Opportunities and challenges of high-pressure ion exchange chromatography for nuclide separation and enrichment
Weixiang Xiao, Duoqiang Pana,,*, Zhiwei Niu, Yang Fan, Sirui Wu, Wangsuo Wu
a Frontiers Science Center for Rare Isotopes, Lanzhou University, Lanzhou 730000, China
b School of Nuclear Science and Technology, Lanzhou University, Lanzhou 730000, China
c Key Laboratory of Special Function Materials and Structure Design, Ministry of Education, Lanzhou 730000, China
ABSTRACT With the rapid development of the nuclear industry, more-stringent requirements are proposed for highlevel radioactive waste liquid treatment and the enrichment of isotope products.High-pressure ion exchange chromatography has been widely accepted for the fine separation of elements and nuclides due to its advantages, such as high efficiency, environmental friendliness, ease of operation, and feasibility for large-scale industrial applications.Here, we summarized the evolution of high-pressure ion exchange chromatography and the relevant research progress in ion exchange equilibrium and related separation technology.The prospects for application of high-pressure ion exchange chromatography to rare earth elements, actinide elements and isotope separation were discussed.High-pressure ion exchange chromatography represents a promising strategy for the extraction of rare earth elements and actinide elements from high-level radioactive waste liquid, as well as being an effective method for the automated production of high purity isotope products with great environmental benefits.
Keywords:High-pressure ion exchange Chromatography Nuclide Separation Enrichment
1.Introduction
With the continuous development of the nuclear industry, the demand for radionuclides is increasing [1–7].Major sources of nuclides include nuclear reactors [8–10], accelerators [11–13], and separation and extraction from nuclear fuel reprocessing waste[14–17].The extraction of nuclides with practical value not only brings great economic benefits but also is conducive to the nuclear fuel closed cycle and the sustainable development of nuclear energy [18–20].Efficient separation technology is beneficial to both the coordinated development of energy and the environment, which also accords with the carbon peak and carbon neutralization strategic deployment of China [21–24].
In dealing with prominent problems in environmental constraints and realizing the green low-carbon high-quality development path, ion exchange technology, which has been advancing since the beginning development of nuclear energy, ushers in new opportunities and challenges.The phenomenon of ion exchange was discovered as early as the mid-eighteenth century and has been extensively studied.The most important event in the development of various ion exchangers was the synthesis of the first ion exchange resin by Adams [25], which was later put into industrial production [26].The rapid development of ion exchange resins and the progress of their related application technology are bound to promote each other.With the advent of various resins with stable exchange capacity [27–29], the development of ion exchange technology ushered in a great development opportunity.
In the Manhattan project, ion exchange chromatography (IEC)was used with great success in the separation and purification of fission rare earth elements.Compared with the traditional separation method of rare earth elements, IEC can greatly shorten the experimental period and achieve trace-scale separation [30–38].After the war, many countries realized its broad prospects and formulated huge nuclear energy development plans that provided an incentive for the rapid development of IEC [39,40].
With the emergence of various new materials and technologies,a brand-new breakthrough has taken place in IEC,i.e., the appearance of high-pressure ion exchange chromatography (HPIEC).This is a new stage of modern chromatography with profound changes from traditional chromatography.In 1968, Scott was the first to use 5–10 μm resin as the stationary phase and force the eluent at a pressure of 150 kg/cm2to separate urine components, from which method he obtained 140 chromatographic peaks [41].Creative use of resin particles can greatly improve column performance.Martinet al.proposed a similar idea; using particle ion exchangers as the stationary phase can produce very low values of the height equivalent to a theoretical plate (HETP) as early as 1941 [42], but there were no available pumps, valves, and connection fittings that could overcome the large bed resistance for the study of ion exchange under high pressure.HPIEC is particularly suitable for treating high-level radioactive waste liquid, especially the separation of lanthanide and actinide elements with similar properties.Therefore, it was soon used for the separation of fission rare earth elements [43] and transplutonium elements[44,45].Meanwhile, HPIEC has been widely used in the separation of amino acids [46,47] and nucleic acids [48–52].
HPIEC suffered a significant increased bed resistance due to the use of fine-particle resin.Since only a slow flow rate can be obtained by gravity on the mobile phase itself, there must be a highpressure metering infusion pump.Chromatographic column is the key to achieve efficient separation.Usually, stainless steel was used for the column material, which has the pressure, temperature and chemical resistance characteristics.For some research purposes in laboratory, homogeneous texture and thick-walled glass tubes are selected to conveniently observe inside the column.The column size, number, and inner smoothness also should be considered.The high-pressure metering infusion pump realizes the rapidly accurate injection and the high performance of column.A suitable detector or online monitoring device will record the real-time data to facilitate cutting column and obtain pure products.Besides, the related auxiliary equipment includes feeding thermostatic device, column packing device, and cleaning device.Meanwhile, the general single part needs preferable replace ability to easily adapt for the different requirements in the separation of radioactive components.The equipment can use the apparatus of high-performance liquid chromatography in the elution development methods, and also can be autonomously designed for the different experiment requirements.For the separation of nuclide, the latter approach is usually taken due to the different types of eluent and the scruple of radioactive contamination.Meanwhile, the assistive technology of displacement development is great different to high performance liquid chromatography.
