Studies on the separation treatment of high-level liquid waste by bisamide podand(I): Extraction and separation of An(III) from Ln(III)
Li-Kun Liu, Shu-Bao Xie, Hong-Bin Lv, Hu Zhang, Guo-An Ye
China Institute of Atomic Energy, Beijing 102413, China
ABSTRACT A process for actinide(III) and lanthanum(III) extraction separation from high-level liquid waste (HLLW)was proposed, with N,N,N’,N’-tetraoctyl diglycolamide (TODGA) as the extractant, tri-n–butyl phosphate(TBP) as the phase modifier and 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol or PTD)as hydrophilic stripping agent.This ‘hot test’was successfully carried out, achieving 99.92% removal of americium-241 (241Am) with a separation factor SF(Eu/Am) of 3.8×103 in the actinide(III) product solution.The results show that bisamide podand extractants can effectively realize the extraction and separation of actinide(III) and lanthanum(III) from Chinese commercial HLLW and thus have a bright practical application potential for the treatment of commercial HLLW.
Keywords:Bisamide podand High-level liquid waste Actinides Lanthanides Extraction separation
Several processes for co-extraction of actinide(III) and lanthanum(III), hereafter denoted An(III) and Ln(III), have been developed in China and other countries: the trialkyl phosphine oxide (TRPO) process in China [1–5], the diisodecylphosphoric acid (DIDPA) process in Japan [4–7], the transuranium extraction(TRUEX) process in the USA and the diamide extraction (DIAMEX)process in France [6–10].
In the innovative-selective actinide extraction process (iSANEX),An(III) and Ln(III) are co-extracted usingN,N,N’,N’-tetraoctyl diglycolamide and tri-n–butyl phosphate (TODGA+TBP) as the extraction system, wherein An(III) is selectively stripped using watersoluble stripping agent [2,6-bis(5,6-di(sulfophenyl)-1,2,4-triazin-3-yl]pyridine (sulfophenyl-BTP), thereby realizing the separation of An(III) and Ln(III) [6].Regardless of its good selectivity and stability, the hydrophilic stripping agent contains many benzene rings in its molecular structure, which prevents its solubility in water.Furthermore, the addition of S in benzenesulfonic acid does not conform to the ‘CHON’principle and thus increases secondary waste in the process [7–11].
The ‘hot test’ process of extracting and separating An(III)and Ln(III) from high-level liquid waste (HLLW) was introduced,wherein extraction with TODGA (Fig.1), an extractant based on diglycolamide and TBP proposed by the China Institute of Atomic Energy, was combined with 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol or PTD; Fig.1), an aqueous stripping agent based on diazine monopyridine, to effectively separate An(III) and Ln(III) [15–17].In the past 20 years, intensive study has been carried out on extractants based on amido podand with respect to the thermodynamics of extraction [11–13], extraction and stripping [9–15], selection of An(III) and Ln(III) separation reagents [16–22], stability of separation reagents under irradiation [18] and separation and extraction process of An(III) and Ln(III) [11–14].Studies have shown the potential application of PTD in the separation of An(III)and Ln(III) [19–23].
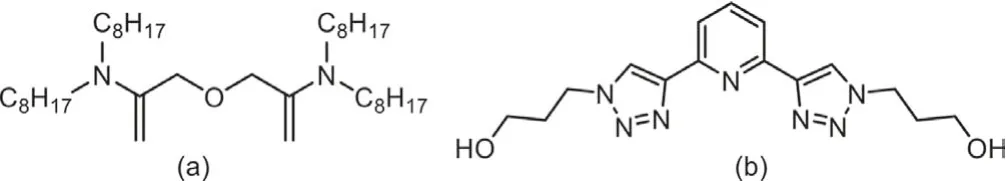
Fig.1.Structural formulae for TODGA (a) and PTD (b).
In addition, an experimental study was carried out for simulated extraction of HLLW with tracer scales of americium-241(241Am) and europium-152 (152Eu) at 0.2 mol/L TODGA, 0.5 mol/L TBP and 0.5 mol/L PTD, which realized the co-extraction of 99.9%An(III) and Ln(III), at an extraction rate of 99.9% for An(III) and with the mass concentration of Ln(III) and other fission products no higher than 0.1% [23].
