seedlings [92].The SPIONs-based dual-mode imaging strategy in this section provides a promising platform for comprehensive analysis of biodistribution profiles and visualization of biological processes through cross-over analysis of MRI/PET imaging.
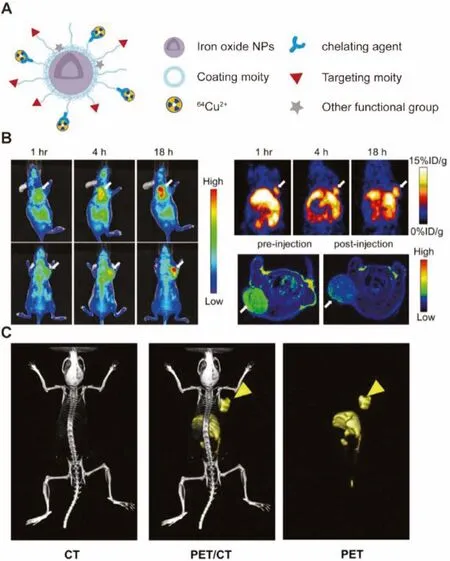
Fig.6.(A) Schematic representation of 64Cu-chelating agent radiolabeled iron oxide nanomaterials.(B) in vivo PET/NIRF/MRI trimodality imaging in a subcutaneous U87MG tumor-bearing mice.Reproduced with permission [62].Copyright 2010, Elsevier.(C) PET/CT images of 64Cu-labeled and cRGD-conjugated SPIO nanocarriers in a U87MG tumor-bearing mouse.Reproduced with permission [65], Copyright 2011,Elsevier.
2.1.6.64Cu-radiolabeled carbon nanoparticles for PET imaging
Carbon nanoparticles possess remarkable physicochemical with different morphologies, their multivalent effect can enhance the binding affinity with targeting ligands, and the ultra-high surface area can effectively load multiple molecules, providing new opportunities for biomedical research [93–95].Carbon nanoparticles can be used not only as nanocarriers for radionuclides and anticancer drugs, but also as agents for directly mediating the destruction of cancer cells for cancer therapy, becoming potential candidates for bioimaging and cancer therapy [96].In this section, we briefly enumerate the design strategies of64Cu-labeled carbonaceous nanomaterials and their applications in tumor imaging.
In 2007, Daiet al.took the lead in designing64Cu radiolabeled PET imaging probes SWNT-PEG5400-RGD and SWNT-PEG2000-RGD using single-walled carbon nanotubes (SWNT) as nanocarriers [66].The probes were mainly composed of an RGD peptide capable of recognizing integrinαvβ3, two different PEG coatings (PEG5400and PEG2000) and a radiolabel (64Cu-DOTA).The SWNT conjugates showed excellent radiostability and prominent uptake in the liver.Furthermore, the long PEG-coated probe SWNTPEG5400-RGD exhibited relatively long blood circulation times and efficient tumor accumulation (15% ID/g) in glioblastoma U87MG tumor-bearing mice.Subsequently, various macrocyclic chelatorfunctionalized carbonaceous nanomaterials such as carbon nanotubes, carbon dots, nanographenes and fullerenes were developed for sensitive PET imaging [97–101].
Different from traditional chelator-based radiolabeling, Caiet al.innovatively designed a chelator-free radiolabeling of probe64Cu-RGO-PEG based on the interaction between Cu and the p electrons of reduced graphene oxide (RGO) by intrinsically radiolabeling nanographene with64Cu [67].The probe showed excellent labeling efficiency compared to graphene oxide (GO) and higherin vivoradiostability compared to NOTA-conjugated RGO, allowing for more reliable biodistribution assessment and accurate PET imaging.In addition, Wilsonet al.have developed a strategy for the preparation of PET/MRI probe64Cu@GNT based on carbon nanotube platform-loaded Gd3+and64Cu2+without chelating ligands.64Cu@GNT exhibited rapid clearance and enhanced accumulation in the lungs [102].
