Radioanalytical chemistry for nuclear forensics in China: Progress and future perspective
Yi Liu, Xuepeng Sho, Wenting Bu, Zhen Qin, Youyi Ni, Fengcheng Wu,Chuting Yng,*, Xiolin Wng
a Institute of Nuclear Physics and Chemistry, China Academy of Engineering Physics, Mianyang 621000, China
b Institute of Materials, China Academy of Engineering Physics, Mianyang 621000, China
c China Academy of Engineering Physics, Mianyang 621000, China
ABSTRACT A relatively new branch of science - nuclear forensics, aiming at providing the nature, origin, history and possible trafficking route of seized nuclear materials/devices, has been established and rapidly developed over decades to screen illicit nuclear activities.This highly interdisciplinary science is built upon a foundation of analytical chemistry, radiochemistry, nuclear physics, material sciences, geology, and other scientific disciplines, within which radiochemical methodologies and radioanalytical techniques play a key role.The present review provides a brief overview about the crucial aspects of nuclear forensics,including basic content, procedure, concerned elements, common separation, analytical method, and so on.The state of the art and recent progresses of nuclear forensics by research communities in China are reviewed, while selected examples and practical applications are emphasized.The challenges associated with this new area and on-going developments are highlighted and discussed.
Keywords:Illicit nuclear material Nuclear forensics Radioanalytical techniques Mass spectrometry Isotopic composition
1.Introduction
After the disintegration of Former Soviet Union in the early 1990s, nuclear materials theft, smuggling and other illicit activities began to emerge and grow.Nuclear terrorism has become one of the most challenging threats to international security.There is an urgent need to accurately identify and track the seized nuclear or radioactive materials, calling for a methodology of scientific analysis of these materials to obtain their intrinsic characteristics as well as information adherent, which could serve as their distinctive “fingerprints”.In consequence, a relatively new discipline, nuclear forensic science, often referred to as nuclear forensics, came into existence and played an important role in the nuclear security framework.According to International Atomic Energy Agency(IAEA), nuclear forensic science can be defined as “the examination of nuclear or other radioactive materials or of evidence contaminated with radionuclides in the context of international or national law or nuclear security.The analysis of nuclear or other radioactive material seeks to identify what the materials are, how,when, and where the materials were made, and what were their intended uses” [1].This definition also clearly reveals the intention of nuclear forensics: to convey the results of scientific analysis into explicit and understandable interpretation that can provide clues on the intended use, provenance and possible trafficking routes of nuclear and radioactive materials that are outside of regulatory control.Such sourcing practices in nuclear forensics are of benefit to improve the physical protection measures of the involved facilities/transportation routes and prevent future theft, conveyance and disperse of illicit nuclear or radioactive materials, and ultimately serve for the goal of nuclear nonproliferation.
The highly interdisciplinary nuclear forensics is built firmly upon a basis composed of principles and knowledge of many scientific subjects,e.g., material science, analytical chemistry, radiochemistry, nuclear physics, geology, geography, and chronology[2,3].Among all the disciplines, radio-analytical chemistry is of particular significance given that the nuclear forensics samples to be investigated are radioactive and toxic, requiring extremely delicate handling, adequately trained and experienced personnel radiation protection, as well as specifically adapted instruments and analytical methods.As a matter of fact, various investigation techniques, such as physical, morphological, radiological, elemental and isotopic analysis, are either directly borrowed or modified from traditional methods in radioanalytical chemistry and will be briefly described in the following part of this review.
In China, this novel science has also gained close attention and developed rapidly in recent years.Many original investigations,featuring trace or ultra-trace determination of radionuclides (especially actinides and lanthanides), development on mass spectrometric method, have been performed by researchers from diverse institutes and universities such as China Academy of Engineering Physics (CAEP), China Institute of Atomic Energy (CIAE), Northwest Institute of Nuclear Technology, Tsinghua University, Peking University, Lanzhou University, Soochow University.The present review mainly focuses on the contributions to nuclear forensic science by research communities in China over the past few years, especially developed methods and achievements on trace or ultra-trace determination of concerned nuclides (uranium, plutonium, rare earth elements,etc.).Selected examples and practical applications are included as well to illustrate the current state of this discipline in China.
