Complexation between uranyl(VI) and CMPO in a hydroxyl-functionalized ionic liquid: An extraction,spectrophotography, and calorimetry study
Baihua Chen, Jun Liu, Hongyuan Wei, Yuchuan Yang, Xingliang Li, Shuming Peng,Yanqiu Yang
Institute of Nuclear Physics and Chemistry, CAEP, Mianyang 621900, China
ABSTRACT The extraction complexes of uranyl(VI) in HNO3 to a hydroxyl-functionalized ionic liquid (IL) phase,HOEtmimNTf2 bearing CMPO, were investigated.Three possibly successive extraction complexes, UO2L2+(L=CMPO), UO2L22+ and UO2L32+, were detected based on variable U/L ratios.Uranyl(VI) prefers to be extracted as complex UO2L32+, combining with the ions from HOEtmimNTf2 to construct a solid material through self-assembly.The thermodynamics of complexes, UO2Lj2+ (j=1-3), were studied by spectrophotometry and microcalorimetry.All the formation reactions are principally driven by entropy, although a small part of the driving force of complexes UO2L22+ and UO2L32+ comes from enthalpy.Based on the thermodynamic properties for complex UO2L32+, we provide a possible coordination mode in HOEtmimNTf2: the first CMPO molecule coordinates with UO22+ in a bidentate fashion while the others do in a monodentate fashion.The results offer a thermodynamic insight into the formation behaviors of the uranyl(VI)/CMPO complexes involving the special IL HOEtmimNTf2, which is of significance to advance the novel IL extraction strategy.
Keywords:Uranyl(VI)CMPO Ionic liquid Solvent extraction Complexation Thermodynamics
Liquid-liquid extraction is the most maturely popular separation technique for the reprocessing of spent nuclear fuels.Due to its unique physicochemical and solvation properties [1,2],ionic liquid (IL) is promising as an efficient and eco-friendly solvent to recover actinides from spent nuclear fuels.Extensive studies have been conducted over years on the extraction and complexation of actinides and lanthanides, replacing volatile molecular solvent dissolving the common ligands with ILs.Positive results on efficiency and selectivity have been achieved [2–6].For example, uranyl(VI) in HNO3was more effectively extracted by trioctylphosphine oxide (TOPO) in butylmethylimidazolium bis(trifluoromethylsulfonyl)imide (C4mimNTf2) greatly elevated the extraction efficiency compared with the same extraction conducted with TOPO in dichloromethane [6].
Octylphenyl(N,N-diisobutylcarbamoylmethyl)phosphine oxide(CMPO) has received attention as one of the most widely studied phosphorous-based extractants to separate tri-, hexa- and tetravalent actinides from acidic solutions [7–9].Schemes of actinides extraction from HNO3solutions by CMPO in ILs have been achieved recently [3,10–12].Comparison studies illustrated the extraction efficiency enhances largely with ILs replacing molecular solvents as the diluent of ligands.Visseret al.using the mixture extractant of CMPO and TBP (tributyl phosphate), found that the distribution ratios for Am(III), Th(IV), Pu(IV) and U(VI) were all at least an order of magnitude higher when butylmethylimidazolium hexafluorophosphate (C4mimPF6) as the diluent of extracting phase than those whenn-dodecane as diluent [10].Routet al.observed much largerDAmvalues using TBP and CMPO in C4mimNTf2than inn-dodecane [13].
Most recently, we provided a novel strategy for efficient capture of uranium from nitric acid solutions with CMPO in(1-hydroxyethyl-3-methyl)imidazolium bis(trifluoromethylsulfonyl)imide (HOEtmimNTf2), a hydroxyl-functionalized ionic liquid [14].The specific property of HOEtmimNTf2triggers the self-assembly of the uranyl(VI)/CMPO complex at the aqueous-IL interface to form a solid material, with the composition of 3/9/1/7 for UO22+/CMPO/HOEtmim+/NTf2-.Almost all aqueous uranium (95%) was transferred to the solid phase.However, the extraction species as well as the complexation of uranyl(VI) with CMPO involving HOEtmimNTf2still keep unknown.Herein, we describe the thermodynamic behaviors of complexation between uranyl(VI) and CMPO in HOEtmimNTf2, investigated by biphasic extraction and monophasic titration.Based on the determined thermodynamics for complexes, we further discuss the chelation fashions of CMPO to uranyl(VI).The results and discussion are of significance in understanding the corresponding extraction behaviors and then optimizing the extraction systems.