2.2.Porphyrin and their derivatives functionalized nanoparticles for PET imaging
Porphyrin and their derivatives such as Chlorin e6 (Ce6) have emerged as multifunctional agents widely used in the field of biomedicine [103–105].They can be used as efficient photosensitizers for photodynamic therapy (PDT) and as fluorophores for fluorescence imaging [106].More importantly, it can also chelate64Cu2+for PET imaging [69].Gao’s group prepared a PET/CT dualmode liposome contrast agent for early and accurate detection of A549 orthotopic lung tumors (Fig.7A) [68].PET imaging offers high sensitivity and quantitative visualization, but the poor spatial resolution is a shortcoming [68].High-resolution CT complementary to PET imaging can provide detailed anatomical information.Iodixanol was encapsulated by cholesterol, DSPE-PEG2000-NHS and partial lysophosphatidylcholine-modified Ce6 for CT imaging,and then the lung cancer-targeting aptamer GLT21.T was conjugated to the surface of the liposome.Then64Cu2+was quickly and efficiently labeled into liposomes to obtain the final probe(64Cu@LCI-apt).When64Cu@LCI-apt was applied for imaging of orthotopic lung tumors, through the combination of PET imaging and CT, tumor lesions with a size of 500 μm can be clearly observed in the left lung of mice.The powerful imaging capability of64Cu@LCIapt provides a broad prospect for early and accurate detection and location of tumor lesions.
The integration of therapeutic and diagnostic agents into one theranostic probe overcomes the differences in the biodistribution and pharmacokinetic of different agents, and is of great importance to the development of precision medicine [107].Based on the multifunctional Ce6, Cai’s group reported a photodynamic therapy nanoprobe guided by dual-mode optical/PET imaging [69].Ce6 (PEG-Ce6) modified with an amphiphilic polymer is assembled into nanomicelles, which were then chelated with64Cu2+to produce theranostic nanoprobe.The 20 nm-sized nanoprobe allows the serum stability and high tumor uptake observed in dual-mode imaging, and achieves excellent therapeutic effects through a low power radiation.The combination of multiple therapeutic methods is an effective way to improve the therapeutic effect.Combining dual optical-PET imaging and chemophototherapy, Cai’s group then prepared radiolabeled DOX-loaded liposomes(Dox-CuPoP) by encapsulating DOX in the cavity and embedding porphyrin-phospholipid into the bilayer for fluorescence imaging, PDT and64Cu2+chelation (Fig.7B) [72].After treating the 4T1 orthotopic tumors with the nanoprobe, effective growth inhibition was observed.In addition, dual-mode imaging found that nanoprobes accumulated in metastatic lung lesions.
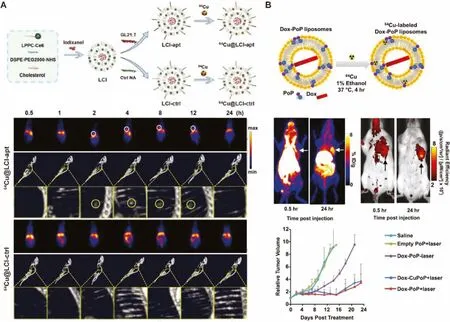
Fig.7.(A) Schematic of construction of 64Cu@LCI-apt and 64Cu@LCI-ctrl and their applications of PET/CT imaging on orthotopic A549 lung tumor mice.Reproduced with permission [68].Copyright 2020, American Chemical Society.(B) Schematic of Dox-CuPoP and its applications of optical/PET imaging and chemophototherapy on orthotopic 4T1 mammary tumors mice.Reproduced with permission [72].Copyright 2017, American Chemical Society.
