Optically “silent” neptunium(V)-nitrate complex in ionic liquid
Xue Dong, Zhipeng Wang, Qiang Yan, Haiwang Liu, Yuxiao Guo, Hong Cao, Jing Chen,Chao Xu
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
ABSTRACT The complexation of pentavalent neptunium, Np(V), with nitrate ion in an ionic liquid solution has been studied spectroscopically for the first time.The characteristic f-f transition absorption band of Np(V) in the NIR region changes significantly upon the titration of nitrate ion into the solution, revealing strong complexation of Np(V) with nitrate ion in the ionic liquid.Most notably, the absorption band of Np(V)almost disappears when a sufficiently high concentration of nitrate ion is present in the solution.Such a rare optically “silent” species can be assigned to the 1:2 Np(V)/nitrate complex with a centrosymmetric coordination environment where Np sits at the inversion center.
Keywords:Neptunium Ionic liquid Complexation Absorption f-f transition
Neptunium (Np) is an artificial and radioactive element produced through nuclear reactions and it locates between U and Pu in the actinide series in the periodic table.Although not as famous as U and Pu, Np is also a key element in nuclear fuel cycle and one of the most problematic actinides for the storage, reprocessing, and disposal of nuclear wastes.Understanding the chemistry of Np is thus of great significance for the sustainable development of nuclear energy [1].
Similar to its neighboring elements U and Pu, the redox chemistry of Np is very rich and can exist in oxidation states ranging from +II to +VII [2,3].Nevertheless, the most stable and accessible oxidation state for Np in solutions is +V.The pentavalent Np(V)exists as a linear dioxo neptunyl ion ([O=NpV=O]+) in aqueous solutions.The linear dioxo feature offers Np(V) interesting coordination chemistry similar to that of U(VI),e.g., the ligand can only approach and access the center Np atom in the equatorial plane.Moreover, although the center Np atom is in the +V oxidation state in Np(V), the overall charge of Np(V) is +1 and the effective charge on Np of Np(V) is only +2.2 [4], making Np(V) of less affinity in coordinating with ligands through electrostatic interactions.
Since the discovery of Np in the 1940s, the coordination chemistry of Np(V) in aqueous solution has been extensively studied.For example, around five water molecules were found to locate around the equatorial plane of Np(V) in an acidic solution [5].The complexation properties of Np(V) with inorganic anions such as NO3-, Cl-and SO42-, as well as more complicated organic ligands in aqueous solution have also been revealed [6].However, studies on the coordination chemistry of Np(V) in organic medium other than the aqueous solution have so far been quite limited.Here,we explored the complexation behavior of Np(V) with nitrate ion(NO3-), the most commonly encountered inorganic anion in nuclear industry, in an ionic liquid solution through spectrophotometric titrations.
Ionic liquid (IL) is a type of liquid solvent typically composed of cations and anions at room temperature and has been regarded as environmentally benign solvent due to its unique properties such as low volatility, high thermal stability, and broad liquid temperature range [7].The employment of IL as diluent in the separation of metal ions has been a hot topic in nuclear chemistry [8].In the present work, the ionic liquid 1-butyl-3-methylimidazolium bis[(trifluoromethylsulfonyl)]imides (C4mimNTf2) was used as the complexation medium.Np(V) was preloaded into C4mimNTf2by direct dissolution of NpO2(ClO4) or NpO2(NTf2) with C4mimNTf2,while the nitrate solution was prepared by dissolving C4mimNO3in C4mimNTf2.
First, the complexation of Np(V) with NO3-was studied in a relatively “wet” C4mimNTf2, in which the water content is~1 mol/L.As shown in Fig.1, the initial Np(V) in this “wet”C4mimNTf2exhibits a characteristic f-f transition absorption band at around 976.5 nm.The position of this band is blue-shifted as compared to the 980 nm band of Np(V) in an acidic (HClO4) aqueous solution [9].This could be explained by the fact that less water molecules are present in C4mimNTf2and thus Np(V) would be less hydrated in the IL solution than in the aqueous solution.Moreover,since there is a large amount of NTf2-in C4mimNTf2, a few NTf2-ions might be associated with Np(V) in the solution.Upon the addition of nitrate ion (C4mimNO3/C4mimNTf2) into the solution,the absorption band of Np(V) changed significantly.The changes can be grouped to two stages.In the first stage (Fig.1a), the intensity of the absorption band at 976 nm decreases, while a new band appears at 982.5 nm and intensifies upon the titration.In the second stage (Fig.1b), the absorption band at 982.5 nm decreases upon the further titration of nitrate ion, and interestingly, it almost disappears when a large amount of nitrate ion was added.