Based on the above research results, a process for the extraction and separation of An(III) and Ln(III) from genuine HLLW was proposed (Fig.2).
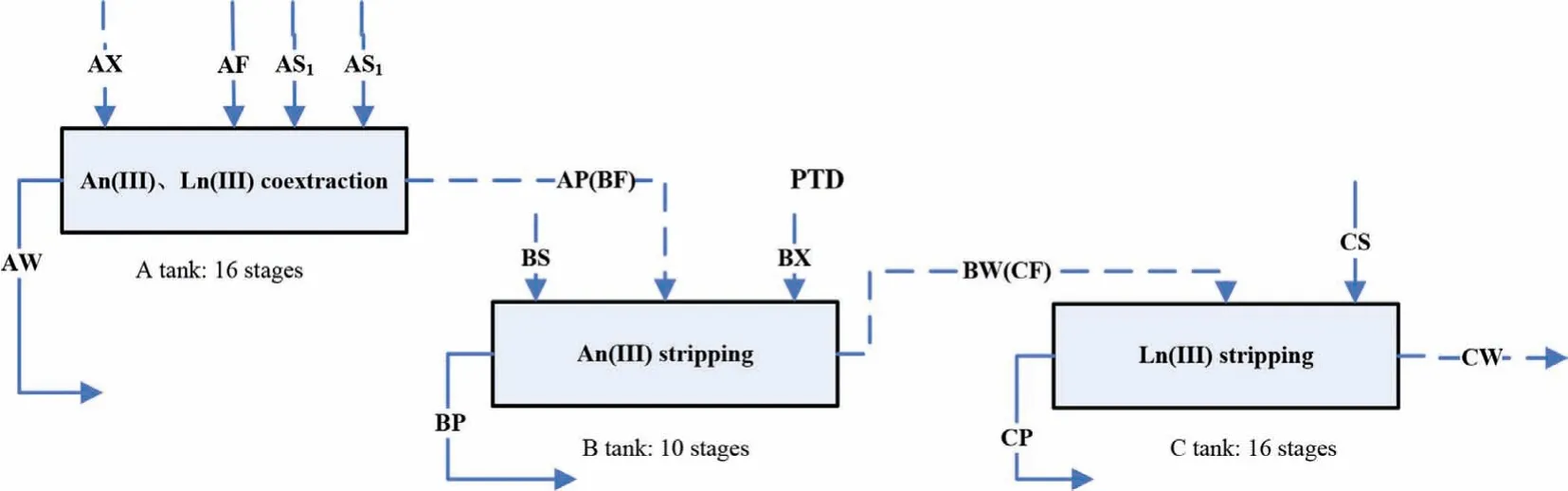
Fig.2.Flow diagram of process for An(III) and Ln(III) extraction and separation from genuine HLLW.
In this process, AXE is 0.2 mol/L TODGA+0.5 mol/L TBP.During the extraction process, scrubbing was carried out twice.The scrub solution AS1was 3.0 mol/L HNO3+0.5 mol/L H2C2O4+0.08 mol/L hydroxyethylethylenediaminetriacetic acid (HEDTA), which was utilized to strip the fission products extracted from the organic phase into the aqueous phase so that the fission products do not enter the liquid product of An(III) and Ln(III); the scrub solution AS2was 0.5 mol/L HNO3for the purpose of stripping strontium in the loaded organic phase to prevent it from entering the liquid product of An(III) and Ln(III).BX was 0.5 mol/L PTD, as the stripping agent for the separation of An(III).In addition to stripping An(III), PTD also has a certain stripping effect on Ln(III).Ln(III) stripped into the aqueous phase needs to be re-stripped into the organic phase,so BS was 0.2 mol/L TODGA+0.5 mol/L TBP, as the stripping agent for Ln(III) in BS.Finally, CS used 0.001 mol/L HNO3for stripping Ln(III).Table 1 shows the detailed process parameters.