Compared to classical IEC, HPIEC adopts uniformly selected spherical resin particles and a high-pressure infusion pump so that the liquid flow is forced through the exchange column at a steady and high flow rate.The schematic diagram of multicolumn high-pressure ion exchange chromatography for nuclide separation and enrichment was depicted as Fig.1.On the one hand,the main characteristic is high efficiency and equipment miniaturization.Empirically, the moving velocity of the chromatography band is proportional to the empty column linear velocity of the mobile phase.The use of particle ion exchange resin with an extremely fast mass transfer rate can improve the bed efficiency at a high flow rate.Thus the size of the column can be greatly reduced,and the equipment still maintains the original processing capacity.The demand for resin can be greatly reduced, which makes strictly homogeneous resin possible.Uniform resin particles and uniform packing of the ion exchange column are the conditions necessary for high performance, which cannot be achieved in the separation of the classical IEC because of the large amount of resin and the huge size of the column.On the other hand, the problems of heating during operation and gas release can be solved.When the solution containing the high-level radioactive material passes through the resin bed, large amounts of bubbles will be generated due to the strong radiolysis on reagents and resin.This will seriously affect the stability of the solution flow rate.The bubbles even disturb the tightly arranged resin bed and block the liquid channel so that the interface of the chromatographic band is chaotic, resulting in failed separation.For the separation of some substances, the formation of bubbles will become a major hidden danger of heating during operation, but in HPIECs, all the gas in the system is dissolved and carried away by the continuous flow of fresh solution.
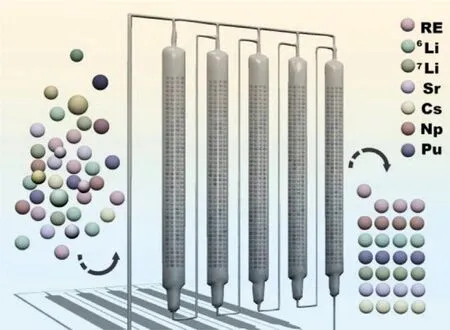
Fig.1.Schematic diagram of high-pressure ion exchange chromatography for nuclide separation and enrichment.
In addition, the reagent consumption and the amount of waste liquid can be greatly reduced.The reagent consumption is proportional to the separation time, so the reagent consumption decreases with the greatly shortened separation time.For the separation of radioactive substances, HPIEC can greatly reduce the waste liquid volume and thus reduce the investment required for treatment of radioactive wastewater, which is of great significance to industrialization and cost savings.
2.High-pressure ion exchange chromatography
HPIEC has three operating methods that are still based o n the classical IEC separation method.First, elution development is suitable for the separation of small amounts of materials and is often used for the separation of radioactive elements in radiochemistry.According to the locations of the chromatographic peaks, the individual radionuclides or organic matters can be qualitatively identified.During the separation process, the column packing is nearly unchanged and thus the column physical condition can be maintained, which leads to a high bed performance in elution development.In addition, another advantage of elution development is that the resin bed does not need to be regenerated during routine analysis.The disadvantage of this method is that the amounts of the materials to be separated are strictly limited by the column volume.
Second, displacement development can arrange the components of the test material to discharge from the column in a certain order, and we can obtain pure products from the plateau regions of the chromatographic curve of each component.This method shows irreplaceable advantages for the separation of similar large-scale elements, such as the production of high purity rare earth elements and the separation and refinement of heavy actinides.The disadvantage of this method is the existence of a mixed cross region between the two pure components, which limits the yield.In addition, the resin bed must be regenerated, which consumes a considerable amount of reagent and time.
In a typical displacement development process, the solution that includes retention ion of Cu2+is transported the chromatography column to transform the original resin bed to retention bed.After the load of feeding solution, a mixed adsorption segment is formed on the top of column.Then the displacement agent is continuously flowed in the column at a certain rate.In the development process, the segment of retention ion will gradually shorten.The chromatography band of the components to be separated will gradually move down and the upper portion of column will be replaced by the displacement ion.Due to the different affinity to ion exchanger, the separation components will display a complex arrange process during the movement process of all chromatography band, then the pure products can be obtained from the plateau regions in the chromatographic curve (Fig.2).
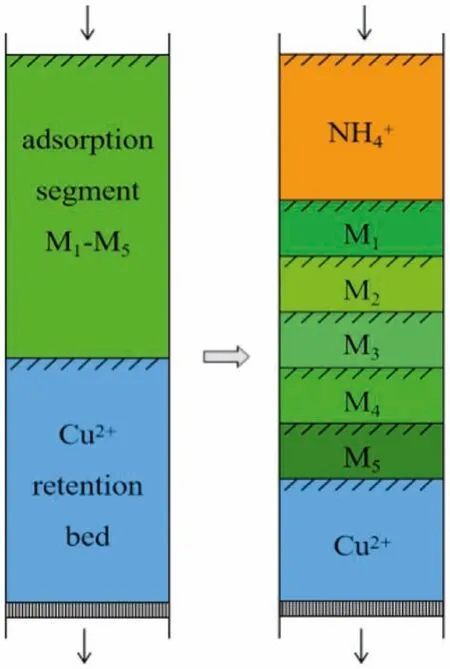
Fig.2.The segment of displacement development process.
