Stability of selenium and its speciation analysis in water using automatic system separation and HR-ICP-MS measurement
Junqiang Yang, Yawn Chn, Kliang Shi,d,*, Kshng Hu, Run Li, Xiaoqing Gao,f,Qian Wang, Wio Zhang, Yun Zhou, Yanyun Wang, Jiangang H, Tonghuan Liu,Xiaolin Hou,*
a Frontier Science Center for Rare Isotopes, Lanzhou University, Lanzhou 730000, China
b School of Nuclear Science and Technology, Lanzhou University, Lanzhou 730000, China
c Beijing National Laboratory for Molecular Sciences, Fundamental Science Laboratory on Radiochemistry & Radiation Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
d Key Laboratory of Special Function Materials and Structure Design, Ministry of Education, Lanzhou University, Lanzhou 730000, China
e CNNC Lanzhou Uranium Enrichment Co., Ltd., Lanzhou 730000, China
f Environmental Monitor Center of Gansu Province, Lanzhou 730000, China
ABSTRACT A simple and convenient method has been developed for the pre-concentration and separation of inorganic selenium species from environmental water samples using anion exchange chromatographic column combined with high resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) measurement.75Se(IV) and 75Se(VI) were prepared and used as tracers during the experiments.The volatility of selenium during solution evaporation was investigated to establish a reliable water samples pretreatment procedure.The parameters which affect the uptake of Se(IV) and Se(VI) on Dowex1×8 resin was optimized and the procedure for Se(IV) and Se(VI) separation was proposed.Both Se(IV) and Se(VI) are retained on the column in natural or alkaline solution with high distribution coefficient.The successive gradient elution of pre-concentrated species of selenium with HNO3 solution allows to differentiate between them.Se(IV) and Se(VI) finally were eluted with 0.05 mol/L HNO3 and 5.0 mol/L HNO3, respectively.The proposed method has been successfully verified using the certified reference materials (CRMs)of real water samples, and spiked recoveries for real samples were 98%-104% with 5% relative standard deviations (RSDs).The developed procedure is proved to be reliable and can be used for the rapid determination of selenium species in environmental water samples.
Keywords:Inorganic selenium Speciation Automatic system separation Rapid determination Water sample
Selenium is a natural tracer element found in bedrock, but it is also introduced into the environment by anthropogenic activities, such as mining and combustion of fossil fuels [1–3].At low concentrations, selenium is an essential micronutrient for mammals, but consumption of quantities exceeding daily recommendations can cause health problems [4,5].The concentration range between deficient and toxic symptoms in drinking water was restricted strictly, the maximum concentration was 0.04 mg/L for the World Health Organization, 0.05 mg/L for the Environmental Protection Agency, USA [6].Moreover,79Se is a long-lived fission product (0.044% for235U) with a half-life of 2.95×105y and is chemically and radiologically toxic [7,8].Because of its high mobility,79Se could cause serious radioactive contamination once leaking to environment.Thus, the studying on the environmental behaviors of selenium is therefore significant.
It is well known that bioavailability of selenium in the natural environment is largely dependent on its chemical form [9,10].Selenium exists in inorganic forms at various oxidation states and in organic forms with direct Se-C bonds, such as seleno-amino acids and methylated selenium compounds [11].Se(IV) and Se(VI)are found to be thermodynamically stable under the pH and redox conditions that are found in most aqueous media and are the predominant chemical forms of inorganic selenium [12].Se(IV) is present in mildly oxidizing, neutral pH environments and typical humid regions.Se(VI) is the predominant form under ordinary alkaline and oxidized conditions [13].The determination of aqueous selenium species (Se(IV) and Se(VI)) is important to evaluate the presence and concentration of selenium in environmental systems since inorganic forms showed toxicity up to 40 times higher than the organic forms [14].The quantitation and speciation of inorganic selenium in the natural waters has been of long-term stand interest.