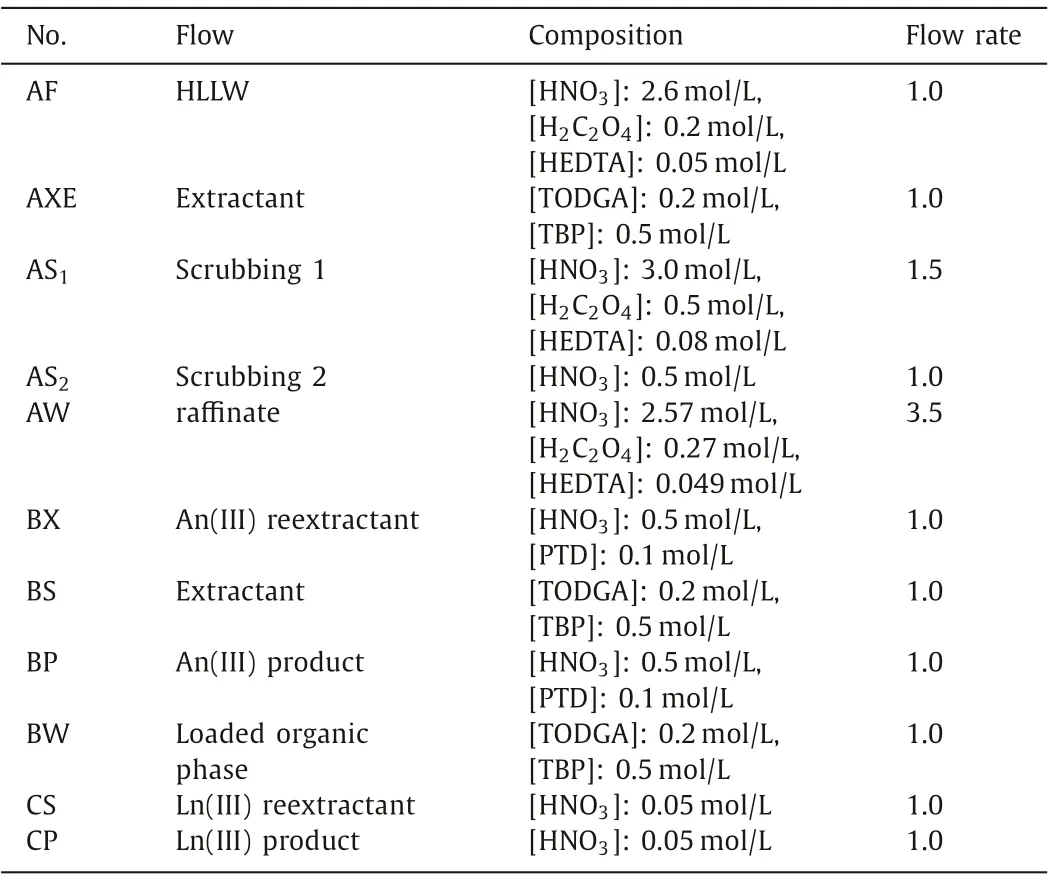
Table 1 Process parameters for An(III) and Ln(III) extraction separation from HLLW.
As part of the extraction and separation equipment, miniature box-type mixer-settlers were used, which were made of polyether ketone.There were two 10-mL 16-stage mixer-settlers and one 20-mL 10-stage mixer-settler.The two 10-mL 16-stage mixer-settlers were used for co-extraction of An(III) and Ln(III) and for stripping of Ln(III), respectively, and the 20-mL 10-stage mixer-settler was used for stripping of An(III).Fig.3 shows the dimension and structure of a 10-mL mixer-settler.The stirring paddles at all stages were engaged through gears, and the gear set was driven to rotate by two DC brushless motors that are backups of each other.
The extraction bench (Fig.4) for the separation process is the core of the equipment system, and the supporting equipment includes a feed liquid tank, vacuum buffer tank, injection pump,pipeline, control system,etc.The extraction bench is removable,each layer of which can be replaced separately.
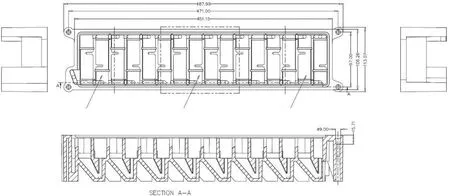
Fig.3.A 10 mL 16-stage mixer-settler extractor.
