Isolation of 212Pb from natural thorium for targeted alpha-therapy
Junyi Chen, Mengxin Xu, Yu Liu, Dongn Dun, Yuxing Hn, Zhio Liu,,*
a Beijing National Laboratory for Molecular Sciences, Radiochemistry and Radiation Chemistry Key Laboratory of Fundamental Science, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
b Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China
ABSTRACT Targeted alpha-therapy (TAT) is increasingly attractive due to its extraordinary antitumor efficacy.However, the supply of α-emitters for TAT is insufficient and under control by a limited number of countries.212Pb is a promising α-emitter with an optimal half-life (10.6 h) and favored decay chain.Of interest,212Pb can be extracted directly from natural thorium, which may be abundant in the mining waste of rare-earth, uranium, etc.Indeed, radioactive thorium waste has been a longstanding environmental challenge that needs immediate action.Developing an on-demand and facile process to isolate 212Pb from natural thorium would be ideal to meet the above challenges, yet is difficult.In theory, the ratio of 212Pb to natTh is below 10-13 in commercially available thorium salts.As a pilot study, 2.2 MBq of 212Pb was successfully extracted from a 5 L solution of thorium nitrate by using a Pb-selective resin.The radiochemical purity of 212Pb is over 99.9% according to gamma-ray analysis.The purified 212Pb was applied to radiolabel a couple of peptides used in clinics (i.e. PSMA, TATE and FAPI-04), and the radiochemical yields are>85%.Of note, 212Pb can be repeatedly separated from the thorium solution every 2 days.In summary,a practical and scalable method was developed to isolate 212Pb for potentially clinical use, which may be of great importance as it does not require either cyclotron or nuclear reactor.
Keywords:212Pb Targeted alpha-therapy Radiolabeling Radionuclide generator Solid-phase extraction
TAT (Targeted alpha-therapy) treats cancer by usingα-particles[1–3], of which the linear energy transfer (LET) is notably higher than that ofβ-particles [4], resulting in lethal damage in cancer cells.α-Particles often induce DNA double-strand break, which can hardly repair, and are less dependent on the oxygen level in tumors [5].The short range ofα-particles (50–100 μm) confines the killing region to the lesion and reduces the negative effects on normal tissues [6].α-Emitters exhibit better therapeutic efficacy than that ofβ-emitters in clinical studies, features with much less therapeutic dose and less radiation resistance [7,8].
For instance,225Ac-labeled radiopharmaceuticals have demonstrated exceptional performance in clinical studies [9–11].However,225Ac is conventionally generated from229Th or233U [12,13],and its annual worldwide manufacturing capacity is less than 3 Ci[14], posing a barrier to the growth of TAT [15].212Pb (t1/2=10.6 h)decays to the stable208Pb, which is also anα-emitting nuclide and can be radiolabeled with DOTA or other TAT chelators [4,17].
Extracting212Pb from natural thorium will resolve the current shortage ofα-emitters for TAT and provide a means of supplyingα-emitters without relying on high-energy accelerators and reactors.Despite the fact that212Pb has been developed for TAT for many years, the available source of212Pb is rather insufficient.212Pb may be separated from the decay of228Th,232Th or232U[18,19].Compare to232Th,228Th and232U are preferred due to the high content of212Pb [16,19].However, both228Th and232U are not commercially available and can hardly be obtained by importation[20].In contrast,232Th is abundant, especially in China [21].The low212Pb content and the consequent technical challenges have hampered the investigation of directly extracting212Pb from natural thorium.Though it may be the only realistic strategy to prepare212Pb in China [20], the reports about isolating212Pb from natural thorium are limited [22,23].
In this work, we attempted to separate212Pb directly from natural thorium compounds for labeling radiopharmaceuticals.By solid-phase extraction with a Pb-selective resin (Pb-SpecTM) [24],the212Pb was isolated directly from the thorium nitrate solution.2.2 MBq of212Pb was obtained from 2.5 kg of thorium nitrate hydrate every 48 h, representing 54% of the theoretical maximal activity and 83% of the212Pb separation efficiency.To validate the quality of the prepared212Pb, it was applied to label several peptide conjugates of clinical importance, DOTATATE [25],PSMA-617 [26] and FAPI-04 [27,28], with good radiochemical yields(over 88%).
