An effective process for the separation of U(VI), Th(IV) from rare earth elements by using ionic liquid Cyphos IL 104
Jiawei Lu, Kebao He, Yi Wang, Geng Chen, Hanqin Weng, Mingzhang Lin
School of Nuclear Science and Technology, University of Science and Technology of China, Hefei 230027, China
ABSTRACT Separation and recovery of U(VI) and Th(IV) from rare earth minerals is a very challenging work in rare earth industrial production.In the present study, a homemade membrane emulsification circulation (MEC) extractor was used to separate U(VI) and Th(IV) from rare earth elements by using Cyphos IL 104 as an extractant.Batch experiments were carried out using a constant temperature oscillator to investigate the extraction parameters of the single element and the results indicated that Cyphos IL 104 could reach the extraction equilibrium within 30 min for all the three elements, i.e., U(VI), Th(IV), and Eu(III).Besides, the MEC extractor possessed a strong phase separation ability.The extraction efficiencies of U(VI), Th(IV), La(III), Eu(III) and Yb (III) increased with the increase of pH.La(III), Eu(III) and Yb(III)were hardly extracted when pH ≤1.50, which was beneficial for effectively separating U(VI) and Th(IV)from La(III), Eu(III) and Yb(III).In the multi-stages stripping experiments, when the stripping stage number was 3, the effective separation could be achieved by using HCl and H2SO4, since the stripping efficiency reached 80.0% and 100.0% for Th(IV) and U(VI), respectively.Slope method and FT-IR spectra showed that Cyphos IL 104 reacted with U(VI) and Th(IV) by chelation mechanism.The extraction of multi-elements indicated that U(VI) and Th(IV) could be well separated from the solution which contains all rare earth elements, and the extraction efficiencies of U(VI) and Th(IV) both were close to 100.0%.Based on the above experimental results, a flowchart for efficient separation of U(VI) and Th(IV) from rare earth elements was proposed.
Keywords:Rare earth elements Actinides Solvent extraction Membrane emulsification Ionic liquid
The lanthanides (except for the synthetic element promethium),along with scandium (Sc) and yttrium (Y) are usually referred to as "rare earth elements" (REEs), which gain widespread applications owing to their excellent and unique properties [1].In the past several decades, the demand for REEs has dramatically augmented with their increasing consumption for advanced materials,e.g.,fluorescent powders, optical fibers, catalysts, precision ceramics, battery materials and hydrogen storage materials [2,3].In general, low-level radioactive uranium (U) and thorium (Th) coexist with the rare earth minerals in the bastnaesite, monazite, and xenotime by lattice substitution due to the similar chemical structure [4–7].Based on previous researches, most of the monazite contains a large amount of Th, which can be as high as 20%, and a small part of the monazite has a mass fraction of UO2as high as 16% [6].Serious radioactive pollution is inevitably caused by U and Th during rare earths production.As the vital raw ingredients of atomic energy, U and Th are extremely limited in the nature and the separated U and Th from rare earth industry can be used as the candidate nuclear fuel for commercial reactors.Accordingly,the extraction and separation of U and Th from REEs possess great significance in hydrometallurgy process, environmental protection,and sustainable development of nuclear energy [8,9].
Great efforts have been devoted to dealing with the bottleneck of separating U(VI) and Th(IV) from REEs.Due to its good separation performance, ion exchange method was successfully applied to separate U(VI) and Th(IV) from REEs [10].As this technique advancing, various drawbacks have been exposed, such as no continuous processing, time-consuming operation, and high cost of resin regeneration and exchange [11,12].As another traditional approach,solid phase adsorption also could eliminate radionuclides in aqueous solution [13].Our group realized the high efficient adsorption of U(VI) and Th(IV) by functionalized graphene oxide and hierarchically mesoporous silica, respectively [11].Nonetheless, the adsorption capacity was limited and the reuse rate was not high.In addition, a sequence of extra elution procedures was essential to recover U and Th from the adsorbents.
Solvent extraction is commonly used to separate U(VI) and Th(IV) from REEs in modern industry due to the automatically consecutive operation, high separation efficiency and little pollution[14–16].These extractants can be divided into three categories:acidic extractant (cation exchanger), solvated extractant (neutral extractant) and alkaline extractant (anion exchanger).Nasabet al.investigated the extraction of U and Th from different acidic media using tributyl phosphate (TBP), trioctylamine (TOA), and methyl trioctyl ammonium chloride (Aliquat 336) extractants [17].Currently, many acidic extractants such as D2EHPA, PC88A, Cyanex 301, have been widely hired in the field of solvent extraction owing to their good extraction performance.However, the acidic extractants can release H+into aqueous phase in the extraction process,which greatly degrades the extraction efficiency.To overcome this problem, acidic extractants need to be saponificated with sodium hydroxide or ammonia.Unfortunately, the saponified extractant might lose its extraction activity after the contact with water, and even the extraction process will generate emulsification and third phase at the higher degree of saponification.
Consequently, the choice of appropriate extractant and extractor is of great significance to the extraction.Among multitudinous extractants, room-temperature ionic liquids with low volatility, stable physical properties, and easy design, have arrested plenty of attention.As the shining-star acid-base couple ionic liquid extractant, Cyphos IL 104 is composed of cation of Cyphos IL 101 and anion of Cyanex 272.It is obtained by the reaction of Cyanex 272 with a quaternary phosphonium weak base extractant.And this synthetic procedure makes it avoid the complex process of presaponification.Meanwhile, the synergistic coordination ability of quaternary phosphonium cation can greatly improve the stability of metal complexes.Therefore, Cyphos IL 104 can combine advantages of Cyphos IL 101 and Cyanex 272, which is desired to show the outstanding extraction performance.