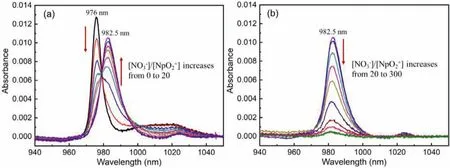
Fig.1.Spectrophotometric titration for the complexation of Np(V) with nitrate ion in relatively “wet” C4mimNTf2.(a) Nitrate/Np mole ratio increases from 0 to 20, (b)nitrate/Np mole ratio increases from 20 to 300.The dark black line in (a) represents the absorption spectra of Np(V) without of nitrate in C4mimNTf2, while the dark green line in (b) is the final spectra of Np(V) with excess of nitrate in C4mimNTf2.
Furthermore, we conducted a spectrophotometric titration for the complexation of Np(V) with nitrate ion in a relatively “dry”C4mimNTf2solution, in which the water content is less than 250 mmol/L.As shown in Fig.2, Np(V) in this “dry” C4mimNTf2initially exhibits a characteristic f-f transition absorption band at around 968.8 nm, which is further blue-shifted when compared to the absorption band of Np(V) in the “wet” IL.Such a blueshift can be ascribed to the less water content in the “dry” IL, where the Np(V) will be further dehydrated.Moreover, there are also a few side bands in the longer wavelength region in the spectra, which might be caused by the hydrolysis of Np(V) or the presence of other coordinating impurities such as carbonate in the solution[10,11].With the addition of nitrate ion into the solution, the intensity of the main absorption band at 968.8 nm decreases gradually, suggesting the complexation of Np(V) with the nitrate ion.When the nitrate/Np(V) ratio reaches 40 in this relatively “dry”C4mimNTf2solution, interestingly, the main absorption band of Np(V) also disappears.
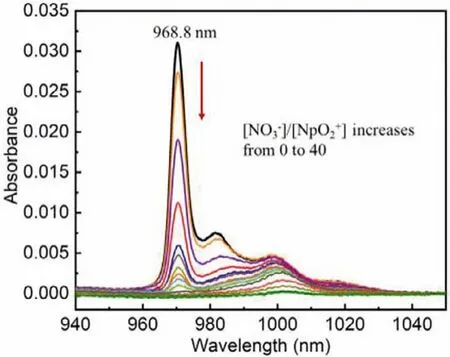
Fig.2.Spectrophotometric titration for the complexation of Np(V) with nitrate ion in relatively “dry” C4mimNTf2.The dark black line represents the absorption spectra of Np(V) without of nitrate in C4mimNTf2, while the dark green line is the final absorption spectra of Np(V) with excess of nitrate in C4mimNTf2.
The disappearance of the absorption bands of Np(V) during the titration of Np(V) with nitrate ion in the ionic liquid medium inspired us to further identify the Np(V)/nitrate complexes and elucidate the coordination mode in the ionic liquid.We attempted to fit the titration data in Figs.1 and 2 to obtain the complexation species and their corresponding binding constants.Unfortunately,such attempts failed, largely due to complicated coordination environment in the ionic liquid medium.Nevertheless, the significant change of the Np(V) spectra upon titration of the nitrate ion suggests the complexation between Np(V) with NO3-is quite strong in the ionic liquid.This is in sharp contrast to the very weak complexation of Np(V) with NO3-in aqueous solutions, in which the binding constants are small (logβfor NpO2(NO3) is in the range of-0.55~0.13) and the interaction between Np(V) and NO3-is exclusively ionic and lack of direct bonding feature [6,12].