The activatable probe has received widespread attention due to its high selectivity and sensitivity, which is conductive to accurate diagnosis of pathological processes and significantly reduce the severe side effects of anticancer drugs [108,109].Liuet al.reported a theranostic probe that combines multimodal imaging and light-activated and hypoxia-mediated tumor therapy [70].Using liposomes as a carrier, a hypoxia-activated hydrophilic prodrug,AQ4N is encapsulated into its aqueous cavity, and hexadecylaminemodified chlorin e6 (hCe6) acts as a photosensitizer and stabilizing chelating agent embedded into its hydrophobic bilayer, and then64Cu2+is labeled by chelating with hCe6 for PET imaging.PET imaging, fluorescence and photoacoustic imaging provide detailed and comprehensive information, including the biodistribution, pharmacokinetics and tumor accumulation of the theranostic vehiclein vivo, providing reliable evidence for subsequent treatment.When the tumor region is pertinently irradiated by 660 nm light, photodynamics therapy will be turned on, and at the same time, severe tumor hypoxia will appear in the tumor tissue,and AQ4N will be activated for chemotherapy.The obvious therapeutic effect and the accuracy of image-guided treatment show the great potential of theranostic probe in precision medicine.Chen’s group prepared a glutathione-responsive multifunctional nanoprobe, a chemotherapeutic camptothecin as a prodrug for tumor chemotherapy, and a photosensitizer 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-α(HPPH) for photodynamic therapy is conjugated by a disulfide linker (Fig.8A) [73].After64Cu2+is chelated into HPPH, the radiolabeled dimer and degradable PEGb-PLA are assembled into nanoparticles.The dimer is efficiently loaded into the nanoparticles.Under the guidance of quantitative PET imaging, the delivery efficiency of the prodrug dimer is greatly improved, and the GSH responsive linker shows high selectivity to tumor tissues, thereby obviously maintaining effective cytotoxicity to tumor tissues and reducing side effects to normal tissues.Due to its versatility, other Ce6-like structures can also be applied to64Cu2+labeling biodistribution study and imaging-guide therapy in other tumor models [71,74].The effective and selective drug delivery of nanomaterials with PET imaging capability can significantly advance the intuitive analysis of pharmacokinetics and imagingguide therapy, providing broad prospects in future cancer therapy.
64Cu labeled nanomaterials can also be used to detect the effect of tumor immunotherapy.Gao’s group reported a pyropheophorbide-A conjected PEG500 (PPa-PEGm) coated 5-fluorescein isothiocyanate modified tumor Trip2 antigen (FITCTrip2) stabilized colloidal nanoparticles (Trp2/PPa-PEGm NPs) for tumor immunotherapy based on tumor vaccination (Fig.8B) [75].64Cu2+was chelated to the tetrapyrrole ring of PPa for PET imaging.The short chain of PEG leads to a relatively small particle size, which is beneficial to the endocytosis of dendritic cells, then presents the antigen and triggers the antitumor effect of cytotoxic T-cell lymphocytes.After the administration of Trp2/64Cu@PPa-PEGm NPs, the homing of dendritic cells to draining lymph nodesin vivocan be clearly observed by high sensitive PET imaging.In addition, the inoculated mice showed enhanced tumorous infiltration of CD8+T cells and anti-tumor immune response, showing great potential as a multifunctional platform for ultra-high sensitive imaging-tracked tumor immunotherapy.