It is generally accepted that ILs do not behave as inert diluents toward nitric acid [15,16].As depicted in Fig.S1 (Supporting information), the HNO3concentration in HOEtmimNTf2increased linearly with the increase of the aqueous HNO3concentration.HNO3distribution ratio was calculated to beDHNO3=0.31 by the linear slope, suggesting that strong interaction between HOEtmimNTf2and HNO3occurred.CMPO is of weak basicity and interacts with HNO3.It has been demonstrated that HNO3can be extracted into the organic phase, using molecular solvents as the diluent of CMPO [17–19].For example, equilibrating CMPO dissolved in NPHE (nitrophenylhexyl ether) with aqueous HNO3solution, HNO3·CMPO was formed in the organic phase with the equilibrium constantK=3.1×10-5[18].Given this, we conducted an additional experiment, equilibrating HOEtmimNTf2bearing CMPO with 0.1 mol/L HNO3.As shown in Table S1 (Supporting information), in the concentration range of CMPO (0.024–0.064 mol/L),the equilibrium HNO3concentrations in the IL phases (0.0283–0.0274 mol/L) changed around 0.0277 mol/L (the equilibrium HNO3concentration in the IL phases without CMPO).Taking experimental uncertainty into account, we believe that the CMPO concentration in the IL phase affects the HNO3distribution little.In other words, the interaction of HNO3with CMPO is incomparable with that with HOEtmimNTf2.It is the right reason for our previous observation that the HNO3concentration in the aqueous phase did not affect the uranium extraction efficiency [14].Consequently,to simplify the experiment process and data treatment, the experimental solutions were prepared with the equal-volume equilibrated HOEtmimNTf2and 1.0 mol/L HNO3, while the protonation of ligand CMPO was not considered.
The formation of extraction complexes in the biphasic extraction system is essential to produce solid assembly material.Firstly we conducted extractions on a constant-temperature water bath shaker, with the aqueous phase with UO2(NO3)2(no more than 1.0 mmol/L) in 1.0 mol/L HON3.Pre-experiments showed that no observably solid assembly material appeared at the inter-surface of the biphasic system in a day time when the initial uranyl(VI)concentration in 1.0 mol/L HNO3solution was less than 1.0 mmol/L.Cation exchange has been presumed to be the partitioning mechanism for the biphasic metal extraction equilibrium with hydrophobic IL as diluent [1,20–22].Considering the stoichiometric ratio of the solid material obtained at the interface of the biphasic system [14], we hypothesized three successive extraction complexes formed possibly in the IL phase,i.e., UO2L2+, UO2L22+and UO2L32+.The uranium distribution ratio (DU) should function as a cubic polynomial equation along with the CMPO concentration([¯L]) in the IL phase (Eq.S5 in Supporting information).Fig.1 depicts the UO2(NO3)2extraction results by CMPO in HOEtmimNTf2at 25 °C.Fitting the extraction data with Eq.S5 based on the least square method, as shown the short dash line in Fig.1a, the apparent equilibrium constants (Kexapp) for the three successive extraction complexes were obtained to beKex,1app= 2.16±0.24,Kex,2app=4.97±0.34, andKex,3app=8.53±0.03, respectively.Interestingly that (Kex,3app-Kex,2app)>(Kex,2app-Kex,1app)>Kex,1app, suggests that, for this extraction system, uranium prefers to be extracted by the complexes of higher uranyl(VI) stoichiometric ratio, especially UO2L32+.It can be interpreted that along with the uranyl(VI) stoichiometric ratio increasing, the extraction complexes become more hydrophobic, which facilitates their transfer into the organic phase [21].As illustrated in Fig.1b, in a dual logarithmic coordinate system a fine linearship we observed betweenDUand [] (2.6-4.5 mmol/L) with a slope of 2.93, suggesting complex UO2L32+being the predominant extraction species [6].