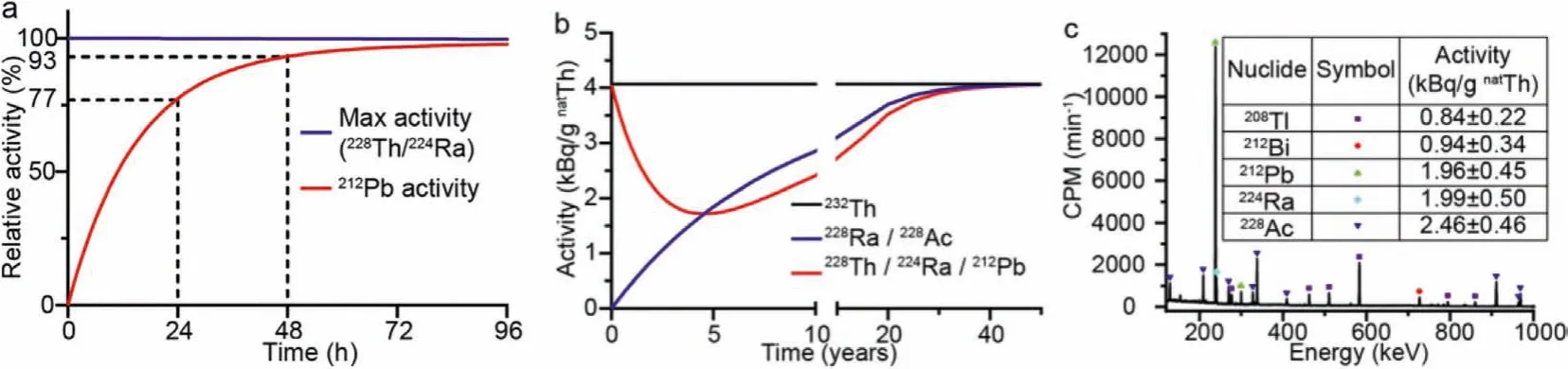
Fig.1.(a) Recovering of 212Pb from 228Th and 224Ra, (b) 232/228Th decay equilibrium curve, and (c) γ-spectroscopy of commercially available thorium compounds.
232Th is abundant and often considered as radioactive waste from mining.The ineffective utilization and treatment of thoriumcontaining slag not only wastes resources, but also causes major environmental issues when radionuclides from232Th enter the atmosphere as dust or enter the land and riversviagroundwater[29].The decay of232Th produces212Pb, which is anα-emitter and can be exploited for TAT.The activity of212Pb in natural thorium is 4.07 kBq/g212Th at decay equilibrium [30], and it may be extracted directly from thorium compounds for radiopharmaceuticals.In the decay chain,212Pb was accumulatedvia224Ra (α, 3.7 d)220Rn (α, 55.6 s)216Po (α, 0.14 s)212Pb.According to the aforementioned, shown in Fig.1a,212Pb in232/228Th can be recovered 77%within 24 h and up to 93% within 48 h following the separation of212Pb, which satisfies the nuclide generator’s requirement.
The decay equilibrium curve of232/228Th is shown in Fig.1b.Right after the isolation of thorium compounds, the activity of212Pb gradually declines due to the rapid decay of228Th, eventually reaching the lowest value of 1.7 kBq/gnatTh in the fourth year.Following that, as232Th decays to228Th, the212Pb activity grows gradually until it reaches decay equilibrium.
As shown in Fig.1c, theγ-spectrum of commercial thorium nitrate.As an inexpensive and easily accessible thorium compounds,thorium nitrate contains radionuclides from the thorium decay chain, ensuring its purity as a raw material for212Pb generators.The acquired thorium compound had not reached decay equilibrium, as the212Pb activity was only 1.96 kBq/gnatTh, or 48% of equilibrium activity, resulting in additional thorium compound.
Thorium nitrate hydrate (2.5 kg) was dissolved in 5 L HNO3solution (1 mol/L) with a thorium concentration of around 1 mol/L.According to previous results, this solution had an activity concentration of around 386 kBq/L and total activity of 1.93 MBq.
Pb-SpecTMis a extraction chromatographic resin by sorbing 4,4′,(5′)-di-(t-butyldicyclohexano)-18-crown-6 (DtBu-CH18C6)in isodecanol solution on an inert substrate for the separation and preconcentration of radiolead [24,31].Lead has a good retention on Pb-SpecTMunder a wide range of nitric acid concentration(0.1–8 mol/L).Under 1 mol/L HNO3, theDwof the PB-SpecTMwas around 1.5×103(k’~8×102) [24], indicating that the extraction column with 6 mL resin (2.3 g resin,Φ12.5 mm×50 mm, 4 mL free column volume) was adequate as the extraction resin for a more efficient212Pb extraction.
