Temperature-responsive alkaline aqueous biphasic system for radioactive wastewater treatment
Chunying Liu, Jinhui Ln, Qibin Yn, Zhipeng Wng, Cho Xu, Weiqun Shi,Chengling Xio,b,**
a College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
b Institute of Zhejiang University – Quzhou, Quzhou 324000, China
c Laboratory of Nuclear Energy Chemistry, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China
d Institute of Nuclear & New Energy Technology, Tsinghua University, Beijing 100084, China
ABSTRACT The treatment of anionic 99TcO4- in the waste tank with high alkalinity is still very challenging.In this work, a new temperature-responsive alkaline aqueous biphasic system (ABS) based on (tri-n–butyl)-ntetradecyl phosphonium chloride (P44414Cl) was developed to remove radioactive 99TcO4-.The phase transition mechanism was studied by cloud point titration, small-angel X-ray scattering, dynamic light scattering, and molecular dynamic simulations.As the NaOH concentration or temperature increased, the P44414+micelle could grow and aggregate.This micelle showed a particularly high affinity toward ReO4-/99TcO4-compared to other competing anions and could directly extract more than 98.6% of 99TcO4- from simulated radioactive tank waste supernatant.Furthermore, the loaded 99TcO4- could be easily stripped by using concentrated nitric acid rather than metal salt-based reductants.This work clearly demonstrates that the alkaline ABS is a promising separation system for solving the technetium problem in the alkaline waste tank.
Keywords:Pertechnetate Ionic liquid Nuclear waste Aqueous biphasic system Sustainable chemistry
Liquid-liquid extraction is widely applied in environmental engineering, hydrometallurgy, petrochemical industry, pharmacy, and fine chemicals engineering [1–5].In this process, one compound is separated from others due to their different solubility in two immiscible liquid phases [6].Traditionally, organic solvents like kerosene are adopted as an immiscible phase.However, the high volatility, inflammability, and inherent unsustainability of these solvents make them the source of pollution and danger in many chemical engineering processes [7].Furthermore, the hydration energy and mass transfer on the interface which need to be overcome during the extraction make it an energy-intensive process.For this, various green solvents, such as ionic liquid (IL), supercritical fluids, deep eutectic solvent (DES), and aqueous biphasic system (ABS), have been developed to replace the traditional organic solvents [8–11].Among them, the ABS, which consists of two immiscible aqueous-rich phases, is regarded as a non-denaturing and highly efficient separation media without the need of dehydration [12,13].However, the interfacial mass transfer problem still impedes the extraction efficiency of ABS.
The homogeneous liquid-liquid extraction process is considered to be a solution to the mass transfer problem.This technique has extremely fast kinetics because there is no obstacle of mass transfer by the liquid interface and is mainly applied to extract metals or natural products [14,15].The phase separation of homogeneous liquid-liquid extraction is achieved by the thermomorphic behavior of the solute in water, which are often organic salts or ILs.Recently, some novel phosphonium-based IL ABSs have been developed to separate transition metals and rare earths, showing the advantages of both ABS and homogeneous liquid-liquid extraction[16–18].However, to the best of our knowledge,there is still lack of alkaline ABSs that may have huge particular applications in hydrometallurgy and nuclear waste treatment.
Taking the99Tc problem for an example, as a long-livedβemitting radionuclide, it is generated from the fission of235U and239Pu in nuclear reactors [19,20], and is stored in the waste tanks with high alkalinity after recovery of nuclear materials [21].The long half-life (2.13×105years) and high thermal neutron fission yield (6.1%) make this radionuclide a long-term threat to human health [22].What is more, the major species of99Tc (i.e.,99TcO4-)has a vast solubility in water and high environmental mobility,leading to severe leakage of this hazardous anion [23,24].To avoid this pollution, it is highly desired to treat99TcO4-in the alkaline tank waste.However, the content of99Tc in the waste tank is very low (5 ppm) and the alkalinity is very high, making the treatment of99TcO4-very challenging [25].The state of art99Tc removal techniques are mainly focused on the adsorption process based on novel cationic materials which were operated under pH ranges that might not be suitable for treating waste water with OH-concentration as high as 2 mol/L [26–30].