Third, the frontal method neither realizes quantitative separation nor separation from each other to obtain pure components.However, it is a simple and effective method if one target component has a greater affinity for the resin.The method is also suitable for filtering some harmful components from the initial liquid and removing interfering ions.The distinction of the three separation methods is relative, and as the experimental conditions change,one given typical method can transform to another.Many factors will influence the result of ion exchange chromatography separation, which is different from the elution development and displacement development.Generally, these factors can be summarized as the physical factors, including pressure, flow rate, and temperature,the chemical factors, including eluent types and its concentration,pH, chemical properties of ion exchanger, the geometric factors,including height of bed, diameter and surface finish of column,particle size of ion exchanger, and the packing factor.In order to achieve the best separation result, these factors are usually considered comprehensively.
Scientists of Lanzhou University in China has developed the modern separation theory of high-pressure ion exchange chromatography and has conducted systematic and pioneering researches on ion exchange equilibrium and related engineering technology since 1965, which emphasizes the combination of theory and applications with unique characteristics.On the one hand,starting with the uranium mining and hydrometallurgy process,the ion exchange equilibrium mechanism and multivariate exchange equilibrium in the displacement chromatography segment were deeply studied.In terms of intragranular mass transfer, the pore diameter played the main role in controlling the diffusion mechanism, which was confirmed by studying the pore structure of the sulfonic acid macroreticular resin and the mass transfer dynamics of UO22+.The delay time of elution development chromatography was corrected, and a new method to simultaneously measure the internal diffusion coefficient and liquid film thickness was developed.The complex chelation displacement process was simulated by a simple process, and the practical chromatography band speed formula was determined.This laid a good foundation for ion exchange equipment and engineering design.On the other hand, the optimal process separation of lanthanide and actinide elements was established.The enrichment technology of6Li was deeply explored, during which it was confirmed that the concentration efficiency of HPIEC was much higher than that of conventional means.Based on the plate theory of distillation, a set of practical isotope chromatography band equations was derived.In the experimental study, a set of five high-pressure ion exchange separation columns and equipment was built that could alternately extract different nuclides (Fig.3).
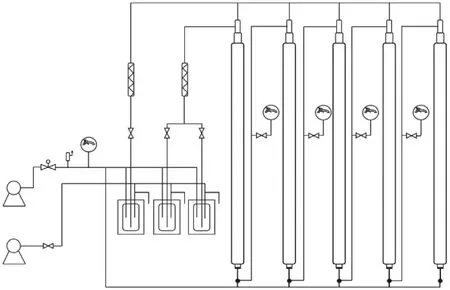
Fig.3.A set of multicolumn HPIEC equipment designed by Lanzhou University.
3.Separation of rare earth elements
The rare earth elements consist of 17 elements which include scandium and yttrium as well as the lanthanides.Because of their unique photoelectric and magnetic properties, and their status as strategic resources related to national security, rare earth elements are known as the “vitamins of modern industry” [53,54].Rare earth elements have similar chemical properties not only because their valence electrons have the same configuration but also because their atomic and ionic radii are very close.The ionic radius of the lanthanide elements decreases with increasing atomic number, which is known as the “lanthanide contraction” [55].
The main industrial method for the separation and purification of rare earth elements is solvent extraction [56,57].The subsequent emergence of various extraction agents also promoted the development of rare earth separation technology [58–60].Considering the time-consuming and environmental burden, it is necessary to develop new separation technologies that are both efficient and environmentally friendly.Cotruvoet al.prepared a rare earth adsorbent material based on a bacterial protein by immobilizing lanmodulin on agarose microbeads [61].An adsorption column filled with this material still retains the remarkable rare earth element selectivity of the soluble protein.Different rare earth elements have different desorption selectivity at different pH values, so they proposed using a two-step desorption method to successfully separate a mixed solution of 50% Nd and 50% Dy into single rare earth element solutions, each with a purity of 99.9%.At the same time, the rare earth mixture could be separated into heavy and light rare earth parts by using a two-step desorption method that requires no organic solvents and reduces the emission of pollutants in the process of separation.IEC used in rare earth production has the advantage that a single separation process can obtain pure products of all components, which is not achievable by the extraction method.Environmental protection and high efficiency are requirements for HPIEC column packing selection in the 21stcentury.In addition, we also need to consider its applicability under different conditions such as high temperature, pressure, and corrosion resistance.