To trap212Pb with high efficiency, 5 L of a 1 mol/L Th(NO3)4/1 mol/L HNO3solution was loaded onto the extraction column at a flow rate of 0.5 L/h.The column was then washed with 5 mL 1 mol/L HNO3and 5 mL H2O to remove residual Th4+and to reduce the acidity.Finally, Pb2+was eluted with 7 mL 0.5 mol/L (NH4)2HCit [31], which is suitable for radiopharmaceutical labeling directly.
As shown in Fig.2a, the column trapped Pb but not Th, Ra or Ac.The radioactivity of212Pb fell from 445 Bq/mL to 68 Bq/mL in the elution, with an 85% retention ratio, suggesting that the column can efficiently separate and enrich212Pb.The generator could rapidly regenerate for the next212Pb separation.The change of212Pb activity in the column effluent is described in Fig.2b.At the beginning of loading, the column retained 100% of the212Pb, but as the loading volume increased, small amount of212Pb leakage was observed.The average extraction efficiency of212Pb was 83%,yielding 1.8 MBq212Pb.As demonstrated in Fig.2c, 7 mL 0.5 mol/L(NH4)2HCit could efficiently elute 95%212Pb from the column.We analyzed the samples that had reached decay equilibrium in the next day, and found that the radioactivity of212Pb was 2.2 MBq at the end of purification.
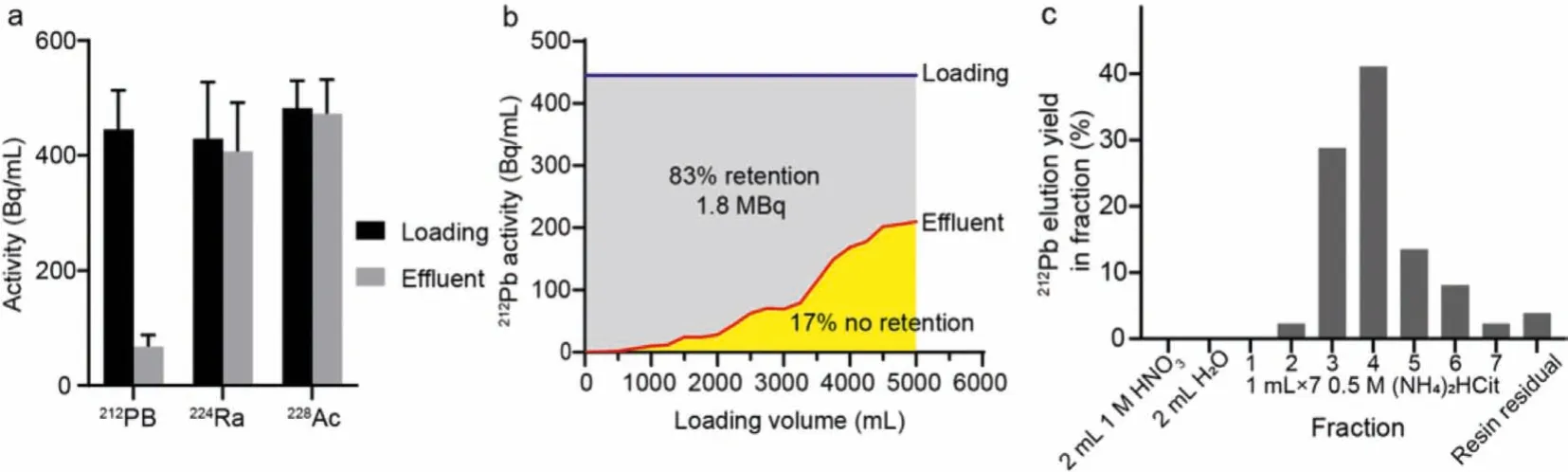
Fig.2.Pb adsorption and elution by Pb-SpecTM extraction column.(a) 212Pb, 224Ra and 228Ac activities in the loading and effluent of the column.(b) 212Pb activity curves in the extraction column loading and effluent.(c) Extraction column 212Pb elution yield in each fraction.