For speciation analysis, the separation of the species of interest from the sample matrix using selective chemical reactions combined with separation and extraction procedures should be firstly considered.A variety of treatment methods have been reported for inorganic selenium separation and removal from contaminated waters, which mainly including sorption and chromatographic methods [15,16].The sorption process is one of the most promising methods for selenium removal from water [17–19].The main disadvantage of this approach is the difficult to remove Se(IV)and Se(VI) simultaneously due to their own characteristics and particularity of Se(VI).Chromatographic methods especially anion exchange chromatography show excellent abilities for Se(IV) and Se(VI) uptake and separation [20].Zhanget al.incorporated an anion exchange column in a flow injection system for the preconcentration and determination of Se(IV) at pH 5 [21].Oyamada and coworkers used Dowex1×4 in the hydroxide form to preconcentrate Se(IV) and Se(VI) with subsequent simultaneous elution of both species [22].However, the reported procedures are difficult to quantitatively separate the inorganic species of selenium each other and the methods for selenium measurement is usually time consuming [23].Furthermore, because the high concentration of HCl and HNO3were often applied for Se(IV) and Se(VI)desorption, the variation of oxidation state of Se(IV) and Se(VI) in these media is still not clear [23,24].Therefore, a sensitive method based on the anion exchange chromatography at ultra-trace level is clearly needed for the efficient pre-concentration and separation of selenium speciation in natural waters.
For sample preparation before ICP-MS measurement, the evaporation method was often applied for reducing the volume and acidity of sample solution.In this case, the stability of selenium during solution evaporation should be firstly considered.To our best knowledge, there is no relative work reported on these issues.
In this work, we aim to develop a reliable and rapid method for determination of selenium speciation in water samples.The inorganic species of selenium (including Se(IV) and Se(VI)) were separated by an automatic system combined with anion exchange chromatographic column (Dowex1×8 resin) and measured by high resolution ICP-MS.The thermal stability of selenium during sample solution evaporation in HCl/HNO3medium was evaluated.In addition, the conditions for the adjustment of oxidation state of Se(0)to Se(IV) and Se(VI) were studied in details to get the radioactive tracers of75Se(IV) and75Se(VI).
Stock solutions of Se(IV) and Se(VI) were prepared by dissolving K2SeO3(analytical reagent, purchased from Strem Chemicals) and K2SeO4(analytical reagent, purchased from Alfa Aesar) salts in high purity water (18 MΩ/cm), respectively.The solutions were then diluted to the desired concentrations as bulk solution for further use.75Se(0) was obtained from China Institute of Atomic Energy and used as a tracer for the experiment after the oxidation of Se(0) to Se(IV) and Se(VI), respectively.All other chemicals used were of analytical grade and prepared with high purity water.
Dowex1×8 anion exchange chromatographic resin (100–150 μm particle size, Cl-form) was purchased from Alfa Aesar.The resin was pretreated with high purity water till the pH of suspension is in the range of 6–8 before use.Certified reference materials (CRMs) of real surface water including GSB 07–3172–2014 (203717; 7.18±0.61 μg/L), GSB Z50031–94 (19.7±2.2 μg/L),BW 0621 (75T3145; 1.28±0.064 μg/mL), which were purchased from the Institute for Environmental Reference Materials of Ministry of Environmental Protection (IERM; Beijing, China) and Beijing Coast Hongmeng Standard Material Technology Co., Ltd.(Beijing, China), respectively.Tap water samples were collected from Lanzhou in Gansu province (Lanzhou, China) and were filtered with 0.45 μm polyether sulfone (PES) membrane filter before using.Both CRMs and Tap water samples were used to verify the proposed method.
For transformation of75Se(0) to75Se(IV), a controlled nonradioactive experiment was performed and the concentrated HNO3was used to dissolve and oxidize the elemental selenium.A suitable amount of stable elemental selenium (about 10 mg, which is equal to the mass of75Se(0) obtained) was dissolved directly with 0.2 mL concentrated HNO3at room temperature.The preparation of75Se(VI) was carried out through oxidation of75Se(IV) obtained with H2O2followed a controlled experiment.Series of 10 mL sample solution contained stable Se(VI) were put into 50 mL glass beakers, then the heating temperature, the amount of 30% H2O2,the reaction time and solution acidity were evaluated.The final sample solution was diluted and measured using 861 advanced compact ion chromatography (Metrohm, Switzerland).