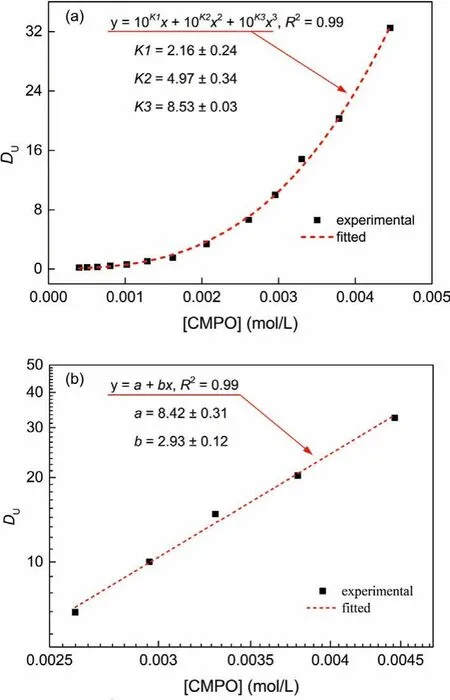
Fig.1.UO22+ in nitric acid extraction with CMPO in HOEtmimNTf2 at ambient temperature.Aqueous phase: 0.91 mmol/L UO2(NO3)2 in 1.0 mol/L HNO3; organic phase:CMPO (0.5~4.5 mmol/L) in HOEtmimNTf2 (equilibrated by 1.0 mol/L HNO3).
As a result of the Laporte forbidden electronic transitions from ligand orbitals to empty 5forbitals of central uranium, uranyl(VI)in the range of 485–380 nm behaves weak absorption bands which are mainly governed by the geometry of the equatorial coordination and only to a less extent affected by the chemical nature of the ligands [23,24].In the present work, identification of the complex species between UO22+and CMPO, as well as the determination of the stability constants of the complexes, was achieved by spectrophotometric titrations of UO22+with CMPO in HOEtmimNTf2[25,26].As shown in Fig.2, additions of CMPO caused significant changes in the shape and location of the absorption bands.As the L/U ratio (R=CL/CU) increased to about 2.32,the series of absorption bands became sharper, well-defined, continuously red-shifted, and intensified.However, different changes were observed afterRwas higher than 2.32.The main absorption bands at about 402, 413, 425.5, and 438 nm were continued to redshifted, accompanied by decreases in intensities, while the absorption band at about 390 nm changed little.For example, as shown in the insert picture of Fig.3a, whenRincreased from 2.32 to 3.49,the peak center of the most intensive absorption band red-shifted from 425.5 to 426.0 nm, while its intensity reduced from 0.0432 to 0.0419; the absorption bands at about 452, 464, 481, and 498 nm increased slightly in intensity.The continuous changes of the absorption spectra in titration indicate the successive complexation of UO22+with CMPO.
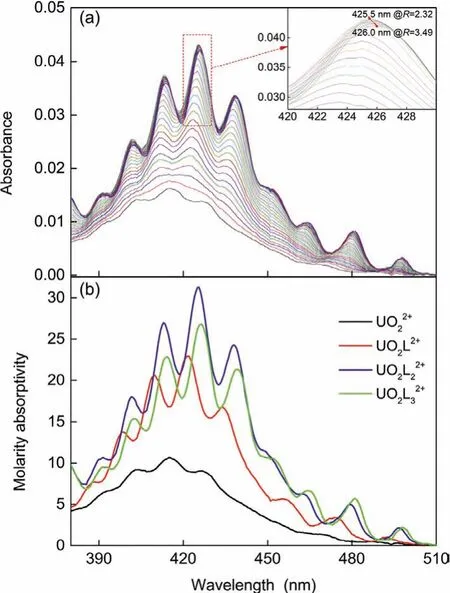
Fig.2.Spectrophotometric titration of UO2(NO3)2 with CMPO in HOEtmimNTf2.Initial solution: V0=2.0 mL, CU0=1.5 mmol/L, titrant: CL=69.7 mmol/L, 0.15 mL added.(a) Spectra of the titration system normalized to the initial concentration of U(VI); (b)molar absorptivity of the species found in the titration system.