The extraction of transition metals by Cyphos IL has been well studied in previous work [18–20].However, there is no report on the separation of U(VI) and Th(IV) from all REEs, not just some individual representative elements, and establishing a complete separation process.Therefore, Cyphos IL 104 was selected to study in HNO3system and it was also applied in the separation of U(VI)and Th(IV) from REEs for the first time.In addition, our group developed a membrane emulsification circulation (MEC) extractor, which achieved excellent extraction performance for precious metal ions (Pd, Au and Pt) using Cyphos IL 101 as an extractant,such as high extraction efficiency, short equilibrium time and less extractant dosage [21].Thus, it is desirable to utilize Cyphos IL 104 as an extractant for extracting U(VI) and Th(IV) from REEs by the homemade MEC extractor.
In this work, the highly efficient extraction of U(VI) and Th(IV)from aqueous solution including REEs was realized by the MEC extractor and using Cyphos IL 104 as an extractant.The effects of different experimental parameters were investigated to optimize the extraction.The selective separation of U(VI) and Th(IV) from REEs was also studied.Not only does this work demonstrate that MEC extractor can be applied to REEs extraction industry in the future,but also the results may provide basic data for practical application.
Chemical sources and purities were described in Section S1(Supporting information).The molecular structure of Cyphos IL 104 was shown in Fig.1.The homemade MEC extractor used in this work was described in our previous paper [21].The schematic diagram of MEC extractor was displayed in Scheme S2 (Supporting information).
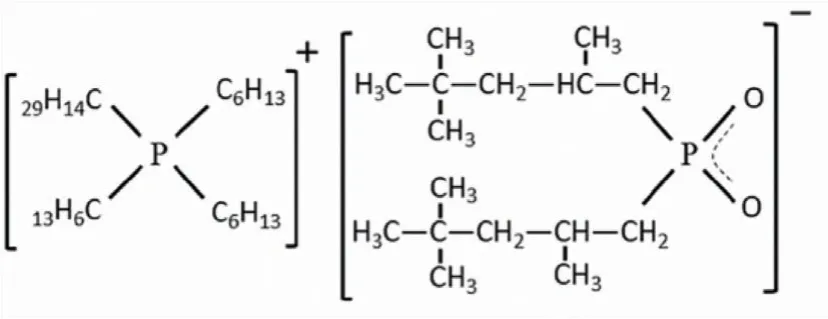
Fig.1.Molecular structure of Cyphos IL 104.
Firstly, the basic conditions of extraction (equilibrium time, pH of extraction system and concentration of extractant) were determined by batch experiments.And the middle lanthanide element Eu was selected as a representative element of lanthanides.Previous studies showed that the molecular weight of lanthanides had an important influence on their extraction process [22].Therefore,in subsequent work, La, Eu and Yb were selected as the representative light, middle and heavy lanthanide to ensure the universality of the experimental results.The detailed experimental procedures and analysis methods were provided in Section S3 (Supporting information).
The infrared structure of organic phase was measured by Fourier transform infrared spectrometer (FT-IR) (Tensor 27, Bruker).The concentration of metal ions in aqueous solution was measured by inductively coupled plasma optical emission spectrometer (ICPOES) (Optima 7300 DV, Perkin Elmer).
The extraction efficiency (E) was calculated by the following Eq.1:

whereCbm(mg/L) andCam(mg/L) represented the mass concentration of metal ions in aqueous phase before and after extraction,respectively.
The volume ratio of organic phase and aqueous phase (O/A) was calculated by the following Eq.2:

whereVo(m3) andVaq(m3) represented the volume of organic phase and aqueous phase, respectively.
The stripping efficiency (S) was defined as the following Eq.3:

whereVsl(mL) andCslm(mg/L) represented the volume of stripping solution and the mass concentration of metal ions in stripping solution, respectively.Vo(mL) andCom(mg/L) represented the volume of organic phase and the mass concentration of metal ions in organic phase, respectively.
Other physical quantities were defined as follows:Cerepresented extractant concentration (mmol/L),trepresented extraction time,CU,CTh,CLa,CEuandCYbrepresented the initial molar concentration of corresponding metal elements (U, Th, La, Eu and Yb)in the aqueous solutions (mmol/L).Similarly,Cxrepresented the initial molar concentration of corresponding metal element X in the aqueous solutions (mmol/L), and Sxrepresented the stripping efficiency of corresponding metal element X.
In order to explore the best extraction effects under different extraction conditions, the influence of extraction time, pH and concentration of extractant on extraction of single element U(VI),Th(IV) and Eu(III) by Cyphos IL 104 were parametrically investigated.In this part, oscillating extraction in the centrifugal tube was adopted.
The kinetics of Cyphos IL 104 is important in assessing the extractant’s performance.The study of MEC extraction kinetics for U(VI), Th(IV) and Eu(III) were carried out over a time ranging from 10 min to 360 min.As shown in Fig.2, the extraction efficiencies of U(VI), Th(IV) and Eu(III) increased with extraction time, and reached equilibrium within 30 min with the balanced extraction efficiency of 69.5%, 54.9% and 57.0%, respectively.Therefore, the extraction time was selected as 30 min to ensure the extraction equilibrium in the later experiments.