For each experiment, three aliquots of 10 mL of sample solution(in HNO3or HCl media) were prepared in glass beakers.After being spiked with75Se(IV)/75Se(VI) tracer, the beakers were placed on a hot plate at 100°C until near dryness (about 0.5 mL left) or dryness.Cooling to room temperature, the residue was dissolved using 10 mL of 1 mol/L HNO3and the solution was transferred to a glass tube for yield measurement.The variation of oxide state of Se(IV) and Se(VI) in different concentration of HCl/HNO3was checked using an ion chromatographic system (Metrohm 861) after solution preparation.
The distribution coefficients (Kd, mL/g) of Se(IV) and Se(VI) on Dowex1×8 resin in different pH of sample solution were investigated by batch techniques.10 mL of 0.001 mol/L NaNO3(pH ranged from 0 to 9) containing 1.26×10-6mol/L Se(IV) (or 4.01×10-7mol/L Se(VI), the75Se(IV) or75Se(VI) was spiked for measurement)were transferred to a 20 mL glass bottle, 0.05 g of Dowex1×8 was added to the bottle.The samples were then shaken for 10 h at room temperature, centrifuged at 10,000 rpm for 20 min to separate resin and solution, and then measurement.Three replicates were performed for each experiment.
An automated system based on sequential injection (SI) approach using anion exchange chromatography (Dowex1×8) was established for separation of selenium species from water samples.The automated separation setup consists of a Fotector-02HT-20 system (Automated Instruments, Raykol) furnished with a syringe pump (SP), a holding coil (HC), an internal 12-port multi-position selection valve (SV), a mechanically-controlled sample rack, and one collection rack with separation column (SC).The schematic illustration of the SI analyzer is shown in Fig.1.
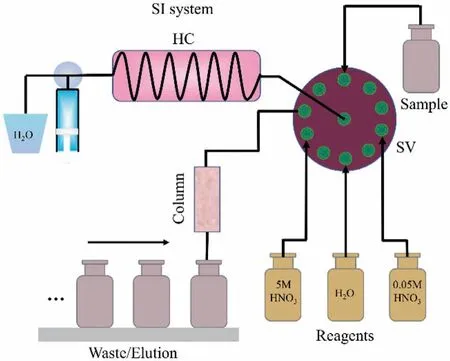
Fig.1.Automatic system for the dynamic separation of selenium species (HC: holding coil, SV: selection valve).
A small anion exchange chromatographic column (2.0 mL) filled with Dowex1×8 resin (100–150 μm) were applied for Se(IV) and Se(VI) concentration and separation.The procedure consists of the following steps: (i) rinsing of the holding coil (HC) as well as the sample inlet and outlet tubing with deionized water (pH 8~9) at a flow rate of 5 mL/min; (ii) loading the sample solution (pH 8~9) into the Dowex1×8 column at 1.0 mL/min; (iii) washing the column with 20 mL of 0.05 mol/L HNO3at 1.0 mL/min to recover Se(IV); (iv)eluting Se(VI) from the column with 10 mL of 5 mol/L HNO3at 1.0 mL/min; (v) washing the column with water till pH 7 to regenerate the resin for further use.The final eluates (0.05 mol/L HNO3and 5 mol/L HNO3) were evaporated to near dryness on a hot plate at the temperature of 100 °C.The residue was dissolved in 5 mL of 0.5 mol/L HNO3solution, which was ready for selenium speciation analysis.