In this work, we developed an alkaline ABS system based on P44414Cl to extract99TcO4-from waste tank water with high selectivity.The phase separation and extraction mechanism were studied through cloudy point titration, small-angel X-rays scattering (SAXS), dynamic light scattering (DLS), and molecular dynamic simulations (MD).
The phase transition of ABS is a thermodynamically driven process, where the temperature plays a significant role in the critical concentrations [31].A simple illustration of the phase separation process is provided in Fig.1A.As the NaOH concentration or temperature increases, the P44414+micelles tend to aggregate and result in a new phase.The binodal curves of P44414Cl-NaOH-H2O systems at 25, 35, 45 and 55°C, shown in Fig.1B, were obtained by cloudy point titration.The biphasic domain is located at the right for each binodal curve, while the monophasic domain is at the left, indicating that this system is a low critical temperature system (LCTS) [32].In this kind of colloid solution, the critical concentration of the solute decreases as the temperature increases.So,the P44414Cl-NaOH-H2O system is a homogeneous aqueous solution at room temperature and become ABS when the temperature exceeds to a certain degree.This temperature-responsive property is endowed with the surface activity of the long side chain on P44414+and salting-out effect of NaOH [33,34].Compared with the HCl ABS, the concentration of NaOH needed to induce ABS under the same condition is smaller, but the distance between isothermal binodal curves of NaOH is much closer [16], which gives NaOH a more moderate phase transition condition but narrower adjusting range than HCl ABS.
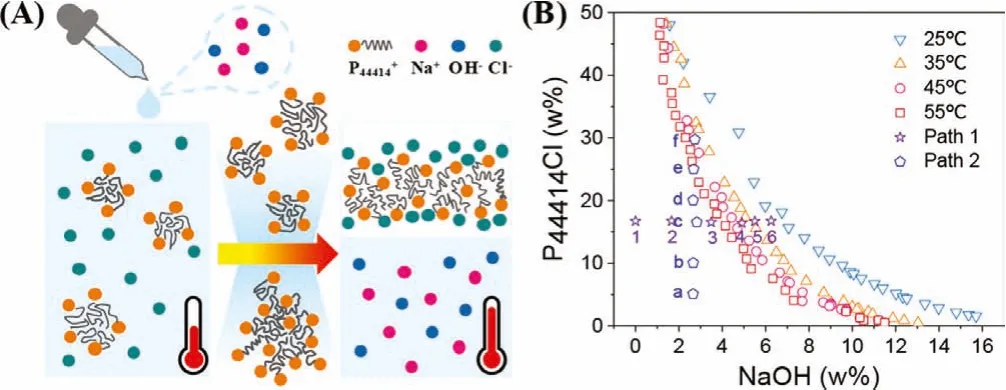
Fig.1.(A) Phase separation process of the P44414Cl-NaOH-H2O system, (B) the binodal curves for the P44414Cl-NaOH-H2O system at various temperatures and the composition of mixture points in paths 1 and 2 studied.
Due to quite similar size and physico-chemical properties between ReO4-and99TcO4-, ReO4-is used as a surrogate for99TcO4-in most of our batch experiments.The effect of NaOH concentration on the extraction of ReO4-was first studied.From Fig.2A, when the NaOH concentration increases from 2 mol/kg to 6.5 mol/kg, the ReO4-could be extracted quantitatively by the P44414Cl with extraction efficiency larger than 99.9%.The extraction equilibrium could be accomplished in 3 min due to the strong reaction between ReO4-and P44414Cl.As a comparison, the extraction efficiency of ReO4-dramatically decreases with increasing HNO3concentration (Fig.2A), which indicates the P44414Cl ABS is suitable to extract ReO4-from NaOH solution rather than HNO3solution and the HNO3might be applied in the stripping of ReO4-/99TcO4-.

Fig.2.(A) Extraction efficiency of ReO4- by P44414Cl-NaOH and P44414Cl-HNO3 ABS,(B) stripping ratio of ReO- using HNO3 and NaNO3, (C) effect of competing NO3-anions on the extraction of ReO4- by P44414Cl-NaOH ABS, and (D) effect of different competing anions (1000 times) on the extraction of ReO4- by P44414Cl-NaOH ABS.