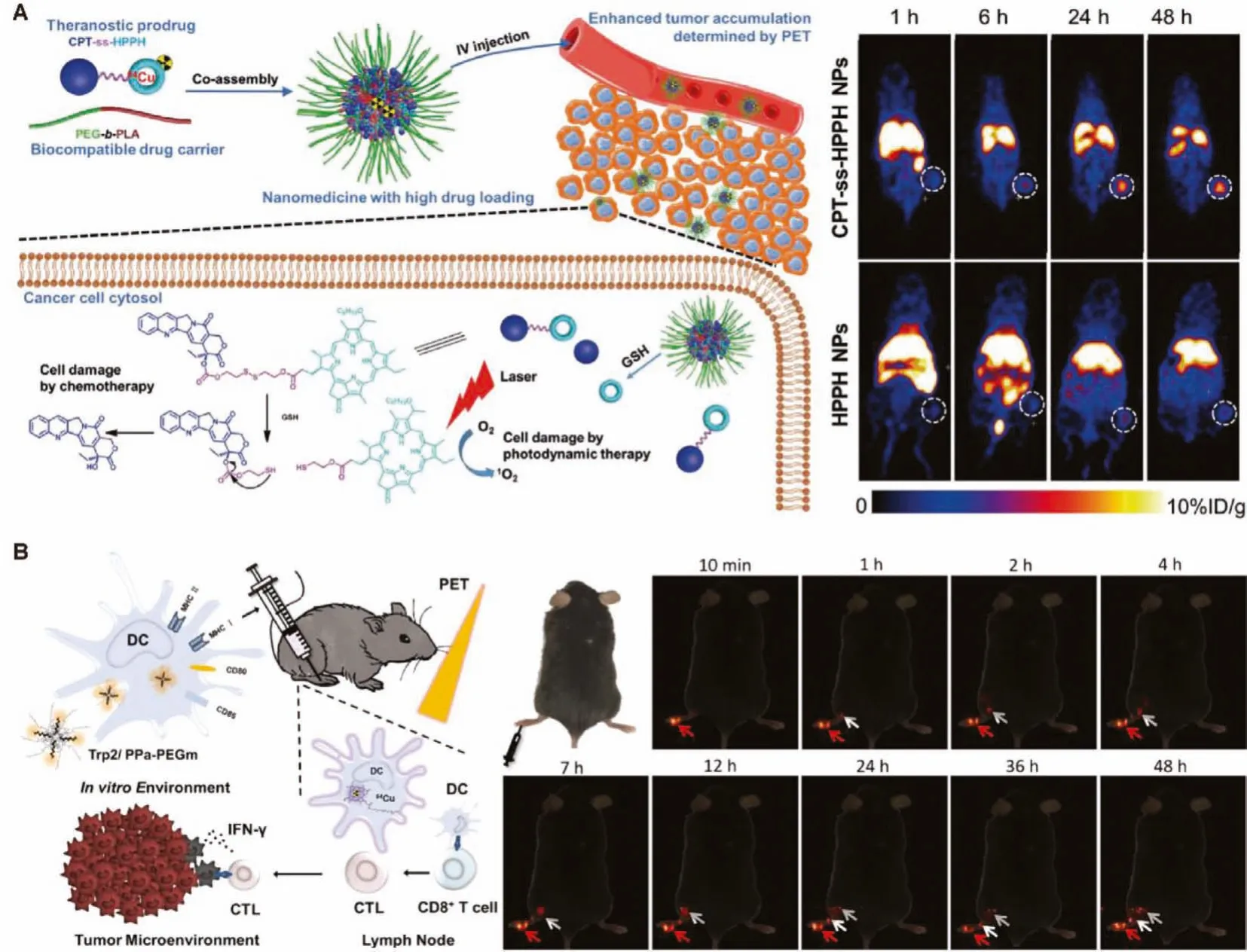
Fig.8.(A) Design of GSH-sensitive CPT-ss-HPPH and PET images of CPT-ss-HPPH NPs and GSH-insensitive HPPH NPs in HCT116 tumor-bearing mice.Reproduced with permission [73].Copyright 2017, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.(B) Schematic Diagram of Trp2/64Cu@PPa-PEGm NPs for PET imaging tracking and dendritic cell-based immunotherapy.Reproduced with permission [75].Copyright 2021, American Chemical Society,.
2.3.64Cu-functionalized liposomes for PET imaging
Liposomes can achieve long-circulation pharmacokinetics and non-specific deposition and retention of tumor sites [72,110].In addition, liposomes with satisfactory surface modification and strong loading capacity have been widely used asin vivoimaging and drug delivery systems [111].64Cu radiolabeled liposomes are mainly divided into chelating agent-based surface labeling and chelating agents encapsulated in liposomes by ionophore-based remote loading [10].Here we only briefly introduce several technologies of64Cu radiolabeled liposomes, and discuss their corresponding advantages and disadvantages.Surface functionalized64Cu radiolabeled liposomes are realized by attaching or conjugating different kinds of chelating agents (BAT, DOTA, TETA) to the surface of liposomes [112–122].This can flexibly realize the application of different nuclides and the alternative of targeting motifs for different disease models.However, several reports have also manifested that64Cu radiolabeled liposomes of surface functionalized chelating agent cause radio stability in serum andin vivo.The predecessors have made a detailed review of this part [10,34,123,124].
2.3.1.Liposomes encapsulated with chelating agents for 64Cu PET imaging
Radiolabeling of liposomes can also be achieved within the liposome core to improve the stability of radiolabeling [10].This type of radiolabeling procedure is relatively complicated, and usually requires pre-synthesis of a radiotracer before incorporation into liposomes.The most widely used intraliposomal radiolabeling method uses ionophores to transport radionuclides through the lipid bilayer to chelate with internally encapsulated chelating agents [125–134].Petersonet al.innovatively constructed a liposome that uses ionophore 2-hydroxyquinoline (2HQ) to deliver64Cu2+through phospholipids, and successfully encapsulated the chelating agent DOTA inside the liposome [126].Due to the passive encapsulation of DOTA, it does have the inflexibility of radioactive labeling.Hendrikset al.designed64Cu radiolabeled liposomes using a new chelating agent 4-DEAP-ATSC as a64Cu2+loading and trapping agent [133].This method can firstly chelate 4-DEAP-ATSC with64Cu externally, and then load into liposomes through the liposome bilayer to achieve stable PET imaging.