The radioactivity of75Se(IV) and75Se(VI) was measured on a NaIγ-ray spectrometer.The counting time of each measurement was selected to ensure the relative standard deviation to be less than 1%.The concentrations of stable Se(IV) or Se(VI) in equilibrium supernatants and sample solution were measured by high resolution inductively coupled plasma mass spectrometry system(HR-ICP-MS, Element XR, Thermo Fisher Scientific) equipped withan Xs skimmer cone and a concentric nebulizer, operated under hot plasma conditions.Prior to sample measurement, the HR-ICPMS instrument was optimized using selenium standard solution(1.0 μg/L), typical response for78Se (to reduce the interference,78Se was chosen as the target nuclide during mass spectrometry measurement) ranged from 8000 to 10,000 counts per μg/L (1.0 ppb), the blank signal of78Se is lower than 10 counts.The particular measurement conditions of HR-ICP-MS were described in Table 1.During measurement, indium solution (as In(NO3)3) was added to selenium solution with a concentration of 1.0 μg/L (1.0 ppb) as internal standard for instrument calibration.A series of selenium standard solutions were prepared in 0.5 mol/L HNO3containing 1.0 μg/L In(III) to get the standard curve for calculating the concentration of Se(IV)/Se(VI) in the samples.
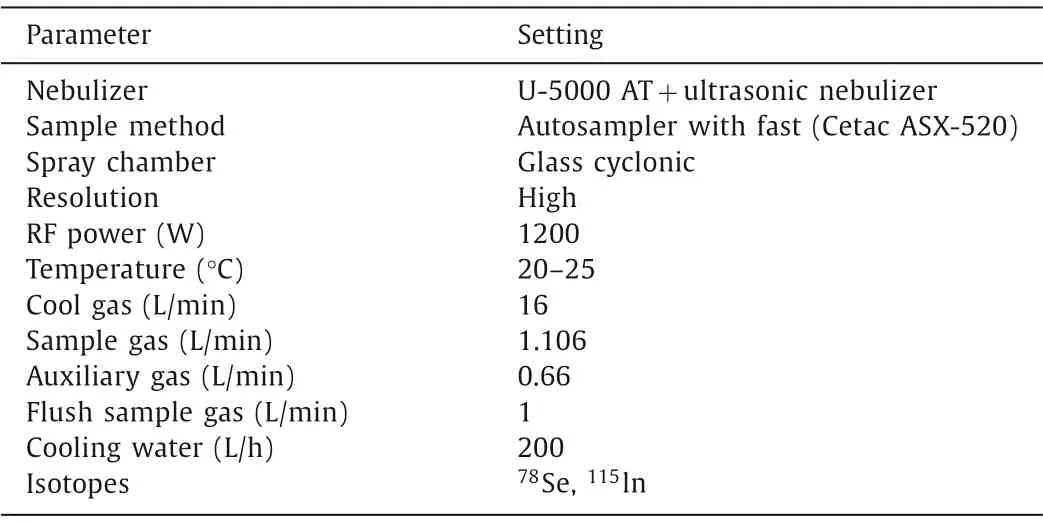
Table 1 HR-ICP-MS settings for selenium measurement.
In order to get the75Se(IV) tracer from75Se(0) (elemental selenium was usually used to produce radioactive75Se), according to our previous studies, concentrated HNO3can be used to rapidly dissolve and quantitatively oxidize selenium.The results(Fig.2A) show that the appearance time of sample solution in ion chromatography is around 12.2 min and no other chromatographic peaks observed, which is in good agreement with the appearance time of SeO32-standard solution, indicating that the elemental selenium can be quantitatively oxidized to Se(IV) by concentrated HNO3.The possible reaction between elemental selenium and HNO3can be written as follows:
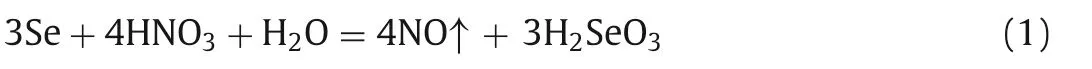
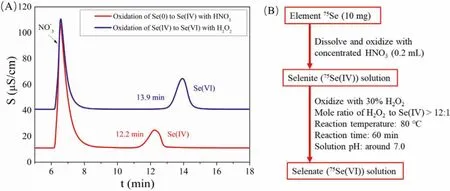
Fig.2.Preparation of 75Se(IV) and 75Se(VI) tracers by oxidization of elemental 75Se.(A) Chromatographic peaks of controlled sample solution; (B) Procedure developed in this work.