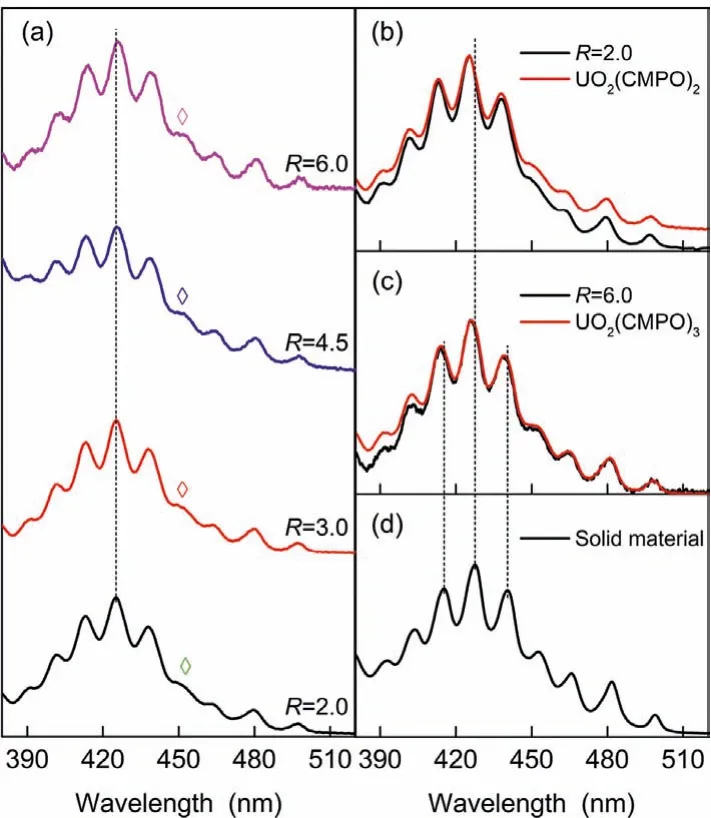
Fig.3.(a) Absorption spectra of the IL phases after extraction; (b) Spectrum comparison of the IL phases after extraction (R=2.0) with complex [UO2(CMPO)2]2+;(c) Spectrum comparison of the IL phases after extraction (R=6.0) with complex[UO2(CMPO)3]2+; (d) Absorption spectrum of the solid assembly material.
Factor analysis by HypSpec program [27] suggested the presence of four absorption species in the system,i.e., free UO22+and three successive complexes like UO2L2+, UO2L22+, and UO2L32+.Fit converged well at the stability constants (logβ) of 4.82 (5),9.39(12), and 12.98(5) for complex UO2L2+, UO2L22+, and UO2L32+,respectively.The calculated molar absorption spectra of the species are depicted in Fig.3b.The better-defined vibronic fine structure and sharper absorption bands clued that uranyl(VI) complexed with CMPO in an ordered ligand field [25,28].The spectrum changes of the titration system (Fig.3a) can be interpreted with the quite different spectra of uranyl(VI) species and their concentration changes in the solution.For instance, Absorbancy of complex UO2L32+is weaker than that of complex UO2L22+, responsible for the spectrum intensity decreased in the titration system whenR>2.32.