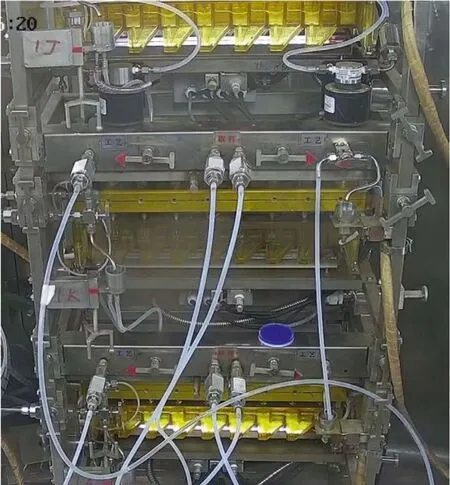
Fig.4.Extraction bench for the HLLW partitioning hot test.
The non-radioactive feed liquid (e.g., HNO3, TODGA/TBP, PTD)used in the hot test was prepared in advance and then injectedviathe reserve pipeline in the hot chamber using the injection pump placed between the measuring tanks.The radioactive feed liquid was directly injected from the HLLW storage tank in the hot chamber using the injection pump shielded by lead in the hot chamber.
The TODGA used in the test was produced by Qingdao Beite Woer Technology Co., Ltd.with a purity of more than 95%.Other reagents were analytically pure and purchased from Sinopharm Chemical Reagent Co., Ltd.Samples were analysed using an ICAPQ inductively coupled plasma mass spectrometer purchased from ThermoFisher Technology (China) Co., Ltd., an HPGeγspectrometer (Model BE3830) from Canberra Manufacturing Co., Ltd.and a Tricarb5110TR low-background liquid scintillation counter from Perkin Elmer Enterprise Management (Shanghai) Co., Ltd.
To prepare HLLW for the hot test, a spent fuel solution of pressurized water reactor with a fuel consumption of 33,000 MWd/tU stored for three years was post-treated using the PUREX process to obtain raffinate 1AW (with a specific activity of 2.83×1012Bq/L)and then most of the uranium, neptunium and plutonium in 1AW was removed by extraction with TBP and most of the caesium-137(137Cs) was removed by extraction with calixcrown.The raffinate obtained was the HLLW used for the hot test.Table 2 shows the analysis results of the key components.
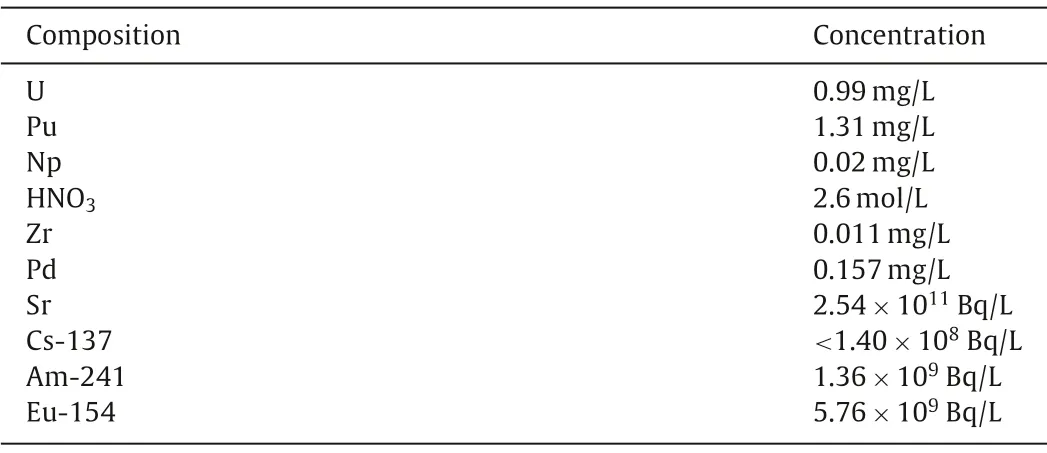
Table 2 Composition of the HLLW.
The hot test comprises tank filling, acid balance, feed liquid running and tank washing.Upon tank filling, the organic phase was 0.2 mol/L TODGA+0.5 mol/L TBP and the aqueous phase was 0.5 mol/L HNO3.After the two phases in each mixer-settler were filled, the acid system started to run for 6 h at a set flow rate.The acid test was to observe and adjust the operating state of each device in the bench system and prepare for the feeding of genuine HLLW.
The genuine HLLW was injected for the hot test.After the hot test was conducted for 9 h, grab samples were taken every 6 h.The hot test was conducted for 94 h in total and grab samples were taken 10 times.