With the comparative trial, the oxidation state of75Se(0) was quantitatively adjusted to75Se(IV) and the tracer of75Se(IV) is obtained.
According to the studies reported [25], the directly oxidation of Se(0) to Se(VI) is relatively difficult.In this case, the variation of Se(IV) to Se(VI) would be more feasible.In our present work, 30%H2O2was used as the oxidizing agent to oxidize the obtained Se(IV) with the reaction as:
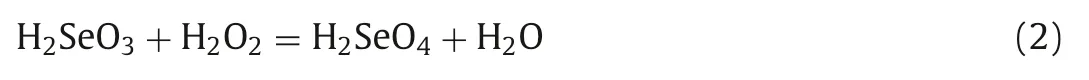
The parameters which affect the oxidation reaction were optimized (Fig.3).It was found that the reaction temperature seriously influences the variation of Se(IV) to Se(VI).The oxidation ratio increased (from 15% to 100%) with the enhancement of system temperature (in the range of 30~85 °C) and the complete oxidation of Se(IV) can be obtained when the temperature is more than 80 °C(Fig.3A).The amount of oxidizing agent would be another parameter which affects the oxidation of Se(IV).Our results (Fig.3B) show that the oxidation reaction can be quantitatively finished when the mole ratio of 30% H2O2to Se(IV) is higher than 12:1.Because the reaction is present in high temperature, the oxidation time is crucial especially for tracer preparation as the operation of radioactivity.Different reaction time were studied and the results (Fig.3C)show that the reaction can be completed when the reaction time is within 60 min, which is much shorter than the reported procedure (about 24 h) [25].The oxidation level of Se(IV) would be also influenced by the solution pH.It was found in Fig.3D that there is no obvious effect on the oxidation of Se(IV) to Se(VI) with the variation of solution pH from 3 to 9.It should be pointed out that the excess H2O2can be easily removed from the sample solution in natural and alkaline media [26].Accordingly, the solution pH of 7.0 was chosen in the oxidation procedure.
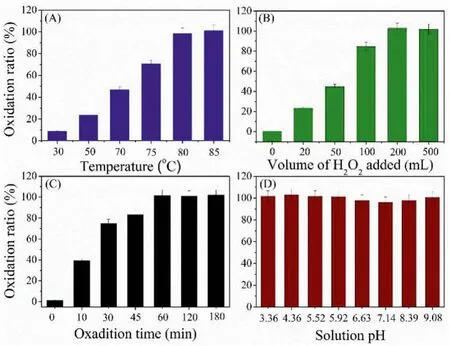
Fig.3.Parameters optimization for transformation of Se(IV) to Se(VI) using H2O2:(A) system temperature; (B) amount of H2O2 added; (C) reaction time; and (D) solution pH.
With the optimized parameters, procedures for the preparation of75Se(IV) and75Se(VI) through elemental75Se were developed and the flowchart has been shown in Fig.2B.It is a rapid and reliable method which can be used in transformation of different oxidation state of selenium with radioactivity.
Evaporation is often used to reduce the sample volume and remove acids in sample solution in analytical procedure of target elements [27].In this work, the stability of selenium species in different evaporation conditions was investigated.The results (Fig.4) show that the composition of solution is key parameters determining the stability of Se during evaporation.Significant loss of Se(IV) and Se(VI) was observed when evaporation was carried out at high temperature in HCl media, especially at high concentration of HCl (e.g., 5 mol/L) (Fig.4B) when the sample was evaporated to dryness.This can be explained that the formation of volatile species of SeOCl2(melting point of 176~180 °C) in high concentration of HCl and at high temperature [28].The Se(VI) can be reduced easily to Se(IV) by high concentration of HCl (e.g.,1.0 mol/L) in high temperature, and thus shows a similar stability of Se(IV) (Fig.4E).It should be pointed out that if the sample solution was not evaporated to dryness, for example 0.5 mL solution left, the loss of both Se(IV) and Se(VI) can be avoided even at high concentration of HCl (Fig.4A).In other words, the loss of selenium species mainly happened when the full dryness of sample solution because the temperature will increase evidently in this process.Compared with HCl media, the stabilities of selenium species in HNO3media are well even at high concentration of HNO3(e.g., 8 mol/L) when sample solution was evaporated to dryness (Figs.4C and D).By evaluation of sample solution using ion chromatography, it can be found that the oxidization state of both Se(IV) and Se(VI) are stable and cannot change with the variation of HNO3concentration (Figs.4G and H), indicating that the H2SeO3and H2SeO4are stable and there is no other volatile species of selenium formed in HNO3media.