In 1960, it would have taken 14 h to separate all the rare earth elements with the classical IEC of elution development.For the first time, Campbellet al.used HPIEC elution development to study the effect of the degree of resin cross-linking degree, particle size,and eluent gradient on the elution results in a 17 mg equivalent resin bed.The separation was completed in only 5 h under optimal conditions [62].In the following year, a glass short column was used with a cross section of 0.64 cm2and a height of 33 cm packed with 25–60 μm resin to separate 15 rare earth elements,which shortened the separation time to 1–2 h.Under the optimal conditions for this experiment, the chromatographic peak interval between two adjacent elements was only 5 min [63].The results also show that the eluent gradient is an important experimental factor and that only an appropriate eluent gradient can achieve efficient separation of rare earth elements.
The separation of the heavy rare earth elements ytterbium and thulium was studied by displacement development at Lanzhou University.The same column performance could be maintained even when the flow rate was increased 20-fold, which will significantly improve the production efficiency and shorten the production cycle.After that, the separation process of rare earth elements was improved.In the case of H+as the retention ion, 60%of chelating agent existing in the retention section can form a hydrogen complex, which can play the role of a purification displacement agent and can be recovered to reduce cost.The results showed that chelating agent and H+form pure independent sections that can be recycled after adjusting the acidity and concentration.Meanwhile, the rare earth elements retained at the forefront of the chromatography band have no cross problem with retention ions, which improves the yield and purity.
HPIEC for rare earth separation can improve production effi-ciency and reduce cost.If the new extraction system can be combined with a particle ion exchanger for rare earth element separation [64–68] and then combined with the computer simulation of multistage extraction theory [69–73], it can be further advanced in automatically controlling the process of producing high-purity rare earth products.At the same time, it is of great practical significance to realize the closed cycle of nuclear fuel for the separation of fission products encountered in reprocessing nuclear fuel[74,75], such as90Y,140La,141Ce,147Pm and151Sm.
4.Separation of actinide elements
HPIEC has long been used in the separation of actinide elements.237Np captures a neutron to become238Np and continues to decay to238Pu.238Pu is the most ideal fuel for radioisotope power supply and heat sources required for space activities [76].The Savannah River Laboratory (SRL) separated neptunium and plutonium using a series of Dowex-1 resins and macroreticular MSA-1 resins with different degrees of cross-linking and particle sizes by HPIEC.The pressurized single column equipment used a diameter of 2.032 cm and a height of 40.64 cm (Fig.4).Considering the ion exchange heat, dilution heat of NO3-, and radioactive decay heat in the experimental process, the anion exchange resin was prone to vigorous chemical reaction under concentrated nitric acid operating conditions, so the top of the column was equipped with an explosion-proof device.When the pressure was too high, the resin in the column flowed out through the connection port of the explosion-proof device to prevent the solution from exploding.The experimental results showed that the Dowex-1×2 resin would deform under high pressure due to its poor mechanical strength, so it was impossible to use finer resin and a higher flow rate.The Dowex-1×8 resin had the highest mechanical strength, but due to the slow internal diffusion, the produced neptunium was seriously contaminated by plutonium.Only 190 mesh Dowex-1×4 and MSA-1 resins had good adsorption and desorption properties, with little cross contamination of the product [77].This indicated that the particle size and type of resin have a great influence on the separation process.Macroreticular resin is suitable for certain complexed ions, such as Pu(NO3)62-and Np(NO3)62-, which are large in volume and diffuse slowly within the resin phase.For the selection of the degree of resin cross linking, the different separation objects need to be considered from the point of view of separation factors, mechanical strength and HETP.
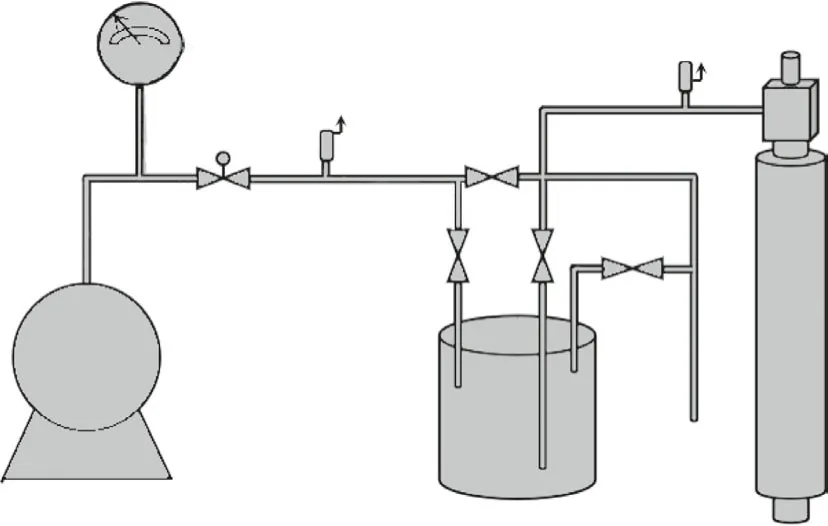
Fig.4.Single-column HPIEC equipment for neptunium-plutonium separation.