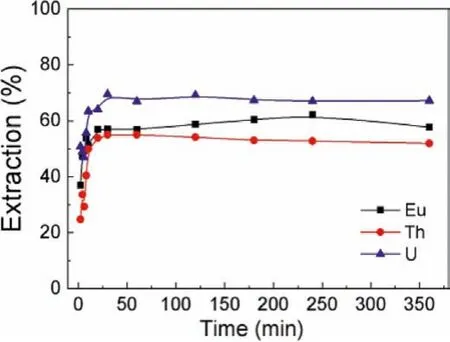
Fig.2.Extraction kinetics for U(VI), Th(IV) and Eu(III) (Ce=1.0 mmol/L,CU=CTh=CEu = 0.2 mmol/L, O/A=1:1, pH(U,Th) 2.00, pH(Eu) 3.00).
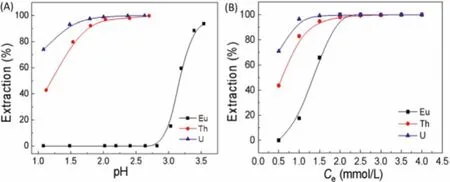
Fig.3.The trend of extraction efficiencies with (A) pH (Ce=1.0 mmol/L, CU =CTh=CEu = 0.2 mmol/L, O/A=1:1, t=30 min), and (B) extractant concentration(CU = CTh=CEu = 0.2 mmol/L, O/A=1:1, pH(U,Th) 2.00, pH(Eu) 3.00, t=30 min).
The pH of the solution can affect dramatically the extraction process because of the different species distribution of metal elements under different pH.As shown in Fig.3A, the extraction effi-ciencies of U(VI) and Th(IV) increased with pH.At pH 2.00, the extraction of U(VI) and Th(IV) substantially reached equilibrium with the extraction efficiency of 99.0% and 96.9%, respectively.And at pH 2.70, the maximum extraction efficiencies of U(VI) and Th(IV)both were close to 100.0%.However, the extraction efficiency of Eu(III) kept zero in the pH range of 1.00~2.80, indicating that Eu(III) was not extracted by Cyphos IL 104 at pH ≤2.80.Then,the extraction efficiency of Eu(III) augmented at pH>2.80 and reached 93.7% at pH 3.50.Noticeably, the pH of three extraction systems increased after the extraction of U(VI), Th(IV) and Eu(III)as shown in Table S4 (Supporting information).This phenomenon could be attributed to the fact that H+competed with metal ions to react with Cyphos IL 104 in the extraction process.Previous studies have confirmed that phosphonium-based ionic liquids were prone to capture protons from aqueous solution [23–25].Therefore, phosphonium-based ionic liquids had a strong ability to combine protons, which led to an increase in the pH of aqueous solution and a decrease of ionic liquids that could react with metal ions.The extraction mechanism of U(VI) and Th(IV) was proposed as Eqs.4-6 [25–27].
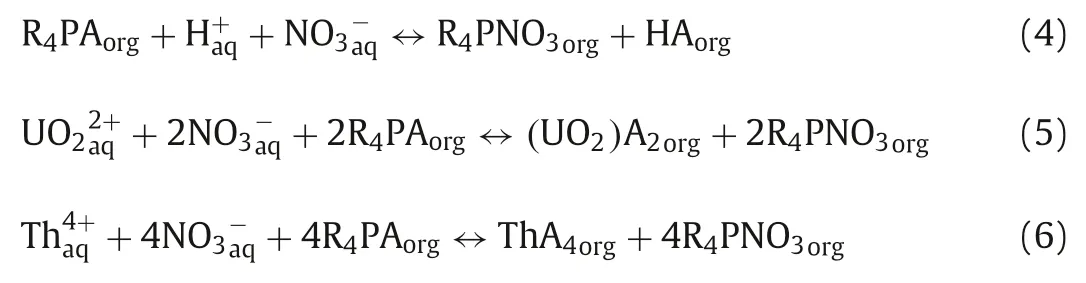
where R4PA and HA represented Cyphos IL 104, bis(2,4,4-trimethylpentyl)hypophosphic acid (Cyanex 272), respectively.With the initial pH increasing, the H+had been reduced and the consumption of Cyphos IL 104 also showed a downward trend (Eq.4), which led to more remaining Cyphos IL 104 that would participate in the extraction of UO22+or Th4+.This was benefit for the reaction towards the formation of (UO2)A2and ThA4.Accordingly,the increased pH was conductive to extraction.
The effects of the Cyphos IL 104 concentration on the extraction were also studied.As shown in Fig.3B, the extraction efficiencies of U(VI), Th(IV) and Eu(III) gradually increased with extractant concentration.When the extractant concentration was 1.5 mmol/L,the extraction efficiency of U(VI) generally reached equilibrium with a value of 99.2%.The extraction efficiencies of Th(IV) and Eu(III) reaching equilibrium at the concentration of 2.5 mmol/L and 2.0 mmol/L were 99.3% and 99.9%, respectively.According to the Eqs.5 and 6, higher concentration of extractant could provide more R4PA, which benefited the production of (UO2)A2and ThA4.In view of the high extraction efficiencies for U(VI), Th(IV) and Eu(III) at 2.0 mmol/L of Cyphos IL 104, this concentration was hired in subsequent experiments.