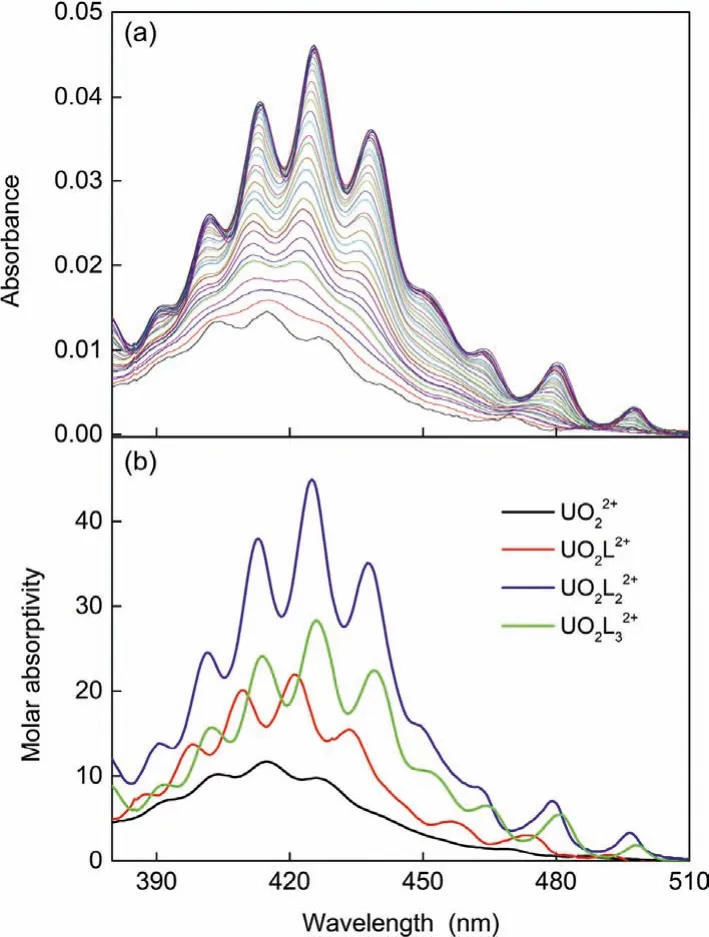
Fig.4.Spectrophotometric titration of UO2(NTf2)2 with CMPO in HOEtmimNTf2.Initial solution: V0=2.0 mL, CU0=1.5 mmol/L, titrant: CL=97.4 mmol/L, 0.096 mL added.(a) Spectra of the titration system normalized to the initial concentration of uranyl(VI); (b) Molar absorptivity of the species found in the titration system.
In Fig.3, we compared the absorption spectra of the IL phases after extraction with those of complexes UO2L22+and UO2L32+in HOEtmimNTf2.The spectra of UO2L22+and UO2L32+, as depicted in Fig.2b, were obtained in the monophasic titration experiments.As shown in Fig.3a, for the spectra of the post-extraction IL phases, along with the increase of L/U ratio, the position of absorption bands red-shifted continuously (depicted the short-dash line),and the relative intensity of the absorption band between 447 nm and 459 nm (marked with ◇) strengthened slowly.No matter in terms of the peak positions or the relative intensities among the peaks, the post-extraction spectrum of the IL phase whenR=2.0 was almost identical to that of complex UO2L22+(Fig.3b), and that of the IL phase whenR=6.0 nearly overlapped that of complex UO2L32+(Fig.3c).Such observations suggest that with the increase of the L/U ratio, the uranyl(VI) species in the post-extraction IL phase successively change in the order of UO2L2+, UO2L22+, and UO2L32+, consistent with the trend of uranium distribution ratio with the CMPO concentration in the IL phase (Fig.1).When CMPO extracts aqueous uranyl(VI) to the IL phase as complex UO2L22+,and similarly whenR=2.0 the extraction complex is UO2L32+[24,25,29].With the experimental techniques of our previous work[14], we collected some self-assembly material and determined its absorption spectrum (Fig.3d).Compared with the absorption spectrum of complex UO2(CMPO)32+in HOEtmimNTf2(Fig.3c), significant redshifts occurred for the corresponding bands in the absorption spectrum of the assembly material.This is a common optical character resulting from the assembly of mono-molecules [30].Typically two reasons can explain this phenomenon: one is related to an edge-to-edge (J-type) aggregation of complex monomers in self-assembly material, and the other is related to the state transition of complex monomers from the disordered dispersion in solution to the orderly tight arrangement the self-assembly material[30–32].