Both242Cm and244Cm are idealαradiation sources and can be used as energy sources in space [78].The chemical properties of Am3+and Cm3+are so close that it is difficult to separate them from each other.If these nuclides that release a high amount of heat are removed first, it can greatly reduce the radioactivity level and toxicity, which is of great significance for long-term safe disposal [79].
The feed liquid that contained 18 kinds of metal ions was adopted to simulate the high-level radioactive waste liquid after power reactor fuel reprocessing.The technological process of pretreatment and displacement separation was studied to simulate the extraction of americium, curium, and promethium from a highlevel radioactive waste liquid.In view of the low content of americium and curium in the feed liquid, the pH of displacement was reduced to extend the chromatography band length for a high yield.At the same time, the concentration of NH4+was increased to improve the band movement rate, which could shorten the operation time to reduce resin radiolysis and waste liquid volume.
The chelating agent selected for displacement development separation depends on the objects to be separated.The position of lanthanides in the band during cation chelation displacement is determined by the size of the chelate stability constant formed by the chelating agent.Therefore, diethylene triamine pentaacetic acid(DTPA) was first used to separate lanthanide and actinide elements,and then nitrilotriacetic acid was used as a chelating agent to place147Pm between samarium and neodymium and to avoid contamination by91Y so that the147Pm could be extracted from fission products.The technological process in this experiment provided an important basis for the engineering design of actinide element separation.
In 1971, SRL was equipped with a multicolumn ion exchange device that used an irradiated plutonium target as raw materials and DTPA as a displacement agent.Each run of the experiment isolated 60 g244Cm, 27 g243Am and 180 μg252Cf [80].The displacement order was Cu2+, Ni2+, Cf3+, Zn2+, Pb3+, Cm3+, Am3+, Eu3+,Nd3+, Pr3+and Ce3+.Among these,252Cf is the ideal material for small neutron sources.This is extremely important in reactor initiation with the advantages of small volume, high intensity, and continuous neutron fission.Reactor irradiation is the only practical method for the large-scale production of252Cf.Subsequently, a transuranium element separation plant was built at SRL [81].The lanthanide and actinide elements were separated by DTPA, and the single actinide was then separated to obtain pure americium and curium products.The californium, fermium, and einsteinium were separated byα-HIB elution to obtain 260 mg252Cf at one time.Later, the large-scale production of heavy actinide elements in the United States adopted HPIEC, which realized the industrialization of transplutonium element production [82].
If excessive H+ions were adsorbed during feeding, a considerable amount of H+entered the retention ion Zn2+segment at the beginning of DTPA displacement.The strong stability of this DTPAZn2+hydrogen complex allows the Zn2+-H+mixed bed to play a strong retention role in separating californium, which will lead to the gradual entry of californium into the Zn2+segment during the removal of H+.For this purpose, the H+in the column should be eluted by ten times the volume of Zn(NO3)2after feeding.
In HPIECs, the selection of retention ions is also an important factor affecting separation.Since the displacement development method is suitable for the large amount of sample, the curved portion of ion exchange isotherm is used for the arrangement of different components.Retention describes a dynamic phenomenon that the extension process of chromatography forefront band is hindered for some reasons, the retention ions can induce the chromatography forefront band to a certain extent and then no longer change.The lower concentration of retention ions causes the lower slope of ion exchange isotherm, and then the faster movement speed of chromatography segment.In general, the dynamic factors cause the chromatography segment to extend continuously,however, the thermodynamic equilibrium factors usually result in a tendency to make the chromatography segment automatically steep, which will compensate for the dynamic factor that keep the segment extending.When the chromatography segment extends to a certain stage and the interaction caused by these two opposing factors is balanced, the segment interface tends to be stable.
In general, ions with less affinity for the resin may act as retention ions for other ions with more affinity.In other words, any ions in front of the displacement order can be used as the retention ion, but the difference between the retention ion and the separation components should not be too large on the resin affinity;otherwise, the retention ion will be separated from the chromatography band and have no retention effect.The selection of retention ions should consider that the chelate formed with the displacement agent must have sufficient solubility to facilitate monitoring as much as possible and that the displacement interface of the chromatogram must be steep enough to have a faster band shift under the same experimental conditions.
5.Separation of isotopes
5.1.Lithium isotopes
As an important strategic metal for energy, lithium has two stable isotopes of6Li and7Li with natural abundances of 7.42% and 92.55% [83].6Li and7Li have the same configuration of valence electrons and very similar chemical properties, so separation is extremely difficult.Because lithium isotopes play a very important role in the nuclear fusion reaction and reactor operation, the separation of lithium isotopes is a key technical problem to be solved in the development of nuclear energy.
In recent years, many techniques have been developed to separate lithium isotopes, such as laser excitation [84,85], electrochemical [86–90] and mercury amalgam methods [91].Mercury amalgam is the only method currently used for industrial production.The separation principle is that6Li is more easily bound to mercury than7Li in the chemical exchange process, and lithium isotopes are exchanged between the two phases.The mercury amalgam method has many advantages in the industrial separation of lithium isotopes [92,93], such as a high separation factor, short isotope exchange reaction period, and relatively simple engineering amplification.Due to the large amount of mercury used in the separation process, dealing with environmental and safety problems is an active priority.Therefore, people are looking for other safe and efficient methods for separation of lithium isotopes.