To get an insight into the mechanism of the extraction of U(VI)and Th(IV) in HNO3system, the extracted compositions were investigated by slope method.The following Eq.7 can be deduced from Eq.5 [28,29]:
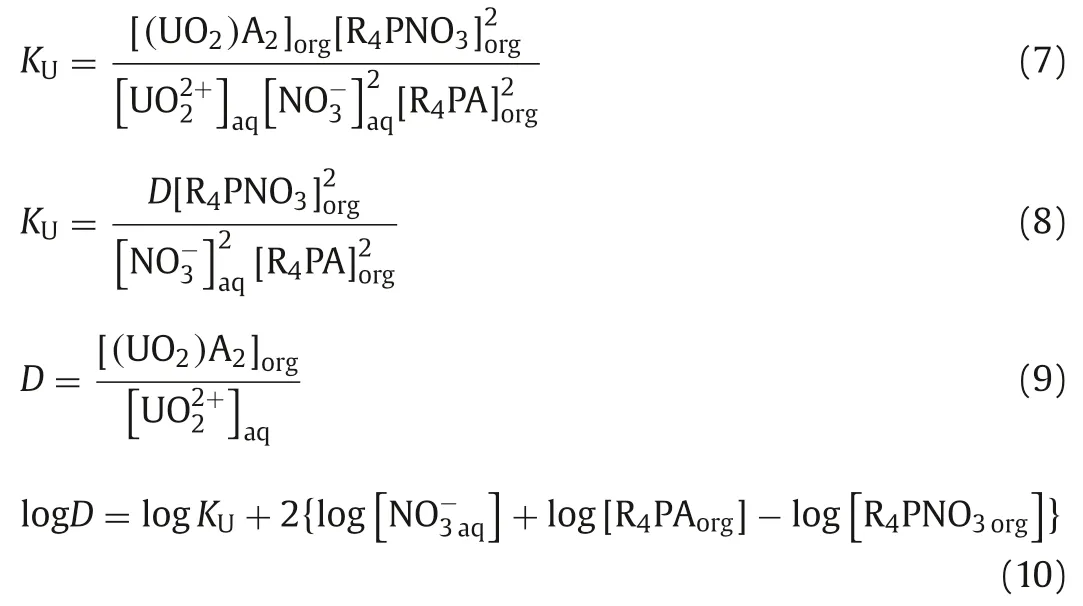
Similarly, the following Eq.11 can be obtained from Eq.6:
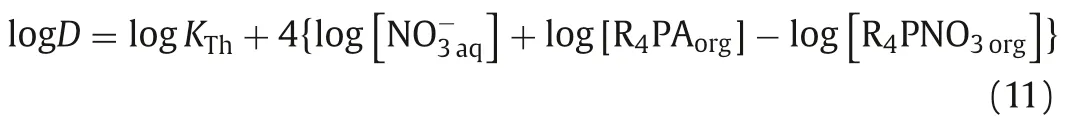
According to Eq.10, plots of logD vs.{log[NO3-aq]+log[R4PAorg]– log[R4PNO3org]} for U(VI) and Th(IV) were provided in Figs.4A and B, respectively.From the Figs.4A and B, the plots of logDvs.{log[NO3-aq]+log[R4PAorg] – log[R4PNO3org]} for UO22+and Th4+had a slope of ~3.A possible explanation might be that Cyphos IL 104 coordinated with UO22+in a proportion of 2:1 to form (UO2)A2and with Th4+in a proportion of 4:1 to form ThA4 Eqs.(5) and (6).In addition, Cyphos IL 104 could react directly with UO22+/Th4+in a proportion of 4:1/2:1 to form UO2[NO3]2·4HA/Th(NO3)3A·HA, resulting in slope ~3, as the following Eqs.12 and 13 [25]:
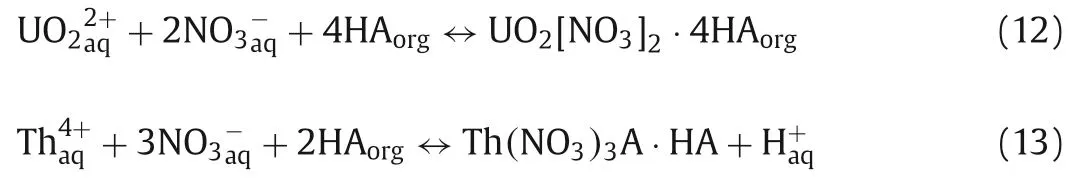
To further elucidate the extraction mechanism, FT-IR spectroscopic analysis was employed to characterize the organic phase before and after extraction.The results of the correlational analysis were set out in Fig.5.Compared with Figs.5C-E showed that after U(VI)/Th(IV) extraction, the free O-H stretching vibration peak(3675 cm-1) of the extractant obviously decreased, and the peak of P=O (1264 cm-1) disappeared.These indicated that the chelation of extractant with uranyl ions by A-, which led to the formation of (UO2)A2and ThA4.Moreover, the stretching vibration peak of P-C (1465 cm-1) also disappeared or decreased.This may partly be explained by the chelation between the trihexyltetradecylphosphonium chloride cation and the anion in Cyphos IL 104, resulting in the formation of R4PNO3[28–30].
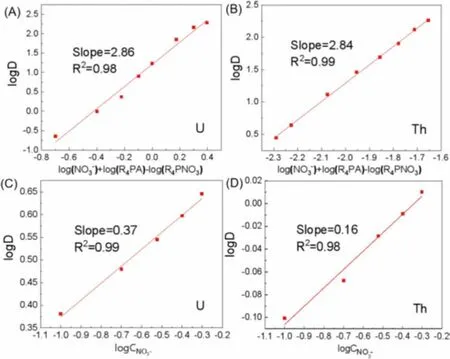
Fig.4.Effects of extractant concentration on extraction of (A) U(VI) and (B)Th(IV) (Ce=1.0 mmol/L, CU=CTh=0.2 mmol/L, O/A=1:1, pH(U,Th) 2.00, t=30 min).NO3- effect on extraction of (C) U(VI) and (D) Th(IV) (Ce=1.0 mmol/L, CU=CTh=0.2 mmol/L, O/A=1:1, pH(U,Th) 2.00, t=30 min).