As aforementioned, the initial titration of Np(V) with nitrate ion in the “wet” IL leads to the appearance a new absorption band at 983 nm (Fig.1).We ascribe this band to the 1:1 Np(V)/nitrate complex.It should be noted that nitrate can bind with metal ions either in monodentate or bidentate mode.Considering the large shift in the absorption bands (Fig.1), it is reasonable to assume Np(V)is coordinated by nitrate in bidentate mode.A possible structure for the 1:1 Np(V)/nitrate complex is shown in Fig.3.The nitrate coordinates directly to Np(V) in the equatorial plane with two oxygen atoms, while the remaining space is occupied by three water molecules.In this 1:1 complex, the center Np atom locates in a highly asymmetric seven-coordinated environment and therefore exhibits strong absorption band at 983 nm.The most interesting thing is the weakening and final disappearance of the absorption band with further addition of nitrate ion into the solution.Such a dramatic change in the absorption band indicates the formation of new Np(V)/nitrate complexes in the solution (note: no obvious reduction or oxidation of Np(V) was observed during the titration process since no characteristic absorption bands of Np(IV) and Np(VI) were found).
For Np(V), as an f2electronic system, the absorption bands of Np(V) in the NIR region stem from f-f transitions, which are electric-dipole forbidden by Laporte’s rule [13,14].Consequently,the intensities of the absorption bands of Np(V) in the NIR region are closely related to the symmetry of the coordination structure in the Np(V) complexes.In this case, if a Np(V) complex is centrosymmetric and the center Np atom locates at the inversion center, the f-f transitions of Np(V) will be forbidden and the NIR absorption bands will be “silent”.Nevertheless, examples of “silent”Np(V) complexes have only been reported in a few occasions previously [11,15–19].Due to the disappearance of the absorption bands of Np(V) upon the complexation with nitrate ion in IL in the present work, it is reasonable to assume that the Np(V)/nitrate complex at high nitrate concentration is another example for the“silent” Np(V) complexes.Since there is little chance for the formation of 1:3 Np(V)/nitrate complex in C4mimNTf2(the highly charged U(VI) was proven to form only up to 1:3 complex with nitrate in C4mimNTf2[20]), the “silent” complex should be the 1:2 Np(V)/nitrate complex.Fig.3 and Fig.S1 (Supporting information) show three plausible structures for this “silent” complex.In case I (Fig.3), two nitrate ions coordinate bidentately to Np(V) in the equatorial plane and two water molecules coordinate to Np(V)in between, forming an eight-coordinated structure and the center Np atom sits exactly in an inversion center.In case II (Fig.S1), both the two nitrate ions coordinate to Np in bidentate mode in opposite direction and there is no water present in the equatorial plane.In case III (Fig.S1), the two nitrate ions coordinate to Np in monodentate form and two water molecules coordinate to Np in between.Considering the strong coordination of nitrate to Np(V) and the presence of a significant amount of water in the ionic liquid,we believe that case I is the most plausible structure.In the “dry”IL, we did not observe obvious shift of the main absorption band of Np(V) upon titration of nitrate ion (Fig.2).The continuous fading of the absorption bands suggests no 1:1 Np(V)/nitrate complex but the “silent” 1:2 Np(V)/nitrate complex formed directly during the titration.
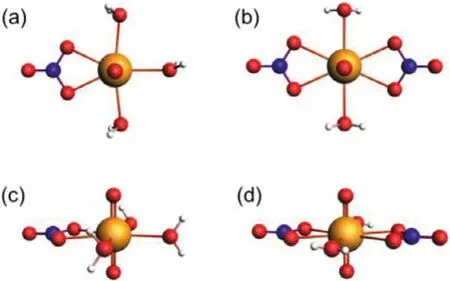
Fig.3.Possible coordination structures for the 1:1 (NpO2(NO3)(H2O)3) (a, c) and 1:2 ([NpO2(NO3)2(H2O)2]-) (b, d) complexes.a and b show the structures looking down from the O=Np=O axis, while c and d show the structures from a view that paralleled with the equatorial plane of Np(V).Yellow: neptunium, red: oxygen, blue:nitrogen, white: hydrogen.
In summary, we demonstrate that Np(V) could form strong complexes with nitrate ion in an ionic liquid medium.With a sufficiently high concentration of nitrate ion in the solution, a “silent”1:2 Np(V)/nitrate complex with an inversion center in the coordination structure would form.This work represents yet another rare example of “silent” Np(V) complex and further enriches our knowledge on the coordination chemistry of neptunium.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (Nos.21790372 and 21822606)and Beijing Natural Science Foundation (No.JQ20041).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.057.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