Solvent extraction was used for separation by using the differences in the distribution coefficients of6Li and7Li in the two solvent phases with cryptand [94] or crown ether [95] as the extraction agent.Nishizawaet al.studied the ability of crown ether to separate lithium isotopes and found that the single-stage separation coefficient of 12-crown-4-ether could reach 1.057 [96–98].In terms of the separation factor alone, crown ether is the most promising ion exchange reagent for industrial separation of lithium isotopes.However, the low distribution coefficient of Li+in the two phases of crown ether and aqueous solution is not conducive to the multistage separation and enrichment of lithium isotopes, which is the main reason limiting the industrial application of isotope separation.A series of studies has also been conducted in this respect, such as crown ether grafted materials [99–103], the ionic liquid enhanced separation process [104–107], and fluorinated solvent extraction system [108].Cuiet al.fabricated a chitosan-graft-benzo-15-crown-5-ether/polyvinyl alcohol porous blend membrane for the adsorptive separation of lithium isotopes.As the immobilization amount of crown ether was fixed from 1.07 mmol/g to 2.60 mmol/g, the single-stage separation factor increased from 1.008 to 1.046.This green and highly efficient blended membrane for lithium isotope separation has great potential applications [109].
The mechanism of IEC for lithium isotope separation is similar to lithium amalgam.The selective resin or group fixation for lithium isotopes is packed into an ion exchange column using the affinity differences of6Li and7Li [110–112].The combined application of various cryptand [113,114] or crown ether [115–117] systems greatly expands the packing choice of columns, which effectively improves the disadvantages of small single-stage separation coefficients.Kimet al.carried out a study on the separation of lithium isotopes with a resin that used monobenzo-15-crown-5 as a functional group.The separation operation was performed at 23°C with a chromatography column that used a 9 mm inner diameter and 250 mm height.The experimental results showed that the separation factor reached 1.053 in this case [118].Fujineet al.explored the effect of temperature and macroreticular resin on the separation of lithium isotopes by displacement development.The separation factor was reduced with the elevation of operational temperature, but the performance of lithium isotope separation was improved because the acceleration of ion exchange reaction surpassed the decrease in the isotope separation factor.Meanwhile, the increase of interphase mass transfer area by using the macroreticular resin resulted the higher speed of moving bands, which leads to smaller HETP and faster isotope separation progress [119].Suzukiet al.studied the engineering application of IEC to lithium isotope separation and estimated the length and experimental time required for6Li with different enrichment degrees by using a small-scale separation experiment.Experimental equipment with a reliable detection method was proposed to demonstrate the industrial application of IEC in lithium isotope separation.However, IEC still has the disadvantages of a long equilibrium time and migration lengths, which require 340 m, 1600 m and 5800 m to obtain 25%, 50% and 90% enriched6Li, respectively.This is not suitable for the production of higher purity lithium isotope products [120].
The effects of displacement agent concentration and flow rate on the enrichment of lithium isotope separation were studied by Lanzhou University using multicolumn high-pressure ion exchange equipment filled with 40–50 μm cation exchange resin.In one round of representative experiment, CaAc2was selected as displacement agent, and the contents of6Li and7Li in the front and back bands were determined to be 15.8% and 97.5%, respectively.Despite the different conditions, the time and reagents needed to achieve the same isotope enrichment after the band were compared [121].The high-pressure method far exceeds the constantpressure method.If a new particle ion exchanger is combined with HPIEC, it may provide a means of lithium isotope separation suitable for modern industrial applications and achieve rapid and effi-cient enrichment of high purity lithium isotopes.
5.2.Boron isotopes
10B has a good neutron absorption capacity and can be used to make control rods for the reactor [122].It can also be used for the treatment of cancer in medical aspects [123,124].Boron isotope separation has long been studied [125], and the main method of boron isotope separation is the chemical exchange distillation method [126–128].Due to its high process requirements and low yield, IEC has shown great advantages in boron isotope production [129,130].N-Methylglucamine-modified polystyrene is a boron-specific resin that is often used in IECs to extract boron from water [131].Zhouet al.used the quantum chemistry method to calculate the separation factor for extracting boron isotopes by IEC, which greatly promotes its industrial application prospects[132].
Aidaet al.performed the enrichment of boron isotopes by using eight-column high-pressure ion exchange equipment that used eight glass columns with an inner diameter of 3 cm and a height of 210 cm filled with 180–260 μm Diaion WA-21 resin.The results show that the enrichment of10B can reach 98.43% when the band migration distance is 620 m [133].In this long-distance migration,the balance of yield and cost should be taken into consideration for the large-scale industrial application.