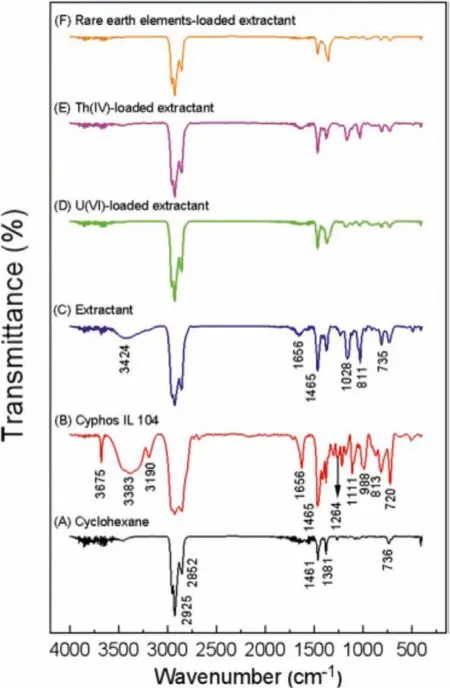
Fig.5.FT-IR spectra of (A) cyclohexane, (B) Cyphos IL 104, (C) Cyphos IL 104 in cyclohexane, (D) U(VI) loaded Cyphos IL 104 in cyclohexane, (E) Th(IV) loaded Cyphos IL 104 in cyclohexane and (F) REEs loaded Cyphos IL 104 in cyclohexane.
Due to the different physical and chemical properties, the extraction of different elements may show diverse extraction performance.Multi-elements extraction experiments using the MEC ex tractor were carried out.The effects of pH, diluents, different anions and stripping agents were investigated to obtain the general conclusion.In this part, MEC extractor was adopted.First of all,we explored the extraction kinetics of MEC extractor compared with stirring extraction to prove the extraction ability of MEC extractor before carrying out multi-elements separation.The kinetics comparison between MEC extractor and stirring extraction (Fig.6A) was investigated to better reflect the extraction effect of the MEC extractor.From Fig.6A, the extraction efficiencies of U(VI) and Th(IV) by MEC extractor were much higher than that of stirring extraction in the same time.The stirring extraction did not reach equilibrium at 20 min and the maximum extraction efficiencies of U(VI) and Th(IV) were only 50.7% and 64.2%, while the MEC extraction was close to equilibrium at 16 min with the extraction efficiencies of 95.1% for U(VI) and 97.6% for Th(IV).Obviously, MEC extractor had the excellent ability with fast extraction and high efficiency.
Phase separation plays an essential role in the extraction process and the speed of phase separation affects remarkablely the whole extraction efficiency.Fig.6B displayed the phase separation before and after extraction.The organic and aqueous phase were transparent before extraction.After extraction, the organic phase turned to pale yellow, indicating that U(VI) in aqueous phase was extracted into organic phase.And it can be seen from Fig.6C, the complete separation between organic phase and aqueous phase could be finished in 18 s, showing the strong phase separation ability.This is likely to be related to the fact that the emulsion droplets dripped into the extractor by the microporous membrane gradually aggregated under the joint effects of density and surface tension.When the droplets reached the cross section, the flow velocity in the vertical direction of the droplet suddenly changed due to the abrupt change of the section area.This would accelerate the collision between droplets and make droplets get together quickly,which was conducive to speed up phase separation.Thus, compared with traditional stirring separation, MEC extractor not only possessed stronger phase separation ability that is time-saving and effective, but also presented a good extraction performance for REEs.
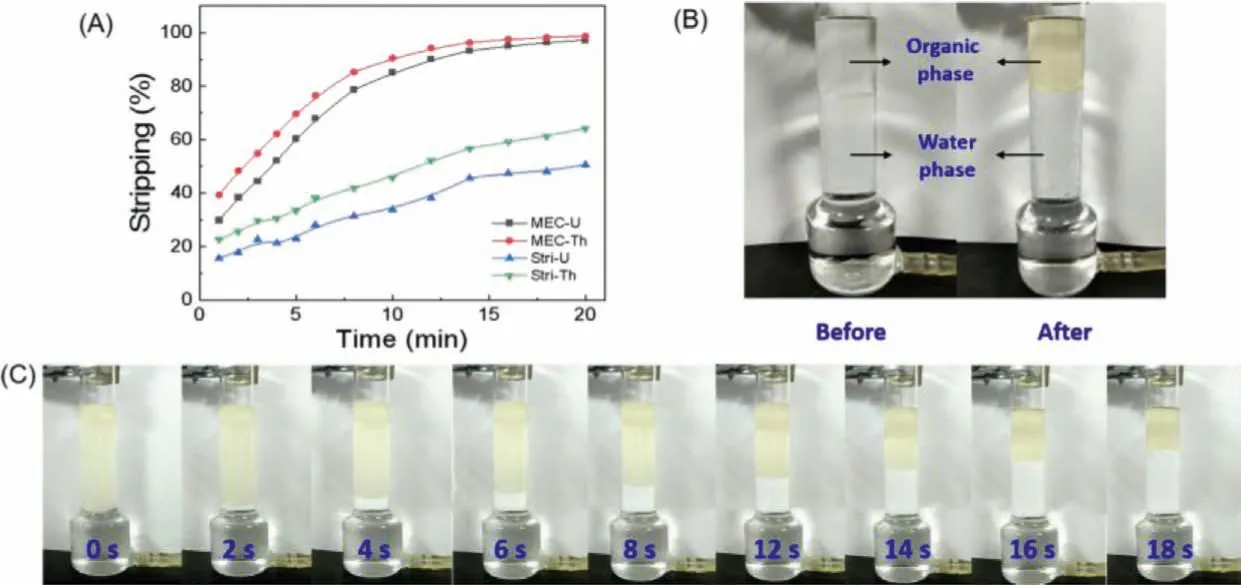
Fig.6.(A) Kinetics comparison of MEC extractor and stirring extraction (CU = CTh=0.2 mmol/L, O/A = 1:5, pH(U,Th) 2.00).(B) Contrast chart of MEC extractor before and after extraction (CU=0.2 mmol/L, O/A=1:5, pH(U) 2.00, t=30 min).(C) Phase separation process diagram after extraction (CU = 0.2 mmol/L, O/A = 1:5, pH(U) 2.00).