After the thermal experiment was stopped, samples at all stages in each extraction tank were taken.Finally, the mixer-settler was cleaned with 0.5 mol/L HNO3.
Fig.5 shows the variation of HNO3concentration in the grab samples during the hot test for the separation of An(III) and Ln(III)from HLLW by the amido podand process.The results showed that the concentration of HNO3in the grab samples changed slightly after 12 h in the bench tests of the An(III) extraction and separation process, indicating that the extraction-stripping process had become stable.Table 3 shows the average HNO3concentrations at the aqueous outlets.
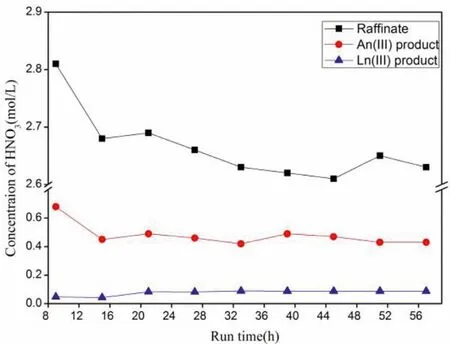
Fig.5.Evolution of HNO3 concentration of the instantaneous outlet samples.
The total feed amount was 9 L for the An(III) and Ln(III) extraction and separation process, which ran for 94 h in total.The grab sample was diluted and the activities of241Am and152Eu were measured using the HPGeγspectrometer.
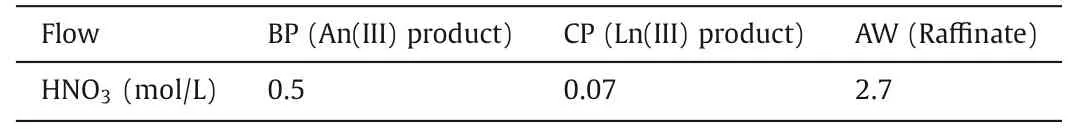
Table 3 Average HNO3 concentrations at the aqueous outlets.
After the hot test was conducted for 9 h, the specific activities of241Am and152Eu in the grab sample changed slightly, indicating that the extraction process had become stable (Figs.6 and 7).The average values were calculated from the specific activities of241Am and152Eu in the grab sample to obtain the specific activities of241Am and152Eu in the liquid product and raffinate (Table 4).The calculated Eu separation factor SF(Eu/Am)for the Am product was greater than 3800 and the Am separation factor SF(Am/Eu)for the Eu product was greater than 980 (SF(Eu/Am)=[C(Eu)org/C(Eu)aq]/[C(Am)org/C(Am)aq]).
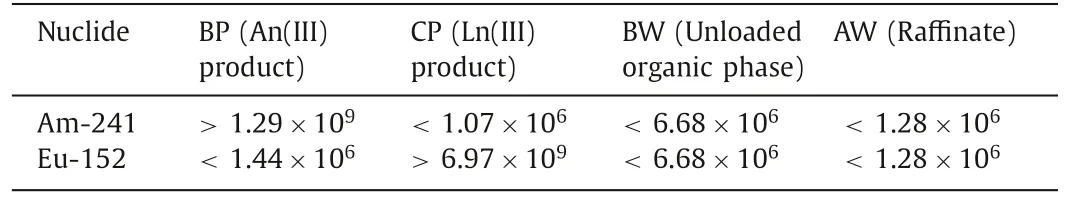
Table 4 Specific activities of 241Am and 152Eu at the outlet samples.
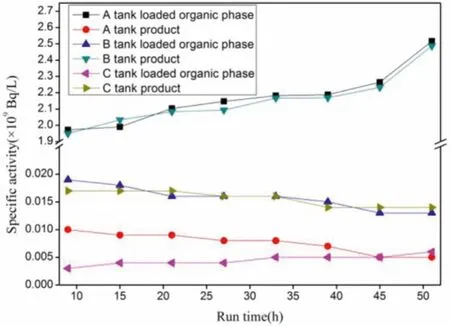
Fig.6.Evolution of 241Am specific activity at the instantaneous outlet samples.