The problem to be solved by HPIEC is how to improve the product yield given the required purity.The first influencing factor is the column performance, which can be reflected by the HETP.When HPIEC is used for separation, the chromatography band velocity will increase several times or even more than one order of magnitude, which usually leads to the increase of HETP in classical IEC.However, the use of uniform particle ion exchanger will accelerate the mass transfer process, then the HETP can be greatly reduced.The second factor is the segment length.For similar interface conditions, an increase in segment length will lead to an increase in yield.This involves the selection of the column cross section in the equipment design, and the last pillar column cross section is the key factor affecting the purity and yield.When the resin particle size, operation flow rate, temperature, and other kinetic factors are determined, the HETP in the steady state is a constant value.The decrease in the cross-sectional area will lead to the extension of the segment length.However, this will increase the operation time, and the section length is not proportional to the yield.When the yield increases to a certain extent, an improper increase will greatly prolong the separation time.Therefore, when designing the equipment, it is necessary to consider the number of plates contained in the cross region of the chromatography band according to the purity requirement and to determine the cross-sectional area of the last column according to the yield requirements.
6.Separation of other nuclides
6.1.Strontium and cesium
There are also recyclable fission elements such as90Sr and137Cs in high-level radioactive waste liquid.The extraction of these elements is of great significance for the final disposal of solid waste and environmental restoration [134–136].Although the adsorption of strontium and cesium by traditional resin has been deeply studied, its adsorption capacity and selectivity still have extensive development space.Recently, inorganic nanomaterials for specific adsorption have developed rapidly.Due to the strong interaction of special functional groups with strontium and cesium [137–139],the efficient column packing material can be developed by fixing functional groups on the carrier, which broadens the selection range of column packing materials (Fig.5).
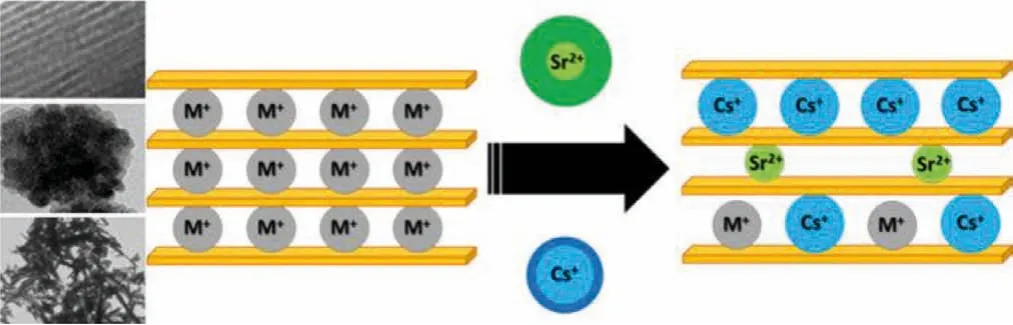
Fig.5.Trapping cesium and strontium from multicomponent aqueous solutions.Copied with permission [140].Copyright 2018, Elsevier ScienceDirect.
A layered microporous thiostannate (FJSM-SnS-4) that had excellent acid base resistance and irradiation resistance was synthesized under mild solvothermal conditions.Impressively, the adsorption capacity for Cs+ions achieved 137.07 mg/g at pH 0.4.With the coexistence of competing ions, FJSM-SnS-4 removed Sr2+rapidly in a neutral environment, and it could retain a high removal efficiency for Cs+ions even in the 1 mol/L HCl solution.This work paved the way for the selective removal of Cs+ions from strongly acidic water environments and the highly selective capture of Sr2+ions under neutral conditions [141].An open framework zinc thiostannate Na5Zn3.5Sn3.5S13·6H2O (ZnSnS-1) exhibited an effective and rapid ion exchange property for Sr2+ions.The maximum exchange capacity for Sr2+was 124.2 mg/g, which ranks ahead of those of all the reported metal sulfide Sr2+adsorbents.Importantly, the Sr2+-laden product could be eluted by 0.2 mol/L KCl solution, the morphology of the cubic exchanger crystals was well-maintained after elution treatment without any cracks or powdering being observed.This inorganic metal sulfide materials showed great potential as ion exchangers for the nuclear waste treatment [142].A 3D uranyl organic framework material was built through polycatenating of three sets of graphene-like layers, which displayed outstanding radiation-resistance and chemical stability in aqueous solutions within a wide pH range.The polycatenated framework structure was completely retained during the ion exchange process and crystallographically disordered cations [(CH3)2NH2]+was well suited for ion exchange of cesium ion [143].These novel materials not only are able to selectively remove cesium from aqueous solutions by retaining the framework structure, but also can provide an inspiration for the column packing in dealing with fission products.An impregnated supramolecular recognition materials (Calix[4]+M)/SiO2-P were modified with tri-n–butyl phosphate to pack chromatography column, which was used for the separation of Cs+ions.According to the mass balance, the recovery percent was calculated to be 99.2% for Cs+.Partitioning experiment by extraction chromatography convinced that this material was of great significance for the efficient and environmental column packing [144].The alginate beads with excellent adsorption capacity and cost effectiveness were used in the column operation mode to recover and enrich Sr2+from seawater.The actual volume of alginate beads in the column module was 0.0565 dm3, the column was desorbed by 0.1 mol/L HCl at a flow rate of 10 mL/min.A highly enriched Sr2+solution of 50 mg/L could be obtained by 3 times repeated column elution process.This column operation may be eligible for industrial scale application and this biosorption process also provides inspiration for the HPIEC separation technology [145].