2.Content and analytical techniques of nuclear forensics
A rough scheme of the forensic program of a seized nuclear or radioactive material can be depicted including four steps [2]:
(1) Sampling and distribution;
(2) Traditional forensic analysis,e.g., analysis of fingerprints,saliva, DNA, fibers, hair, soil, painting, wrapping, shielding materials;
(3) Intrinsic forensic analysis,e.g.,uranium/plutonium contents and isotopic ratios, stoichiometry, daughter radionuclides analysis,age-dating, impurity elements analysis;
(4) Data interpretation and source attribution.
The intrinsic forensic analysis part is undoubtedly associated most tightly with the scope of radioanalytical chemistry, aiming at collecting sufficient amounts of characteristic parameters (“signatures”) in order to achieve correct attribution through possibility elimination and iterative comparison with reference database of nuclear materials.
Though IAEA has published a general guide [1] on the analytical techniques and methods in support of nuclear forensics investigation within different time frames (24 h, one week and two months), there is no standard operation procedure or sequential workflow on a specific intercepted nuclear material in a real-world scenario.The choice of analytical methods to be applied to the particular sample depends on analytical purpose (i.e., what kind information and to what extent are needed) and should be complementary to each other in order to fulfill that purpose within the given time frame or deadline.Commonly nuclear forensics practitioners need to adjust the original plan at times according to results of the last batch of tests.
In general, apart from basic physical characterization (size measurement, visual inspection, weigh mensurationetc.), the analytical techniques that are most frequently used in nuclear forensics can be categorized into two groups by their probing mechanisms as shown in Table 1.The first group is composed of radiological methods that detect the characteristic radiation (alpha particles,electrons, neutrons and photons) emitted from the materials.It is also named “passive” analysis [4] as no energy input is added to the samples under investigation: the probing substances are solely generated inside the samples.The other group, “active” analysis,utilizes an adscititious stimulus (e.g.,visible light, X-ray, a beam of electrons, heat, plasma) to reveal the macro- and micro-structures as well as the chemical and elemental compositions of the materials.Many classical physical and chemical characterization techniques that are often used for non-radioactive substances in material science are adopted in nuclear forensics investigation, but those analytical techniques should be modified to adapt to the specific requirements of radioactive matters (proper shielding, glove box operation, venting,etc.) [5] as aforementioned.
Each technique in Table 1 is marked either with non-destructive analysis (NDA) or destructive analysis (DA), telling whether damage and/or modification of the materials occur during sampling,preparation and measurement process.As can be perceived, nondestructive analysis normally comes first in nuclear forensics investigation to preserve the intactness of the samples before destructive analysis is introduced to reveal the internal structures and compositions of nuclear materials [4].In this way the physical and chemical form of the materials, together with characteristic signatures, can be leaved to an extent as great as possible.It is noteworthy that not every “passive” analytical method is non-destructive.For instance, alpha particles are easily trapped or shielded by matters as thin as a piece of paper; therefore in alpha spectrometry, samples are typically measured following destructive dissolution (digestion), chemical separation to isolate the radionuclides of concern, and electro-deposition on a plate in order to obtain “infinitely” thin layer of analytes.