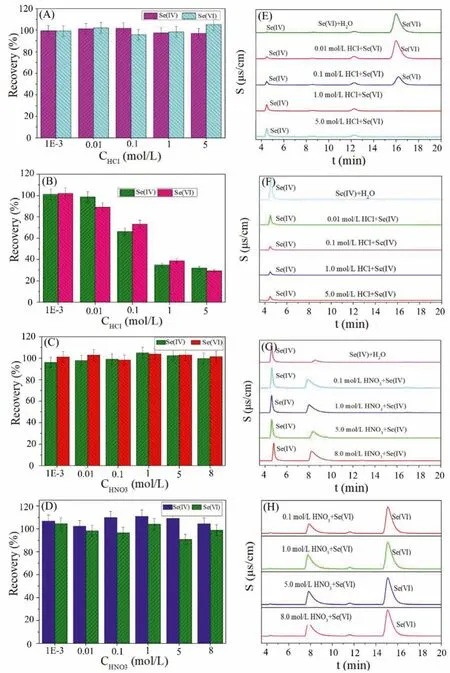
Fig.4.The thermal and oxidation stabilities of Se(IV) and Se(VI) in different concentration of HCl and HNO3 media.(A, C) Evaporated to 0.5 mL solution left; (B, D) Evaporated to dryness; (E) Se(VI) in HCl media; (F) Se(IV) in HCl media; (G) Se(IV) in HNO3 media; (H) Se(VI) in HNO3 media.
The effects of solution pH on the sorption of Se(IV) and Se(VI)at room temperature were studied and the results have been shown in Figs.5A and B.It is clearly that the uptake ratio of Se(IV)and Se(VI) increased with the increase of solution pH.For Se(IV),the sorption edges can be divided into three parts: (i) low uptake with pH<3; (ii) relatively high uptake with pH range of 3~8 and(iii) high uptake with pH>8 (Fig.5A).The difference of Se(IV)sorption would be mainly caused by the variation of Se(IV) species in the solution.As can be seen in Fig.5C, there are three species of Se(IV) existed in aqueous, which are H2SeO3, HSeO3-and SeO32-.At low pH (<3), H2SeO3is the dominant species, which shows poor exchange ability because H2SeO3is a neutral molecule, leading to the lower uptake ratio of Se(IV) onto the resins.At pH higher than 3, Se(IV) is present as either a monovalent (as HSeO3-in the pH range of 3~8) or divalent (as SeO32-in the pH higher than 8) oxyanion, accordingly, the higher uptake ratio of Se(IV) onto Dowex1×8 resin can be observed.Compared with HSeO3-, the sorption of SeO32-is stronger by evaluation the uptake ratio of Se(IV) at high pH.Because the main species of Se(VI) are HSeO4-(pH<2) and SeO42-(pH>2) in the solution (Fig.5D), the high uptake ratio of Se(VI) onto Dowex1×8 resin is mainly caused by the high sorption ability of SeO42-in our experimental conditions.Accordingly, the order of uptake abilities of different species of selenium onto Dowex1×8 resin is: HSeO3-<SeO32-<SeO42-.
Fig.5.Effect of solution pH on (A) Se(IV) and (B) Se(VI) sorption onto Dowex1×8 resin; The speciation distribution of (C) Se(IV) and (D) Se(VI) in aqueous.