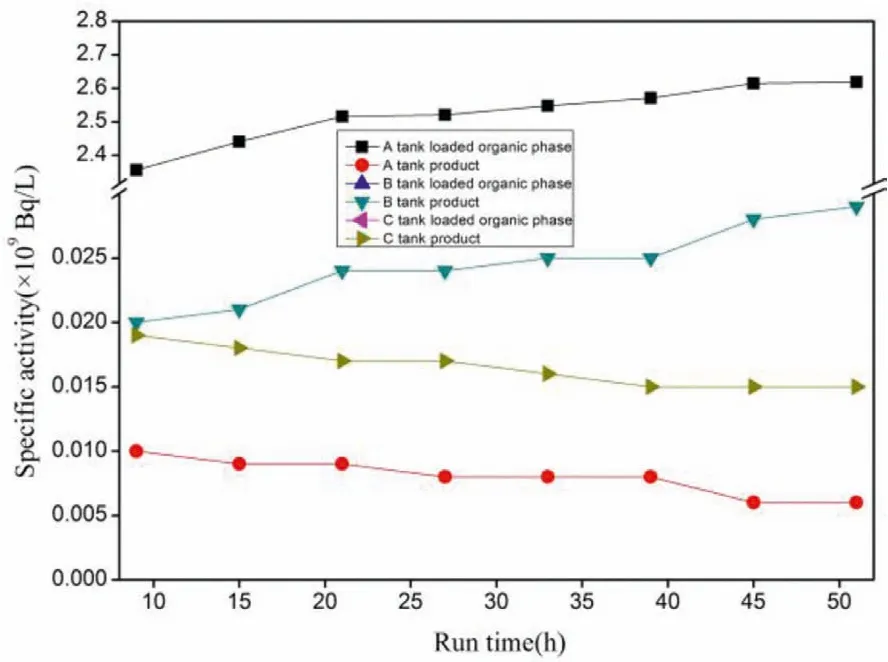
Fig.7.Evolution of 152Eu specific activity at the instantaneous outlet samples.
Table 5 shows the extraction rate, stripping rate and material balance results for241Am and152Eu.The specific activities of241Am and152Eu in An(III) liquid product (BP) were greater than 1.29×109Bq/L and less than 1.43×106Bq/L, respectively, and the recovery rate of An(III) from HLLW was greater than 99.9%.

Table 5 Extraction percentage, stripping percentage and material balance for 241Am and 152Eu.
The specific activities of241Am and152Eu in Ln(III) liquid product (CP) were less than 1.67×106Bq/L and greater than 6.97×109Bq/L, respectively, and the recovery rate from HLLW was greater than 99.9%.
The activity concentration of241Am and152Eu was less than 1.28×106Bq/L in the raffinate and less than 6.67×106Bq/L in the waste organic phase, indicating that their content in the waste organic phase and the raffinate was extremely low.
After the hot test, samples of aqueous and organic phases were taken at all stages in the extraction tank and the contents of241Am and152Eu in the samples were measured using the HPGeγspectrometer.
Figs.8 and 9 show the distribution of241Am and152Eu at all stages.As can be seen from Fig.7,241Am and152Eu in HLLW were extracted into the organic phase by TODGA and TBP in the extraction section.The content of241Am and152Eu in the organic phase was low at the first 3 stages, increasing rapidly after stage 4 and basically did not change with the stage after that, indicating that TODGA has a strong extraction ability for241Am and152Eu.At the feed stage (stage 4), most of the241Am and152Eu could be extracted into the organic phase with fast extraction kinetics.
In the stripping section of An(III),241Am and152Eu were stripped from the loaded organic phase to the aqueous phase due to the complexation of PTD, resulting in continuous fluctuation of152Eu content at the last 5 stages.Due to the stripping effect of TODGA+TBP as a stripping agent on Ln(III), the content of152Eu in Tank B was extremely low at the first 5 stages, which can be basically ignored; the content of241Am in the aqueous phase was inversely proportional to the stage number and remained stable at the first 8 stages.From the data on organic phase samples at all stages in Tank B, it can be seen that the content of241Am decreased continuously with an increase in the number of stages,while the content of152Eu was just the opposite, and there was an intersection between the two at stage 8 and 9, which further indicated that the interaction between stripping agent and extractant can realize good separation of241Am and152Eu.