Traditionally, the loaded ReO4-/99TcO4-on the ABS or IL could not be stripped directly.Reduction precipitation was adopted to recover ReO4-/99TcO4-from the extract [35–37].This procedure would consume extra reduction agents and lack continuity or flexibility.Whereas, as shown in Fig.2B, the maximum stripping ratio of ReO4-by HNO3from P44414Cl phase could reach 85%, which promises the recovery of ReO4-in the practical process.It should be noted that only HNO3could obtain such high stripping ratio of ReO4-.The NaNO3solution did not exhibit any stripping capability (Fig.2B).This result indicates both NO3-anion and H+play an important role in the stripping step.
The influence of NO3-on the extraction also verified this hypothesis.From Fig.2C, the extraction efficiency of ReO4-could keep over 99.5% even the ratio of NO3-to ReO4-increased from 0 to 5000.Comparing with other extraction and adsorption systems,in which the presence of large amount of NO3-would greatly reduce the removal of ReO4-/99TcO4-[36,37], the P44414Cl ABS exhibited stronger selectivity towards ReO4-/99TcO4-.The influence of Cl-, SO42-, OH-, PO43-and NO3-, which are main competing anions in practical tank waste, on the extraction of ReO4-was also tested and is shown in Fig.2D.All the anions had no influence on the extraction efficiency of ReO4-with a molar ratio of 1000:1.
The extremely high selectivity toward ReO4-/99TcO4-by P44414Cl-NaOH ABS could be interpreted by the Hofmeister series[38,39].The P44414Cl acts as a chaotrope in the alkaline ABS, so the P44414+is inclined to form an IL-rich phase with hydrophobic anions [36,40].The anions adopted in this work follow the Gibbs free energies of hydration (ΔhydG0): PO43-(-2773)<SO42-(-1090)<Cl-(-347)<NO3-(-306)<ReO4-(-251) [28].Not surprisingly, the ReO4-with monovalent and largest ion radii is the most hydrophobic anion; thus, it is easy to be extracted into the IL-rich phase even from large amounts of competing anions.The high stripping ratio by HNO3might be the resulting formation of HReO4,which had strong interaction with water molecules and increased the solubility of ReO4-in the acid-rich phase [41,42].
Nevertheless, as shown in Table S6 (Supporting information),the H2SO4and HCl showed almost no stripping effect on ReO4-which suggests both H+and an anion with highΔhydG0are indispensable in stripping.
Another important parameter in the extraction process is the loading capacity of the target anion, which influences the operation efficiency of the extraction process.As Fig.S1 (Supporting information) illustrated, the maximum Re content in the IL-rich phase could reach 160,000 ppm which has reached 50% of the theoretical concentration of Re in P44414ReO4.Because of the strong extraction ability of P44414Cl to ReO4-, the P44414Cl concentration had very little influence on the extraction efficiency of ReO4-(Fig.S2 in Supporting information), which indicates this parameter could be adjusted flexibly according to the sample composition.
SAXS, DLS and MD were combined to interpret the phase transition mechanism of P44414Cl-NaOH ABS.The compositions of mixtures used in the measurements were based on the points marked in the phase diagram (Fig.1B).Points 1 to 6 in path 1 were used to study the effect of NaOH concentration and the corresponding SAXS patterns are shown in Fig.3A.Taking the curve of mixture 1 as an example, from the peak around 1.14 nm-1, the short-range order structure diameter was calculated to be 5.51 nm from the equation:d=2π/q, which represented the size of micelle that the P44414+aggregated into [43].With the increasing NaOH concentration, the peak position around 1.14 nm-1almost did not change,but the shape became smooth and vanished.The DLS measurement supported the change in SAXS curves.From Fig.3D, the dynamic diameter of each mixture increased with increasing NaOH concentration, indicating the aggregation of P44414+micelle [44].It should be noted that the dynamic diameter of each mixture followed by the Gaussian distribution (Figs.S4–S6 in Supporting information) which suggested that the P44414Cl-NaOH ABS was a polydisperse system.When the NaOH concentration increased, the micelles grew larger and started to aggregate, resulting in a larger and more dispersed size range.As the dispersity of the system increased, the peaks of SAXS patterns became smooth and finally resulted in a slash.