Based on the experimental data above, it can be found that the acidity of system is an important parameter during the sorption process, which affects the uptake of selenium species.The separation of Se(IV) and Se(VI) in aqueous can be carried out by the variation of concentration of H+(Fig.6), which is Se(IV) and Se(VI)can be taken up effectively by Dowex1×8 resin at high pH and desorbed with relatively low and high concentration of H+, respectively.
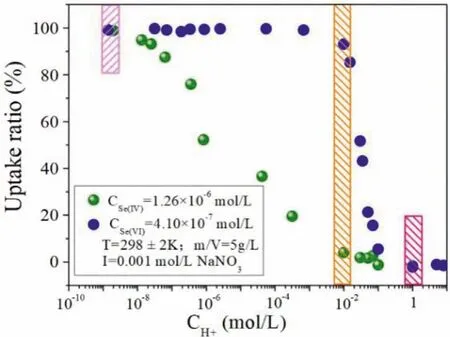
Fig.6.The effect of hydrogen ion concentrations on the uptake of Se(IV) and Se(VI) onto Dowex1×8 resin.
In the present work, a small anion exchange chromatographic column packed with 2 mL Dowex1×8 resins were applied for Se(IV) and Se(VI) concentration and separation.An automatic system was used in all separation process.As shown in Fig.7A, the leakage was not observed until the volume of effluent reached 650 mL and 1150 mL for Se(IV) and Se(VI), respectively.It is clearly found that the column was fully saturated after 1850 mL for Se(IV), while, only 40% saturation of column was observed at same pass volume of Se(VI), indicating excellent concentration ability con-sidering of the small column volume (2 mL) and high concentration of selenium.Furtherly, the separation of selenium was also conducted, the results (Fig.7B) showed that both Se(IV) and Se(VI) can be effectively loaded onto the column in alkaline solution (pH~9).As mentioned in Fig.6, the uptake ratio of Se(VI) on Dowex1×8 resin is very low (close to 0) at high concentration of H+(in HNO3media), indicating that Se(VI) retained on the column can be eluted by high concentration of HNO3.In this case, Se(VI) will disperse in high concentration of HNO3solution.Se(VI) on the column can be quantitatively eluted with small volume of 5 mol/L HNO3.
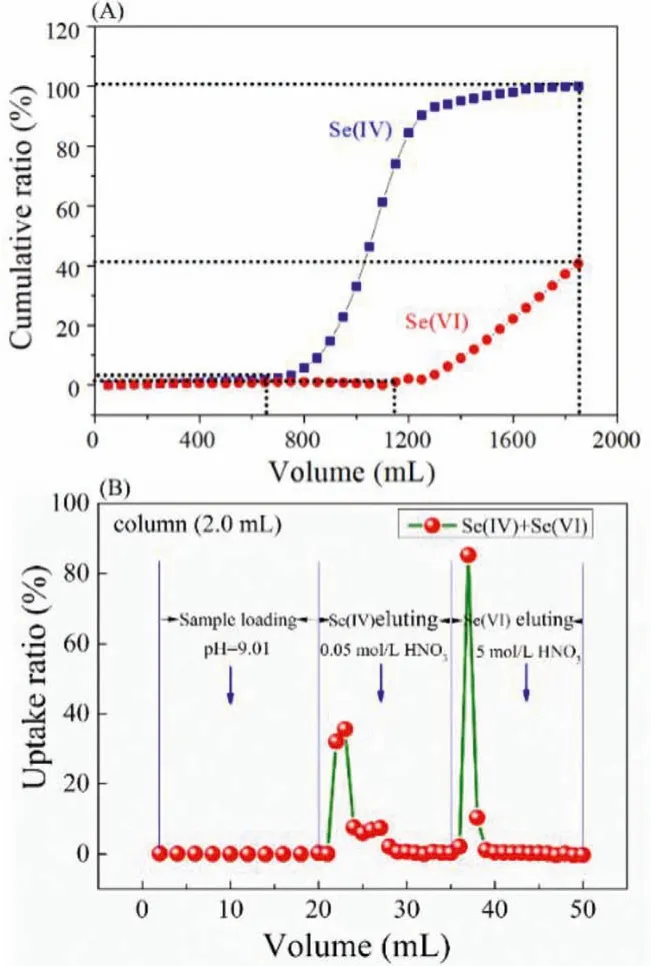
Fig.7.(A) The breakthrough curves of Se(IV) and Se(VI) in Tap water with conditions: CSe(IV/VI)=1×10-4 mol/L, flow rate: 2 mL/min, column volume: 2 mL.(B)The separation of Se(IV) and Se(VI) from column packed with Dowex1×8 resin.CSe(IV/VI)=2.5×10-4 mol/L, flow rate: 2 mL/min; column volume: 2 mL.