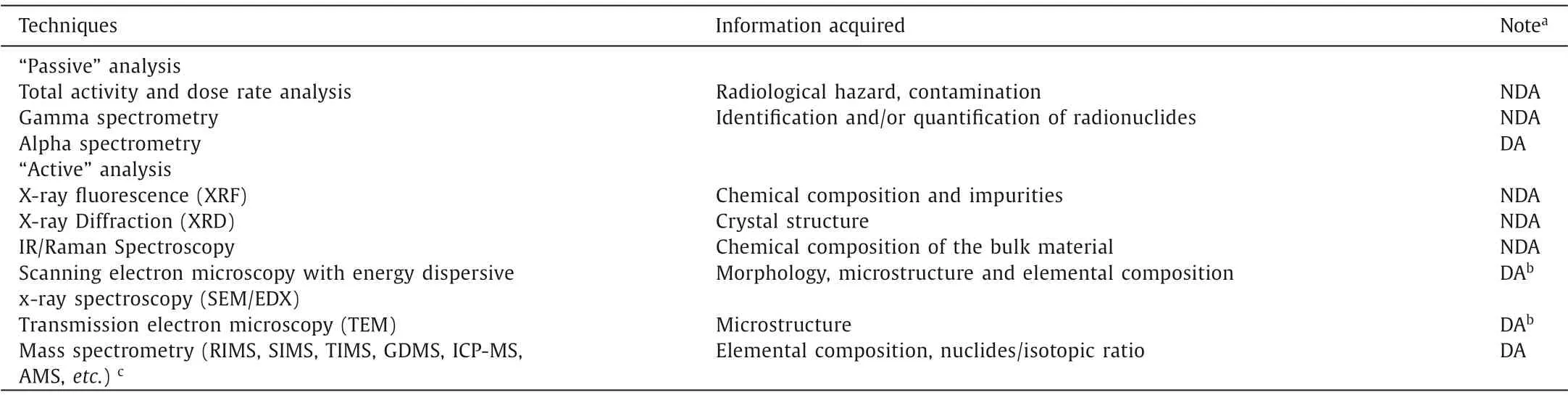
Table 1 Analytical techniques commonly used in nuclear forensics.
The detailed description, principle and application of each technique is beyond the scope of this review and the interested readers are recommended to check other monographs and review papers[2–9] of high quality.Nonetheless, some important conceptions need to be specifically put forward here.Inorganic mass spectrometry (such as ICP-MS and TIMS) are believed to be the most powerful tool for the precise determination of nuclide ratios and nuclides in very low concentrations [9,10], where isotope dilution technique is frequently used.For instance, in the concentration measurement of a specific radionuclide (e.g.,239Pu), a known amount of its isotopic tracer (e.g.,238Pu or242Pu) was pre-added to the sample.After sample preparation, the ratio of239Pu/238Pu or239Pu/242Pu was measured and the concentration of239Pu was herein calculated.
Radiochemical separation, especially for lanthanides and actinides, serves as an indispensable segment before or during some kinds of examination.For example, accurate determination of isotopic ratios by mass spectrometry demands for very pure samples,ideally only containing that particular element to achieve satisfactory quantification.Successful separation of the concerned element from matrix and other interfering elements is commonly a pre-requisite for conducting mass spectrometry measurement with an acceptable precision and can lower limit of detection (LOD)as well.The separation skills and methods, all of which are borrowed from traditional analytical chemistry, usually involve coprecipitation, liquid-liquid extraction, solid-phase extraction, ionexchange, chromatography, electro-deposition, ionic liquid separation, microfluidics, membrane separation, and so on.Combination of these techniques in multi-steps is often required to obtain a high decontamination factor (DF) of interfering elements.
The separation could also contribute to age determination of intercepted materials.Radioactive nuclides as uranium and plutonium are the major elements of concern in nuclear forensics and their self-decay inevitably produces disintegration product,viz.progenies or daughters.The parent/daughter or parent/granddaughter ratio is a suitable indicator (also called radiochronometer) for the determination of the production date of the nuclear material,i.e., the elapsed time since the last chemical processing/separation took place (“time zero”)[3].233U/229Th,234U/230Th,235U/231Pa,238Pu/234U,239Pu/235U,240Pu/236U,241Pu/241Am,242Pu/238U are the most common radiochronometers used for age dating, based on radioactive decay equations of the pair and a series of assumptions [11–13].This process usually requires a complete separation of the progeny isotopes(present at trace-level) from the massive radioactive parent [7].As can been seen from the radiochronometer pairs, in most cases the parent and daughter both belong to actinides that possess quite similar chemical properties.The daughter could also be radioactive therefore the mixture of parents, daughters, granddaughters (actinides, lanthanides or stable isotopes) and impurities introducedby ore mining or processing add more complicity to the system to be analyzed.The chemical separation must be carefully done as incomplete separation could lead to a fundamentally wrong deduction of the calculated model age.