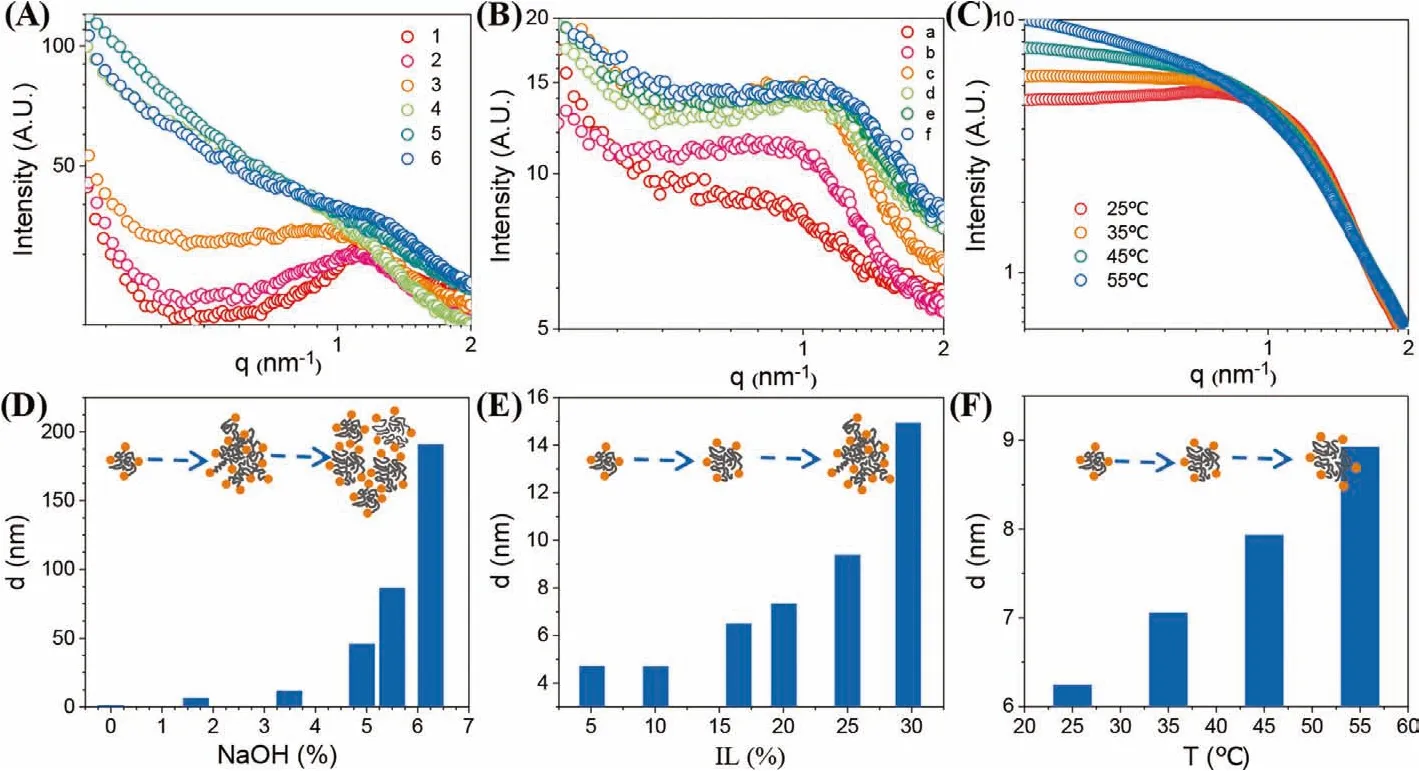
Fig.3.SAXS patterns of mixtures taken from Fig.1: (A) path 1, (B) path 2, (C) point c in path 2, and average dynamic diameter of the mixtures that was taken from DLS,(D) path 1, (E) path 2, (F) point c in path 2.The scheme in (D), (E), and (F) represent the growth and aggregation process of P44414+ micelle.
Similar but weaker changes in the SAXS patterns and micelle diameter could be observed with increasing temperature (Figs.3C and F) and P44414Cl concentration (Figs.3B and E).The mixture used in the temperature study was referred to the point c, path 2 in Fig.1B.The SAXS patterns also became smooth as the temperature increased from 25°C to 55°C, companying with the increase of micelle size.The experiments of P44414Cl concentration were carried out according to mixtures a-f from path 2 in Fig.1B,whose SAXS patterns kept their peaks around 1.14 nm-1.The dynamic diameter varied from 5 nm to 14 nm when the P44414Cl concentration changed from 5% to 30%.Comparing the results of the three variables, we can conclude that the NaOH concentration has a much stronger effect on the trigger of the micelle aggregation than temperature and IL concentration for its stronger effect on the change of critical micelle concentration.The growth and aggregation of phosphonium micelle would finally connect the separated micelles and generate a new aqueous phase immiscible with the NaOH solution [44,45].