Recovery experiments were carried out to investigate the reliability of the proposed methodology.Tap water samples and the certified reference materials (CRMs) of water samples were analyzed (Table 2).Adequate agreement was observed with certified values of CRMs (GSB 07–3172–2014, GSB Z50031–94 and BW 0621 75T3145).The spiked recoveries at 10.00, 20.00 or 50.00 μg/L in the range of 98%-104% with 5% RSDs for all tap water samples and CRMs, indicating that the developed procedure is efficient and can be used for the determination of selenium species in environmental water samples.
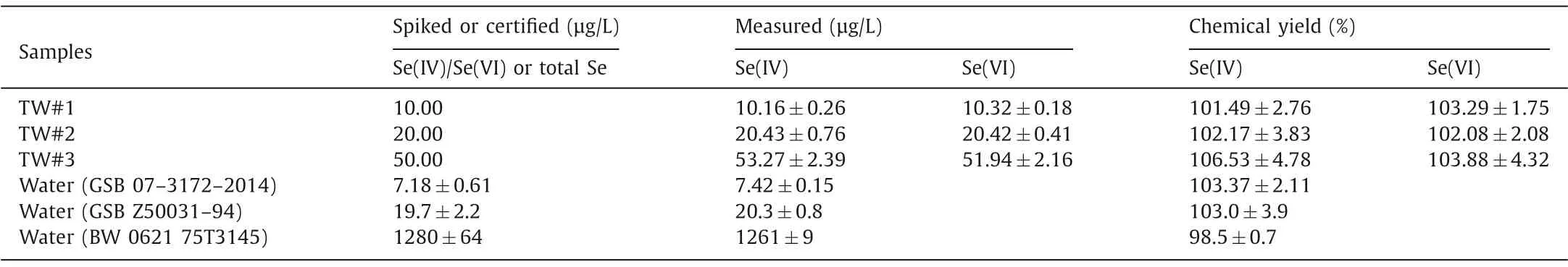
Table 2 Spiked recoveries and measured selenium presence in real water samples (n=3)*.
Based on the results achieved in this paper, the following conclusions can be drawn out.(i) The tracers of75Se(IV) and75Se(VI)were successfully prepared through the oxidation of75Se(0) by concentrated HNO3and the further oxidation of75Se(IV) with H2O2at system temperature of 80 °C, respectively.(ii) The stability of Se(IV) and Se(VI) was proved to be well in HNO3media, but a notable loss of selenium species happened in high concentration of HCl when the full dryness of sample solution.(iii)The pH of solution showed sensitive effect on the uptake of Se(IV)and Se(VI) onto Dowex1×8, accordingly, a pre-concentration and separation approach of inorganic selenium species was proposed.(iv) The advantage of this method, compared to earlier, is that the determination of both Se(IV) and Se(VI) can be carried out separately from the same sample.(v) The reduction of Se(VI) to Se(IV)in HCl media (<1 mol/L) at room temperature is very limited, and Se(VI) can be effectively eluted from the column by relatively high concentration of HNO3.(vi) The developed procedure is proved reliable and can be used for the determination of selenium species in environmental water samples.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The financial support from National Natural Science Foundation of China (Nos.22061132004, U21A20442, 22106059, 22106057,21771093), Fundamental Research Funds for the Central Universities of China (No.lzujbky-2021-kb11, lzujbky-2021-sp41) and Gansu guiding program of Science and Technology Innovation (No.20JR10RA610) are gratefully appreciated.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