To check the effect of nitrate anions on the complexes formation, spectrophotometric titrations were also conducted with UO2(NTf2)2instead of UO2(NO3)2, as shown in Fig.4.Three successive uranyl(VI) complexes were also observed, which are of the same stability constants as the corresponding complexes of UO2(NO3)2(Table 1).Moreover, these species are of almost identical spectrum profiles (Fig.S2 in Supporting information).So, we believe that the species of uranyl(VI) are of the same coordination environments, no matter whether UO2(NO3)2or UO2(NTf2)2has been used as the initial substance.All the aforementioned results possibly explain why the counter ions detected in the solidmaterial collected from the extraction system are NTf2-rather than NO3-.
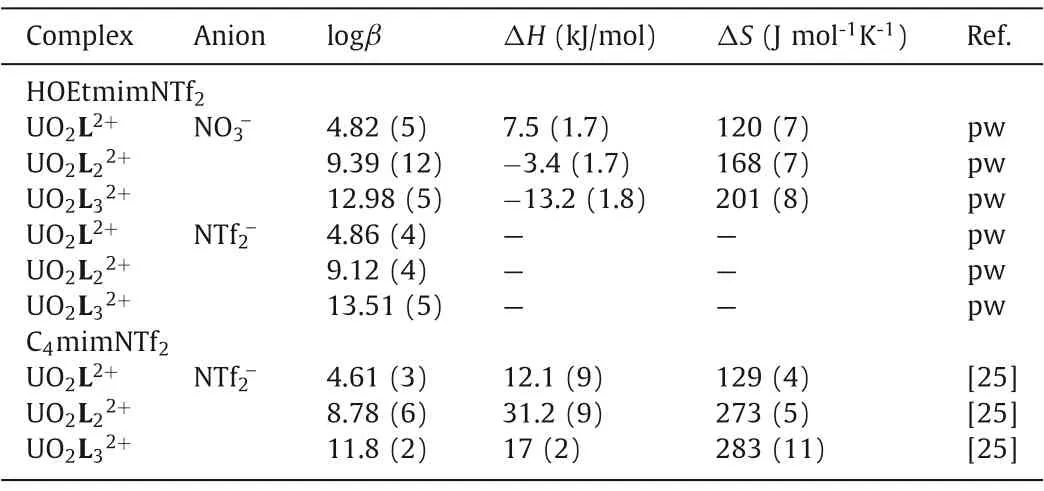
Table 1 Thermodynamic parameters of the complexation of uranyl(VI) with CMPO in ionic liquids.

Fig.5.Representative microcalorimetric titration of uranyl(VI)/CMPO complexation in HOEtmimNTf2.(a) Titration thermograms; (b) cumulative reaction heat (black◇for experimental data (left y-axis), and: the red short dash line for the calculated results) and the uranyl(VI) species (right y-axis, black line: UO22+, red line:UO2L2+, green line: UO2L22+, and blue line: UO2L32+) as a function of the titrant volume (Vtitrant, μL).Initial solution in the cup: 0.800 mL of UO2(NO3)2 solution(2.19 mmol/L) in HOEtmimNTf2, titrant: 40.0 mmol/L CMPO in HOEtmimNTf2.