In addition, Rosaleset al.reported that doxorubicin (DOX), an anticancer drug with metal chelating properties, can be used for radiolabeling of liposomes without the need for additional chelating agents [134].It has been demonstrated that after ionophoremediated transport across the lipid bilayer, the hydroxyl and carbonyl groups on the DOX backbone interact with the radionuclide64Cu, which allows multiple liposomes to simultaneously perform64Cu PET imaging and drug delivery.This approach overcomes the binding of liposomes with additional chelating agents, but the extent of radiolabeling allowed by this method is limited by the strength of the interaction between radionuclides and the drugs in the liposomes.
3.Summary and prospects
The64Cu radiolabeled nanomaterial is an effective nanoplatform and has achieved promising results in PET imaging, imageguided drug delivery, and therapeutic diagnosis.Due to its unique advantages, the latest advances in64Cu radiolabeled nanomaterials have greatly promoted the basic understanding of the role of64Cu PET imaging in cancer diagnosis.In this review, we mainly summarized the general strategies and progress of64Cu radiolabeled nanomaterials, including hot-plus-cold precursors strategy([64Cu]Cu clusters), doping nanoparticles ([64Cu]CuS, [64Cu]CuO nanoparticles and metal doping nanoparticles) and other functionalized radiolabels (chelating agent-based labeling and ionophorebased remote loading), and further analyzed their PET imaging in tumor models.The challenges left by these advancements point us to many future directions and efforts in the design and construction of64Cu radiolabeled nanomaterial probes:
·In vivostability and effective labeling of radionuclides.Although existing probes can achieve PET imaging of tumor, more accurate PET quantification in lesions is still a challenging problem.Since the PET signal comes from a radionuclide, it is crucial that PET imaging platform possess a stable chelating agent or favorable internal doping strategy.The lack of stability may lead to the destruction of the imaging agent, which means the release of free radionuclide [135].For example, free64Cu may more easily flow out of tissues and enter the bloodstream, leading to secondary absorption by other organs.In the liver and even tumors, it may be difficult to distinguish the free radionuclide signal from64Cu signal inherent in the probe [136,137].In addition, the internal incorporation strategy may cause timeconsuming and radioactive labeling effectiveness issues due to the complicated design process [138–140].In this case, the development of reliable radiolabeled nanomaterials is still one of the important research directions.
·The biosafety of radioactive nanoprobes and the feasibility of clinical transformation.The64Cu radiolabeled probe with high sensitivity and quantitative capacity can realize pathological detection in animals and clinical tissues.However, biosafety is an important indicator in actual biomedical applications [141].First, the potential risk of radiation damage cannot be ignored.In order to minimize the risk of radiation, it is recommended that the dose of64Cu nuclides meet the window period for radiolabeling and subsequent imaging [11,12,139].In addition,the relatively large size and surface area of nanomaterials may limit their ability to access predetermined goals and affect excretionin vivo[142].It is feasible to modify the stealth coating on the surface of nanomaterials or rationally design the intelligent biodegradable nanoprobes to further satisfy the future clinical biological application.
·The integration of radioactive nanomaterials and nanomedicine.In addition to PET imaging, it has also been found that the relatively large surface area of nanomaterials can simultaneously load anticancer drugs and radiotracers to achieve the dual functions of diagnosis and therapy [37,143,144].The integrated platform can use PET imaging to accurately monitor drug release in deep tissues.In addition, the Cherenkov radiation of the radionuclide64Cu is used as controllable internal light source to activate the photosensitizers for tumor photodynamic therapy[145].Therefore, the development of more comprehensive diagnosis and therapy nanomaterials is conducive to image-guided surgical treatment, so as to further meet the actual needs of clinical medicine.
In summary,64Cu radiolabeled nanomaterials have made tremendous progress in radiolabeling strategies and tumor diagnosis.It is expected that more versatile radiolabeled nanomaterials will flourish in theranostics, accelerating the transformation of64Cu radiolabeled nanomaterials from laboratory to clinical practice, and ultimately benefit patients.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.U2067214, 21727817), Beijing municipal education commission-Beijing natural science foundation joint funding project (No.KZ202010005006).
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- Radiolabeled peptide probe for tumor imaging