The special functional groups of the column packing are the main factors affecting their physical and chemical properties.The correct selection of the column packing is the key to the successful application of HPIEC.Column packing can greatly accelerate the mass transfer process and minimize the eddying effect because these factors play important role when determine the HETP.Likewise, it can withstand high pressure without deformation and has a uniform particle size so that the column has good reproducibility.If the object to be separated is radioactive, the packing also needs to have radiation resistance.In addition, the expansion and contraction degree must be small in the transition of the column, and the packing must have an appropriate exchange capacity to adapt to different experimental conditions.
6.2.Technetium
99mTc has become the most widely used medical isotope[146] and is obtained primarily from the decay of its parent nuclide99Mo by a99mTc generator [147] that provides a steady supply of99mTc to areas far from the nuclear reactor.Rogerset al.introduced one clean separation technique, aqueous biphasic extraction chromatography (ABEC), which adapts polyethylene glycol-based aqueous biphasic systems to a solid supported chromatographic mode.Subsequently, this ABEC resin was used for the separation and recovery of pertechnetate from molybdate.The results show that all99MoO42-was eluted from the column by K2CO3,and chromatographic separation afforded 94%99mTc activity.The findings of this study suggest that ABEC resins have good radiation stability and that this simple process could be suitable for use in99mTc generators [148].
Chattopadhyayet al.described a99mTc separation technique with a Dowex-1 column and an alumina column.In this method,both the separation of99MoO42-and the concentration of99mTcO4took place on the same small Dowex-1 column.The typical data showed that the average yield of99mTc can reach 93.8% when the 7.4 GBq activity of99Mo is used [149].The automated modules have been widely used in separation technology [150–153].Morleyet al.described a remotely operated module for the purification of sodium pertechnetate from a bulk solution of molybdate.The separation process was optimized by varying the volume of resin and the composition of the load and elution solutions.The results showed that the separation process was efficient and complete in less than 30 min [154].
To realize the intelligent and automated HPIEC industry, the equipment components requiring periodic replacement, which is based on different targets to be separated, are all standard items available from commercial sources, such as lines, columns, and valves.In addition, the automated purification method should consider the processing of high levels of radioactivity.The remote user-friendly computer system is necessary to control the operation, and the holistic device should be placed in a shielding box to decrease personnel exposure.
7.Conclusion and outlook
Although IEC has been widely used in nuclide separation, it has shown unique advantages in the actual production of rare earth elements and actinides.However, with the rapid development of science and technology, the higher requirement of separation effi-ciency and product quality also put forward new challenges.How to quickly and efficiently obtain the required nuclide products is the key to large-scale production.Compared with IEC, HPIEC have the advantages of a short test period, simple manufacturing technique and easy automation.We can learn from the mature industrial experience in protein and peptide drug separation, which can produce them faster and better to meet the needs of efficient and environmental protection of modern industrial production.This paper briefly reviewed the development and application of HPIEC,and pointed out the promising application prospects in nuclide separation.To achieve the industrial application of HPIEC in nuclide separation and establish a stable and guaranteed nuclide supply system, more efforts and breakthroughs are still needed in the following aspects:
(1) The basic research related to ion exchange must be strengthened and should be combined with practical applications.Lanzhou University already had systematic research and formed the relevant theory for actual production.However, in the current background of green low-carbon transformation and highquality development, it is necessary to take a step forward and propose a set of theories suitable for the current situation of nuclide separation.
(2) The traditional resin has difficulty meeting the current needs of diverse nuclide products, and the emergence of various separation systems provides inspiration for the design of efficient and environmental protection column packing.At the same time,when separating different nuclides in one piece of equipment,modular packing can greatly reduce the cost to meet the demands of large-scale industrial production.
(3) The supporting industrial virtual simulation system is of great significance in moving the nuclide separation craft forward and accelerating the trend toward modern and intelligent industry.The optimization of system design for a specific production can not only meet the separation requirements, but also comprehensively consider the operation and construction cost of a reasonable system.Besides, the optimization of system parameters plays a vital role in high performance separation.The optimal operating conditions are simulated under the existing device through the mathematical calculations of certain models, which takes a series of parameters that affect the separation as variables into account.
(4) The application of industrial automation can collect the data of each link in production in the central control room and then use visual technology to show the characteristics of the data so that operators can carry out real-time monitoring and production control.Meanwhile, future industrial development must involve networking and information management.An excellent system-integrated measurement and control function is necessary, that is, all information processing and related hardware control are completely contained on one platform.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The financial supports from National Natural Science Foundation of China (Nos.22176077, U1730245), Natural Science Foundation of Gansu Province, China (No.20JR10RA615), the fundamental research funds for the central universities (No.lzujbky-2021-sp29)are acknowledged.
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