The above results showed that the extraction efficiencies between U(VI), Th(IV) and representative element Eu(III) of lanthanides differed greatly under different pH, which provided guidance for the separation of lanthanides and actinides.Therefore, the multi-elements (U(VI), Th(IV), Eu(III), La(III) and Yb(III)) extraction under different pH conditions were further explored.As shown in Fig.7, the extraction efficiencies of U(VI) and Th(IV) remained almost unchanged at different pH, both higher than 98.0%.However, the extraction efficiencies of La(III), Eu(III) and Yb(III) generally presented an upward trend with pH increasing.At pH ≤1.50, La(III), Eu(III) and Yb(III) were hardly extracted, suggesting that U(VI) and Th(IV) could be separated well from mixed solution in the pH range.This was consistent with the results of single element extraction.Secondly, the extraction efficiencies commonly decreased in this order at specific pH:EYb(III)>EEu(III)>ELa(III).Owing to the lanthanide contraction effect, the ionic radius of La(III),Eu(III) and Yb(III) gradually descended.And the polarization ability of ions increased with the decreasing of ionic radius, where the strong polarization ability could cause low solubility of ionic compounds in aqueous phase.Therefore, the solubility of La(III), Eu(III)and Yb(III) improved gradually in organic phase, which was more beneficial to the extraction [31].
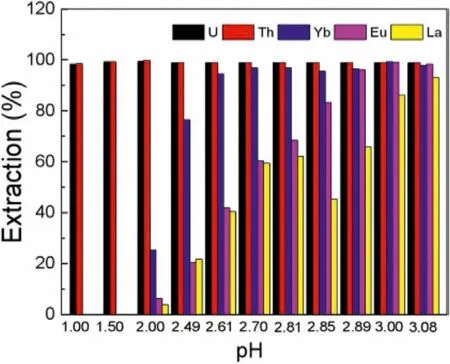
Fig.7.Effects of different pH on extraction separation (Ce=20 mmol/L, CU =CTh=CLa = CEu = CYb = 0.2 mmol/L, O/A=1:5, t=30 min).
The type of diluents is important considerations for practical applications.Toluene,n-hexane and cyclohexane, widely used in industry, were hired as the target diluents to evaluate the extraction behavior.Fig.8 gave the information about the effects of different diluents on the extraction efficiency at different pH.It could be seen from Figs.8A-C that the extraction efficiencies of U(VI) and Th(IV) in different diluents both reached more than 95.0%.No statistically significant difference correlation between the three diluents was found, indicating that different diluents had little effect on their extraction.Among these diluents, cyclohexane displayed the best extraction performance for U(VI) and Th(IV) with the extraction efficiency of 99.9%.As for La(III), Eu(III) and Yb(III), the effect of the diluent type on their extraction was roughly negligible.However, among these diluents, the extraction trends of La(III),Eu(III) and Yb(III) were mainly the same at three (2.00, 2.77 and 3.00) pH: the extraction efficiencies all generally increased with pH and the separation effects of lanthanides (La(III), Eu(III), Yb(III))and actinides (U(VI), Th(IV)) were all better when pH 2.00.Because toluene,n-hexane, and cyclohexane were inert solvents, there was no electron-dependent A-H bonds and electron donor atom B, and hydrogen bonds could not be formed, which led to the little difference in the extraction process.Notably, under the condition of high pH, the extraction efficiencies of Eu(III) and La(III) in cyclohexane andn-hexane were slightly higher than that of toluene.A possible explanation might be that the polarity of toluene (2.4D)was higher than that of cyclohexane (0.1D) andn-hexane (0.06D),so that the extraction ability usually decreased with the increase of diluent polarity [32].
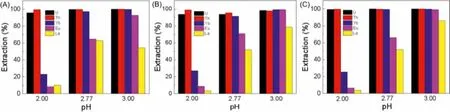
Fig.8.Extraction of various elements with different diluents (A) toluene, (B) n-hexane and (C) cyclohexane (Ce = 20 mmol/L, CU = CTh = CLa = CEu = CYb=0.2 mmol/L,O/A=1:5, t=30 min).
Many vital factors can affect the extraction process in the actual production environment, such as anion concentration and type inn.In most cases, the anion concentration and type will decide the species morphology of metals in the solution.From Fig.9A, concentration of NO3-had no effect on the extraction performance for these five metal ions.Fig.9B showed the effects of different anions on extraction separation.Compared with NO3-, the extraction efficiencies of other elements except La(III) remained high in the condition of Cl-.However, in the presence of SO42-, the extraction efficiencies of other elements except U(VI) all decreased.According to the species distribution of these elements, Th(IV) was the easiest to form Th(SO4)32-in the presence of SO42-.The decrease of extraction efficiency of Th(IV) was probably due to the reduction of positive charge of extracted metal cations and the increase of ion radius.Th(IV) was easier to form ThCl3+and Th(NO3)2+with positive charges when NO3-and Cl-were added to the extraction system separately.This would have a positive influence on extraction process [33].