We first studied the selective extraction behavior of the P44414Cl-NaOH system towards ReO4-using all-atom MD simulations.The geometric structures of P44414Cl optimized based on density functional theory (DFT) is shown in Fig.S7A (Supporting information).Figs.S7B and C (Supporting information) show the snapshots of initial and final configurations of the MD trajectory for the P44414Cl system, respectively.At the initial state, all the chemical components are randomly distributed at a relatively low density in order to avoid the atom overlap.However, as shown in Fig.S7C, the aggregation behavior of P44414+appears after equilibrium in the molecular scale, forming P44414+micelles.Then we analyzed the spacial distribution of ReO4-, Cl-, and OH-as the simulation time increases.As revealed in Fig.S7D (Supporting information), the formed P44414+micelles have significantly stronger affinity towards ReO4-rather than Cl-, which is in good accordance to our experimental results.All the ReO4-ions are selectively extracted by the P44414+micelles from aqueous solution, either being attached on the surface or moving into the interior.In contrast, Na+, Cl-and OH-ions prefer to reside in aqueous solution instead of penetrating into the interior.Fig.S7E (Supporting information) clearly shows the relative distribution of various ions at the micelle-aqueous interface and within channels between neighboring micelles.We can deduce that the positive charges of P44414+may be shielded by surrounding anions to different extents, and thus the observed P44414+micelles can be formedviastrong enough van der Waals interaction.The effect of ReO4-on the aggregation of P44414+has also been studiedviaMD simulations.Our results indicated that the P44414+micelles can still be formed without the absence of ReO4-at room temperature, as displayed in Fig.S7F (Supporting information).The connected micelles predict the forming of P44414Cl-NaOH ABS that is in accordance with the SAXS and DLS analysis.
We also compared the influence of various counterions like Cl-,SO42-and NO3-on the extraction in MD simulations.It is surprising that all the counterions have negligible influence on the formation of the P44414+micelles as well as the extraction efficiency towards ReO4-.As presented in Fig.S8 (Supporting information),the average interaction energies of the P44414+micelles with each counteranion reveal that the P44414+micelles show significantly higher affinity to ReO4-over Cl-, SO42-, and NO3-, which explains the high selectivity to ReO4-of the P44414Cl ABS.
The practical application of the P44414Cl-NaOH ABS was tested with a simulated tank waste supernatant.The composition of elements was listed in Table S1 (Supporting information).The extraction efficiency of99TcO4-is larger than 98.6%.In addition, over 73.8% of loaded99TcO4-could be directly stripped by 65% HNO3.The P44414Cl based ABS extraction process could be regarded as an efficient and green method to prevent the environmental risk from99TcO4-in the tank waste.The reusability of P44414Cl from HNO3stripping was tested by extracting ReO4-with three repeated cycles.As shown in Table S13 (Supporting information), the extraction efficiencies of recycled P44414Cl in all three cycles are 99.9%which is only 0.07% less than original P44414Cl and suggests that this extractant could be reused.
In summary, a homogeneous liquid-liquid extraction process based on P44414Cl-NaOH ABS was developed to treat99TcO4-from tank waste supernatant.The phase diagram suggests this ABS is a LCTS.The NaOH concentration strongly affected the phase separation by changing the critical micelle concentration.The P44414Cl-NaOH ABS could quantitatively extract ReO4-with high concentration of competing anions because the interaction energy between P44414+micelles and ReO4-was much lower than that of other anions.However, the P44414+micelle still has relative strong affinity to NO3-, resulting in the high stripping ratio (85%) of loaded ReO4-by HNO3.The practical test of extracting99TcO4-from simulated waste tank water by the P44414Cl ABS indicate that this system provides an efficient and green option for recovering hazardous anions from alkaline solutions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21876124, U2032106),Natural Science Foundation of Zhejiang Province (Nos.LR21B060001 and LQ21B070004).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.024.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