Though sheer abundance of literature discussing the separation methods of lanthanides and actinides can be found publicly, nuclear forensics practitioners still need to deliberately choose the proper one with caution as some novel methods, though with very pretty separation factors, may not be suitable for the analytes in nuclear forensic or only applicable under some specific circumstances.For instance, in researches dealing with environmental monitoring, trace or ultra-trace radionuclides are to be separated,enriched and detected.Meanwhile, those radionuclides might be overwhelmingly abundant as compared to other elements of interest (stable impurity elements, rare earth elements,etc.) in nuclear forensics investigations, therefore distinct separation strategy and quality controls are demanded.
3.Radioanalytical method developments for nuclear forensics in China
3.1.Analytical methods for major components
The analysis of major components of intercepted nuclear materials, uranium and/or plutonium, is an irreplaceable part of a typical nuclear forensic investigation.Numerous methods for the chemical separation of uranium/plutonium from other elements have been developed, each bearing advantages and disadvantages in various aspects.Though most of these methods were initially designed to serve the purpose of advanced nuclear fuel cycle or decontamination, some feasible ones could be borrowed and modified in nuclear forensics investigation.For instance, Zhanget al.[14] prepared a phosphorodiamidate-functionalized silica material SBA-15-O-DMAP with a high Th(IV) sorption capacity, rapid sorption kinetics and excellent selectivity.Its thorium/uranium separation performance in highly acidic solutions is proved to be one of the best reported so far.Jianget al.[15] demonstrated that graphene oxide (GO) can selectively bind Th(IV) over U(VI) and such a selectivity has high potential for Th(IV)/U(VI) separation.Huanget al.[16] studied a series of novelO-phenoxydiamide, and found the binding affinity of Pu(IV) with BenzoDODA/n-dodecane is much larger than those of other metal ions (U(VI), Zr(IV), Eu(III),Am(III)) hence can be used for recovering Pu(IV) selectively from highly acidic aqueous solution.
TIMS is regarded as one of the most powerful techniques for actinides isotopic determination, but often suffers from a relatively low sensitivity in ultra-trace measurements due to low ionization efficiency [17].Qin group of CAEP has developed a simple and fast filament carburization technique for TIMS [18] with grapheme oxide (GO) as the ionization enhancer to get a high and steady ionization efficiency of ~0.2% for uranium in single-filament mode (10 times that of the traditional double-filament method, Fig.1).The GO-based technique not only simplified the carburization procedure (operation time shortened from several hours to only 10 min),but also showed good accuracy, repeatability and high sensitivity with certified reference materials (the relative uncertainties for235U/238U as low as 1%), revealing its great potential for rapid analysis of samples in nuclear forensics.
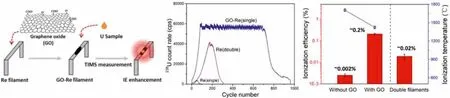
Fig.1.The GO-based filament carburization and sample loading method developed by Qin et al. for isotopic ratio analysis of uranium at trace level.Copied with permission[18].Copyright 2019, American Chemical Society.