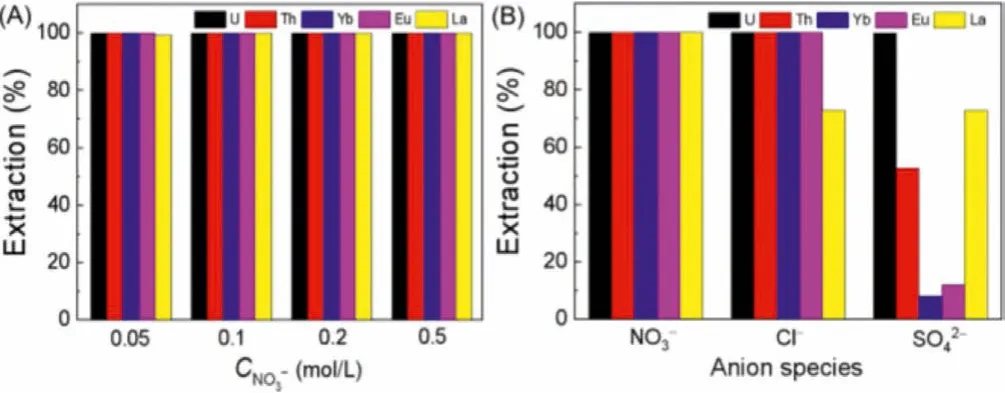
Fig.9.The effects of anions on extraction separation (A) concentration of NO3- and(B) anion species (Ce=20 mmol/L, CU=CTh=CLa=CEu=CYb=0.2 mmol/L, O/A=1:5,t=30 min).
Stripping is an important step in the complete extraction process, which can achieve not only the further manufacture and application of target substances, but also the reuse of extractant.In the field of solvent extraction, strong acids are commonly used as stripping agents.So in the following stripping experiments, the effects of different stripping agents (HCl and H2SO4) were studied on the stripping efficiencies of U(VI) and Th(IV), respectively.As shown in Fig.10A, there was a big difference in stripping U(VI)and Th(IV) using HCl.High stripping efficiency of Th(IV) could be obtained in the case of HCl as a stripping agent.For HCl concentration ranged from 0.1 mol/L to 3.0 mol/L, over 70.0% of Th(IV)could be stripped.And the highest stripping efficiency of Th(IV)reached 99.9% when HCl concentration was 0.5 mol/L.On the contrary, the stripping efficiency of U(VI) was much lower than that of Th(IV) and the highest efficiency was only 20.7% with 0.5 mol/L HCl.As a result, U(VI) and Th(IV) could be separated well by HCl stripping.Besides, Fig.10B showed that the stripping efficiency of U(VI) could reach nearly 80.0% with 0.5 mol/L H2SO4, which was useful to strip U(VI) from organic phase.Due to the high stripping efficiency of Th(IV) and the great difference between stripping efficiency of Th(IV) and U(VI) when the concentration of HCl was 3.0 mol/L, this concentration was selected for the first step of stripping to separate U(VI) and Th(IV).Similarly, H2SO4had better stripping effect on U(VI) than HCl and high stripping efficiency of U(VI) could be obtained in case of 0.5 mol/L H2SO4, therefore this concentration was used for the next step of stripping the residual U(VI).
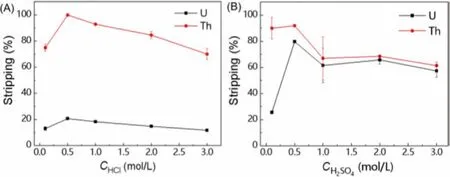
Fig.10.Stripping efficiencies of different agents (A) HCl and (B) H2SO4(Ce=20 mmol/L, CU=CTh=CLa=CEu=CYb=0.2 mmol/L, O/A=1:5, t=30 min).
Based on previous study, the stripping mechanism of Th(IV) and U(VI) by HCl and H2SO4from the loaded Cyphos IL 104 can be expressed as follows [20]:
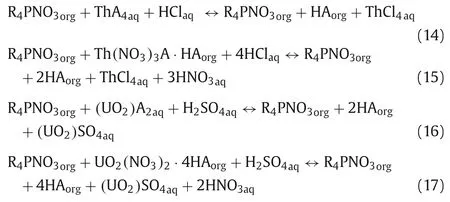
In view of the above experimental results and extraction conditions explored in the previous experiments, a complete process of extracting U(VI) and Th(IV) from REEs was proposed in Fig.11 to examine the effectiveness of this separation method.As shown in the flowchart: firstly, U(VI) and Th(IV) were extracted using Cyphos IL 104 by adjusting the pH of the mixed solution.Then, Th(IV)and U(VI) were stripped from organic phase by 3 mol/L HCl and 0.5 mol/L H2SO4, respectively, aiming at the separation of U(VI) and Th(IV) from REEs and the separation between U(VI) and Th(IV).
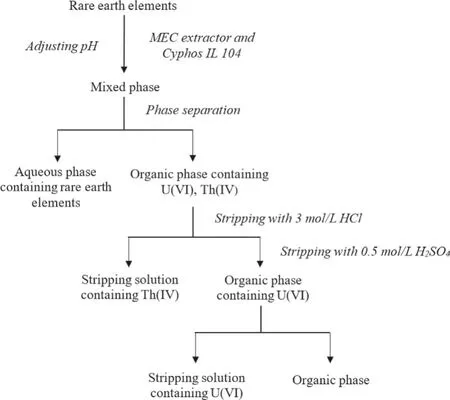
Fig.11.Complete extraction flowchart of separating U(VI) and Th(IV) from REEs.