Fig.5 shows a representative microcalorimetric titration of U(VI)/CMPO complexation in HOEtmimNTf2.At the beginning of titration, the heat-rate curve consisted of negative peaks; with the addition of ligand solution, the negative peaks decreased rapidly in turn and then changed to positive peaks (Fig.5a).This phenomenon indicated that the complexation system was endothermic in the early stage with lower L/U ratios, while rapidly changing to be exothermic with the increase of L/U ratio.In conjunction with the stability constants determined by spectrophotometry, the enthalpies for the U(VI)/CMPO complexes were calculated with HypDeltaH [33] and summarized in Table 1.The formation reactions of complexes UO2L2+, UO2L22+and UO2L32+are of small enthalpy and large positive entropy, in agreement with the normal characters of the inner-sphere complexation between a hard Lewis acid and hard Lewis base [34,35].The formation of UO2L2+is endothermic and entirely driven by entropy; the formations of UO2L22+and UO2L32+are weakly exothermic and driven by both enthalpy and entropy, whereas enthalpy only accounts for a small portion of the driving force.Positive enthalpy for complex UO2L2+indicates that the consumed energy in uranyl(VI) desolvation overrides the release in uranyl(VI)/CMPO coordination, whereas the opposite situation for complexes UO2L22+and UO2L32+[28,36,37].
In molecular solvents, the coordination number of linear UO22+is five or six (CN=5 or 6), forming pentagonal or hexagonal bipyramidal complexes with ligands [26,38–42].However, in ILs such as C4mimPF6and C8mimNTf2, extended Xray absorption fine structure (EXAFS) measurements showed that UO22+behaves lower CN (CN=4.0-4.5) than in molecular solvent [43,44].The entropy for complex UO2L2+in HOEtmimNTf2(120(7) J mol-1K-1) is equivalent to that in wet C4mimNTf2(129(4) J mol-1K-1) [25], indicating that in HOEtmimNTf2, the first CMPO molecule chelates to UO22+in the same bidentate fashion as in C4mimNTf2[25].Such coordination mode has been confirmed by the solid crystallographic data of the corresponding complex [38,39].The stepwise enthalpy and entropy for complex UO2L22+(reaction UO2L2++L →UO2L22+,ΔH2=-10.9±2.0 kJ/mol,ΔS2=48±8 J mol-1K-1) and for complex UO2L32+(reaction UO2L22++L →UO2L32+,ΔH3=-9.8±2.0 kJ/mol,ΔS3=33±9 J mol-1K-1) are of equivalent values in error ranges, suggesting the second and third CMPO molecule most likely chelate to UO22+in the same fashion.Consequently, we herein hypothesize that the second and the third CMPO would act as a monodentate ligand to coordinate with UO22+[45].The thermodynamic results can be interpreted perfectly with such a complexation assumption.One donor of CMPO (possibly the O-donor of P=O moiety because of its stronger basicity than that of the N-O moiety [46]) coordinates with UO22+, which is convenient to disperse the steric hindrances and coordination tensions.As consequence, less difference occurs in the energy needed to remove the inner-sphere solvent molecules of UO22+in the last two complexations.
In summary, the complexation of uranyl(VI) with CMPO in a hydroxyl-functionalized ionic liquid HOEtmimNTf2has been investigated by solvent extraction, spectrophotometry, and calorimetry.Three successive complexes UO2L2+, UO2L22+and UO2L32+form in HOEtmimNTf2.Though UO2L2+and UO2L22+are the possible extraction species under a lower L/U ratio, complex UO2L32+is the preferable extraction species of uranyl(VI) in the IL phase, assembling its neutral molecules at the inter-surface of the biphasic system to yield a solid material [14].In homogeneous HOEtmimNTf2solution, the stability constants of complexes UO2L2+, UO2L22+and UO2L32+are 4.82(5), 9.39(12) and 12.98(5), respectively.All formation reactions are predominantly driven by entropy, although a small part of the driving force of complexes UO2L22+and UO2L32+comes from enthalpy.The thermodynamics of the UO22+/CMPO complexes is interpreted by hypothesizing that the first CMPO molecule coordinates with UO22+in a bidentate fashion, and the others in a monodentate fashion.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the National Science Foundation of China (Nos.22076175, 11675156, U1830202 and 21976165).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.066.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