The isotopic composition of plutonium is directly related to the neutron capture of uranium in a reactor, and varies drastically with different initial235U enrichment, neutron energy spectrum and neutron flux [3].Therefore, the study of plutonium isotopic composition contributes valuable information to specify the reactor type, and more specifically, deduce the possible location of the reactor in which the plutonium material was produced [19,20].For the analysis of plutonium by mass spectrometry, a major concern is to reduce polyatomic interferences such as uranium hydrides(238UH+and238UH2+) especially for samples with high U and low Pu concentrations [21].Buet al.spend efforts on this issue and constructed an anion-exchange chromatographic separation procedure that possessed a decontamination factor (DF) for uranium of 4×106and a chemical yield of plutonium of ~65% [22].The suffi-cient separation of Pu and U could provide a final sample solution with238U concentrations of<2 pg/mL for SF-ICP-MS measurement,thus no extra correction of238U interferences was needed.Though only environmental samples (North Pacific marine sediment collected 50 km off the Fukushima Dai-ichi Nuclear Power Plant site and soil core samples 400 km from Lop Nor, the Chinese nuclear test site) were tested for Pu isotopes, the developed method holds an excellent capability for Pu source identification in nuclear forensics.Another contribution on the rapid analysis of Pu isotopes by Men and coworkers [23] presents a separation strategy comprising Pu valence adjustment, Fe(OH)2primary co-precipitation,CaF2/LaF3secondary co-precipitation and TEVA+UTEVA+DGA extraction chromatography.This method could achieve a pretty high DF for U (~107) and Pu recovery (~83%), with LODs for239Pu and240Pu 0.08 fg/mL by SF-ICP-MS, beneficial for Pu determination in a short period of time (12 h).Even higher Pu recoveries(90%-97% for soil; 92%–98% for sediment) and the same level of DF of U (1.6×107) can be accomplished with modified method by Wanget al.[24], allowing for the rapid determination of ultra-trace level Pu in nuclear samples.The introduction of a new type of ICPMS with two quadrupole mass filters and a collision-reaction cell(triple quadrupole inductively coupled plasma mass spectrometry,ICP-MS/MS) shows high interference removal capability [25] in the work of Hu group.With a quite simple sample treatment (one stage of TK200 resin chromatographic separation) and high effi-ciency sample introduction system, the absolute LODs for239Pu and240Pu could reach as low as 0.13 fg (0.0003 mBq) and 0.08 fg (0.0007 mBq), respectively.
3.2.Analytical methods for trace elemental impurities
Rare-earth elements (REEs) are typically concomitant with uranium ores, and the species and concentrations of REEs in different types of uranium ore vary significantly [26].Since the chemical properties of REEs are quite similar, the REE pattern (i.e., the elemental distribution and relative concentration) hardly changes during the mining and milling processes [27] and can provide valuable information on the geographical and geological as well as of the uranium ore (UO) or uranium ore concentrate (UOC) [28,29].Dinget al.[30] developed a method for the determination of REEs in uranium ore samples by triple quadrupole inductively coupled plasma mass spectrometry (ICP-MS/MS) with a limit of detection down to 1 pg/mL for all REEs.The simple and reliable chemical procedure, using only one UTEVA resin column, ensured a thorough separation of REEs from the uranium matrix.The polyatomic interference was greatly suppressed by the introduction of O2as a reaction gas and the application of the mass-shift measurement strategy for the measurement of REE oxides.
The mass spectrometry is able to detect multiple REEs at the same time, meanwhile the separation and enrichment of a single REE can be helpful for a more comprehensive assessment with even lower detection limit by excluding the interferences of other REEs in the measurement of specific one.Wang group of Soochow University have studied REE separations by selective crystallization of borates [31].It was discovered that subtle bonding differences across lanthanide series could be amplified by the polyborates during crystallization in molten boric acid, and six distinct phases under identical reaction conditions were produced (La, Ce~Nd, Sm,Eu~Gd, Tb, Dy~Lu).The separation between REEs forming different phases,e.g., Nd/Sm and Nd/Dy, could reach the same level as or superior to that of most solvent extraction processes, but in an environment-friendly way.Even for those lanthanides forming the same structure type, the separation efficiency could be enhanced by controlling the reaction kinetics, featuring an efficient and costeffective strategy for rare earth separationviaselective crystallization.