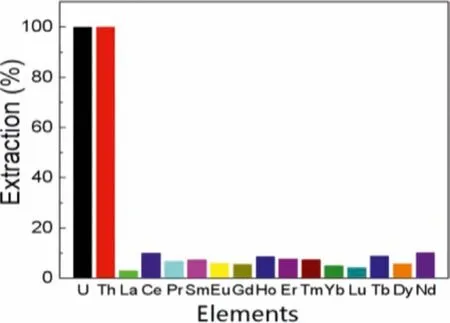
Fig.12.Extraction and separation effects of U(VI) and Th(IV) from REEs(Ce=20 mmol/L, 0.2 mmol/L of all metal ions, O/A=1:5, t=30 min).
In order to confirm the universality of the extraction and separation effect for REEs, the extraction by MEC extractor was also carried out.From Fig.12, the extraction efficiencies of U(VI) and Th(IV) were the highest and close to 100.0%.The extraction effi-ciencies of the other REEs were all less than 10.1%.Furthermore, in order to prove the good extraction ability of Cyphos IL 104, the three different extractants: bis(2-ethylhexyl)phosphate (HDEHP),trinbutylphosphine oxide (TBPO) and dimethyl heptyl methylphosphonate (P350), used widely in extraction industry, were also adopted to separate U(VI) and Th(IV) from REEs using MEC extractor.As shown in Fig.S5 (Supporting information), HDEHP displayed high extraction efficiencies for U(VI), Th(IV), and even almost REEs.The intermediate separation efficiencies suggested that TBPO could not be competent for the extraction of U(VI) and Th(IV).Although U(VI) could be separated selectively from REEs by P350, the extraction efficiency of Th(IV) was only 7.7%.Meanwhile, the extraction efficiencies of U(VI), Th(IV) and REEs by Cyanex 272 and Cyphos IL 101 were also studied (Fig.S6 in Supporting information).From Fig.S6A, Cyanex 272 had higher extraction efficiencies of U(VI) and Th(IV) than Cyphos IL 101, but the extraction ability of Cyanex 272 was weaker than that of Cyphos IL 104.From Figs.S6B and C, the extraction efficiency of U(VI) by Cyanex 272 was just over 70.0%,although the extraction efficiency of Th(IV) could reach 100.0%.The extraction efficiencies of all metal ions by Cyphos IL 101 were very low, indicating that U(VI) and Th(IV) could not be separated by Cyphos IL 101.In comparison, Cyphos IL 104 exhibited outstanding extraction performance for U(VI) and Th(IV) from REEs, where the high-efficiency extraction of U(VI) and Th(IV) as well as poor extraction for REEs could be achieved.
After the separation of U(VI), Th(IV) from REEs, the multi-stages stripping was performed to strip U(VI) and Th(IV), respectively.Multi-stages stripping effect of U(VI), Th(IV) and REEs in Table S7(Supporting information) showed the successful separation of U(VI)and Th(IV).In the stripping step for Th(IV) by HCl, the stripping efficiencies of U(VI) and Th(IV) increased with stripping stage.When the stripping stage was 3, the stripping efficiency of Th(IV) could reach 80.0%, while that of U(VI) was only 2.9%.In the second stripping step for U(VI) by H2SO4, the stripping efficiency of U(VI) was 94.8% when the stripping stage was 2, and it was close to 100.0%when the stripping stage reached 3.However, the other REEs were hardly stripped.As a consequence, the process in Fig.11 was an effective way to separate U(VI) and Th(IV) from REEs by the MEC extractor and Cyphos IL 104 as an extractant.
This study set out to find a new method for the separation of U(VI) and Th(IV) from REEs by using Cyphos IL 104 as an extractant.Through the extraction experiments of single element, a rapid extraction kinetics for U(VI), Th(IV), Eu(III) and fast phase separation ability by Cyphos IL 104 were demonstrated.The increase of pH could improve the extraction efficiencies of U(VI),Th(IV) and Eu(III), and the final extraction efficiencies could be higher than 99.0%.In the process of multi-elements experiments,the influence of different diluents, pH and anions on the extraction and separation were investigated.The results showed that when pH ≤1.50, La(III), Eu(III) and Yb(III) were basically not extracted, while U(VI) and Th(IV) were completely extracted.This phenomenon indicated that adjusting pH could achieve separation of U(VI) and Th(IV).The presence of excessed SO42-significantly reduced the extraction efficiencies of the elements other than U(VI), while NO3-and Cl-had little effect on the extraction.The FT-IR analysis of the organic phase before and after extraction showed that the extraction mechanism was mainly due to the chelation of bis(2,4,4-trimethylpentyl)phosphinate with metal ions.Moreover, the MEC extractor was used to extract U(VI) and Th(IV)from the mixture solution containing also REEs.The results showed that Cyphos IL 104 could successfully separate U(VI) and Th(IV)from REEs.In the multi-stages stripping, when the extraction stage number was 3, the stripping efficiency could reach 100.0% and 80.0% for U(VI) and Th(IV), respectively, exhibiting a very good separation between U(VI) and Th(IV).Based on the experimental results, a flowchart for effective separation of U(VI) and Th(IV) from REEs was proposed and it was expected to be useful for practical application.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.11775214 and 51803205) and the China Scholarship Council (No.201906345006).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.089.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