Theγ-spectrum of obtained212Pb was shown in Fig.3a.All visible peaks were ascribed to212Pb and its daughter, confirming thegreater purity of212Pb product obtained by this approach.To further evaluate the212Pb’s purity, it was utilized to label a couple of peptides.Although Cit3-can be coordinated with Pb2+, it was demonstrated that 0.5 mol/L (NH4)2HCit did not affect the labeling of Pb2+.Additionally, 0.5 mol/L (NH4)2HCit with a pH of 5.5 may be an ideal buffer for212Pb labeling with DOTA.The212Pb labeling reaction was carried out for 10 min at 95°C, and the reaction’s progress and yield were monitored using radio-iTLC (Na3Cit as mobile phase, Fig.3b).Table 1 summarizes the labeling conditions and results for [212Pb]Pb-FAPI-04, [212Pb]Pb-PSMA-617, and[212Pb]Pb-DOTATATE (chemical structure shown in Fig.4).All radiochemical yields (RCY) were greater than 88%, and isolated yields were greater than 83%, indicating that the212Pb produced using this method was reliable for radiopharmaceutical investigations.

Table 1 Conditions and results of 212Pb labeling.a
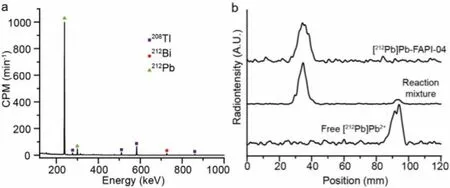
Fig.3.(a) γ-spectrum of 212Pb product.(b) Radio-iTLC of free [212Pb]Pb2+, reaction mixture, and purified 212Pb-labeled compound during the 212Pb labeling of FAPI-04.
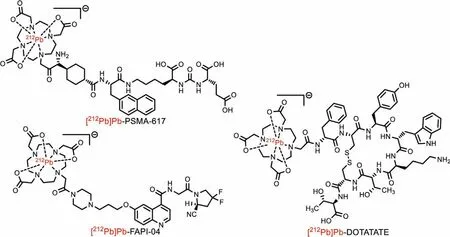
Fig.4.Chemical structure of [212Pb]Pb-FAPI-04, [212Pb]Pb- DOTATATE and [212Pb]Pb- PSMA-617.
In the above experiment, we successfully obtained 2.2 MBq212Pb from a 5 L thorium nitrate solution.However, to obtain more212Pb, additional thorium nitrate solution is needed, which may be difficult in the lab.Another solution to obtain212Pb based onnatTh is to extract228/224Ra from the matrix and enrich them to isolate212Pb in a limited volume of228/224Ra, which would be easier to generate a higher activity of212Pb.Therefore, the separation and enrichment of228/224Ra from natural thorium compounds are ongoing to construct next-generation of212Pb generator.In addition,in the process of rare earth and uranium mining, ‘radium removal residue’is a kind of radium-containing waste residue in the form of Ba(Ra)SO4, and the amount of228/224Ra is considerable.If the waste could be used as a source of228/224Ra to prepare228/224Ra-212Pb generators, it would be a “one-stone two-bird” strategy that make full use of the hazardous radioactive waste to produce highvalue medical radionuclides.
To summarize, this study aims to extract and purifyα-emitter212Pb from natural thorium compounds for use in TAT radiopharmaceuticals.2.2 MBq of212Pb was extracted from 5 L of thorium nitrate solution using Pb-SpecTMwith an extraction efficiency of 83% and a high purity.The212Pb in 0.5 mol/L (NH4)2HCit solution produced could be used directly for the radiolabeling of DOTATATE,PSMA-617 and FAPI-04, which all achieved greater than 88% RCY and 83% isolation yield.The aforementioned results demonstrate that modest amounts of212Pb can be isolated directly from natural thorium compounds for developing212Pb-labeled radiopharmaceuticals for targeted alpha-therapy.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in,or the review of, the manuscript entitled.
Acknowledgments
This work was funded by Beijing Municipal Natural Science Foundation (No.Z200018), the Special Foundation of Beijing Municipal Education Commission (No.3500–12020123), the National Natural Science Foundation of China (Nos.U1867209 and 21778003) and the Ministry of Science and Technology of the People’s Republic of China (No.2017YFA0506300) and Li Ge-Zhao Ning Life Science Youth Research Foundation (No.LGZNQN202004).
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