Nd is present at trace levels in natural uranium-based nuclear materials derived from the uranium ores, thus the amount of Nd available for analysis could be as small as several nanograms if the nuclear materials are extremely limited.Another demonstration on the advance of trace REE separation is brought out by Chenget al.[32].In this study a capillary electrophoresis system coupled with a unique detection unit, in-column fiber-optic laser-induced fluorescence detection, was developed and employed firstly for the rapid separation and quantification of Nd under a dynamic ternary complexation separation mode.It was observed that the separation behavior of the assembled CE system could be tuned by buffer concentration, buffer pH as well as separation voltage.
Under optimal conditions, Nd could be effectively separated from its neighboring lanthanides (praseodymium and samarium) in a short period of several minutes with a limit of detection at ppt level (Fig.2).The separation efficiency (electrophoretic resolution)and detection sensitivity (response signal) of this ICFO-LIF-CE system were comparable to or somewhat better than those obtained in the previous traditional CE systems, showing a promising application in nuclear forensics investigation.
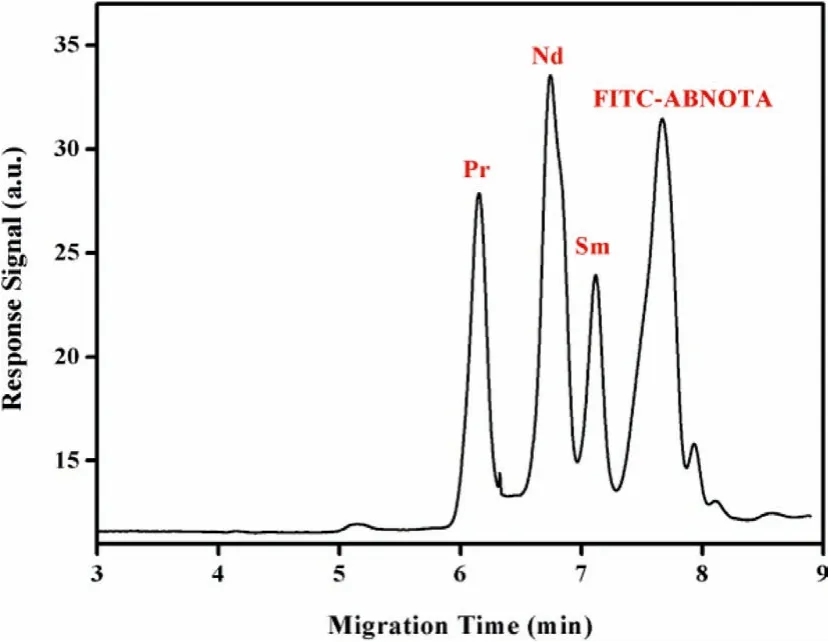
Fig.2.Typical electropherogram of Pr, Nd and Sm-based complexes under optimal separation conditions.Copied with permission [32].Copyright 2016, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim.
Moreover, the variation in143Nd/144Nd ratio is extremely subtle, thus the measurement of143Nd/144Nd isotopic ratio with high sensitivity and precision is required.Shaoet al.[33] developed a method for the Nd isotope analysis as Nd+species with the newlydeveloped film porous ion emitter (FPIE) by TIMS.Compared to previously reported measurement method, the ion yields of Nd+showed remarkable improvement by about 10 times and high precision (3.5×10-5) was achieved for samples containing only 1 ng Nd (Fig.3).Besides Nd, the138La–138Ce isotope system has also been regarded as a useful radiogenic tracer for nuclear forensics studies.Shaoet al.[34] also developed a novel method based on FPIE for high-precision measurement of Ce isotopic ratios by TIMS as Ce+, and the reproducibility of Ce isotopic ratios obtained wasca.10-fold better with the employment of TaF5as an activator and the combinations of diverse amplifiers.
In addition, domestic researchers have studied various ionization enhancement mechanisms of mass spectrometry to improve the ionization efficiency and analytical sensitivity of trace nuclear forensic samples [18,35,36].