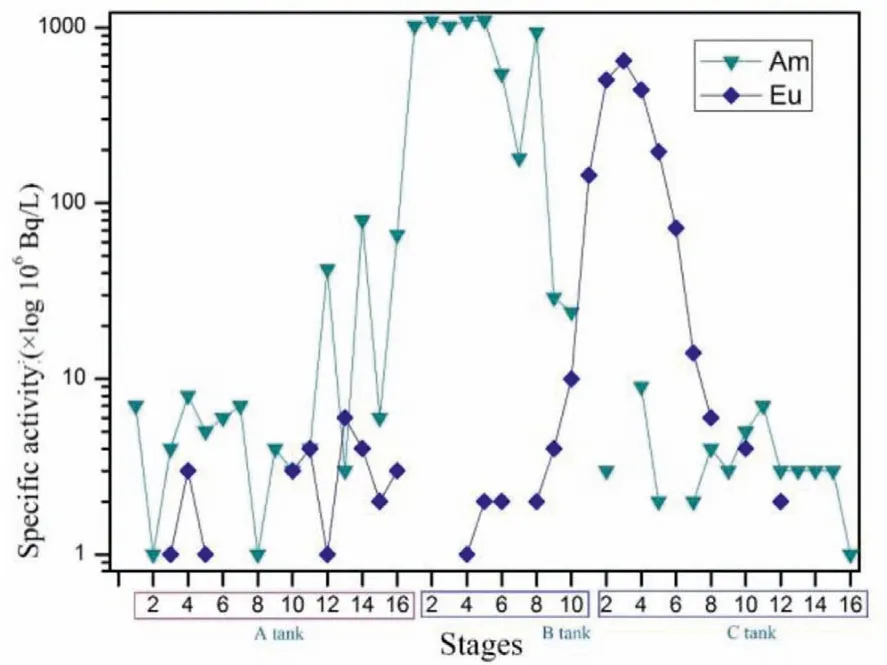
Fig.8.Distribution of 241Am and 152Eu in the aqueous phase at each stage.
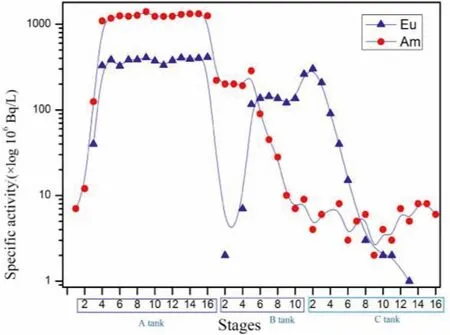
Fig.9.Distribution of 241Am and 152Eu in the organic phase at each stage.
Samples of aqueous phase in Tank C were injected at stage 16 and152Eu was thus stripped from the organic phase into the aqueous phase under low acid conditions.The content of Ln(III) in Tank C at the last 8 and 9 stage was therefore relatively low and the content of152Eu in the aqueous phase at the first 8 stages initially increased and then decreased, indicating that152Eu was stripped into the aqueous phase, whereas241Am was completely extracted into the aqueous phase in Tank B and did not enter into Tank C.
The separation of An(III) from HLLW and then from Ln(III) is of great importance for the volume reduction and degradation of high-level waste, as well as for comprehensive utilization of resources.Over the past two decades, the China Institute of Atomic Energy has conducted systematic research on the mechanism and performance of extractants based on amido podand for the extraction and separation of An(III) and Ln(III) from HLLW.On this basis,a process for extraction and separation of An(III) and Ln(III) from HLLW was proposed in which TODGA+TBP was used as the extractant and PTD was used as the aqueous stripping agent.In addition, the hot test using genuine HLLW was conducted on a mini mixer-settler bench.The hot test was run continuously and stably for 94 h and 9 L of genuine HLLW were treated.The removal rate of An(III) reached 99.92% and the SF(Eu/Am)in the liquid product of An(III) was greater than 3800.
The extraction and stripping system was used to simultaneously extract An(III) from HLLW and separate it from Ln(III).The process is suitable for a wide range of acidity so there is no need to adjust the acidity of the feed liquid.The hot test also verified the reliability of the amido podand process in the treatment of genuine HLLW and the research results provided further options in the development of separation and treatment technology for HLLW in China.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I am deeply indebted to Mr.Yong-Gang Zhao for his direct and indirect help to me.Special thanks should go to my colleagues who have put considerable time and effort into their comments on this paper.Finally, I am indebted to CIAE for the continuous support and the experiment condition.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