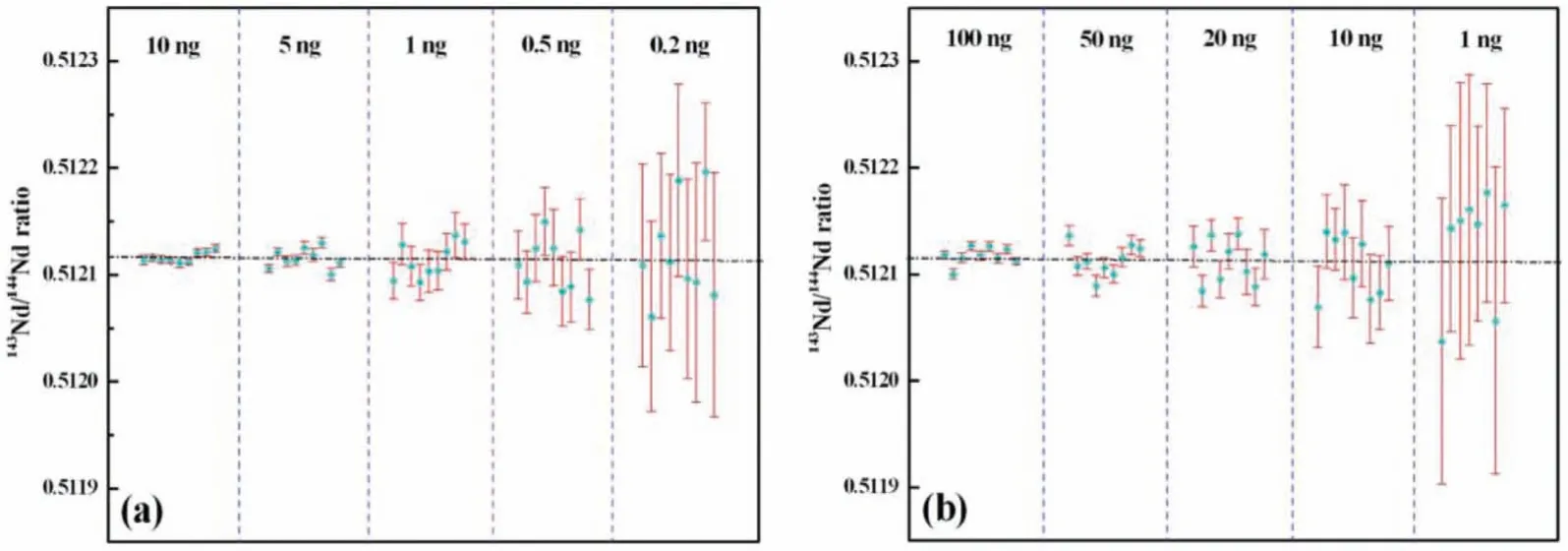
Fig.3.The precision for measurement of Nd isotopic ratios with FPIEs (a) and traditional double Re filaments (b) as a function of analyte sample sizes.Dashed horizontal lines represent the reference value of 143Nd/144Nd ratio.Copied with permission [33].Copyright 2019, Elsevier.
Analysis of trace impurities (other than REEs) in uranium materials is crucial for quality control in the nuclear industry and also informative for nuclear forensics.As aforementioned, the major difficulty associated with the mass spectrometry measurement is the isobaric interferences of interested nuclides and other nuclides/polyatomic species of the same mass [9].This interference could be suppressed or resolved with the improvement of instrumentation, such as the introduction of double-focusing sector filed and collision cell [22,37] as well as making use of powerful separation techniques prior to analysis, as discussed above.To detect trace impurities, the massive uranium matrix should be separated or removed to avoid the matrix effect [38] and increase sensitivity of ICP-MS [39], usually achieved by offline separation methods.Niet al.[40] proposed a rapid and highly efficient method for accurate determination of 15 impurity elements (Al, Ti, Cr, Mn, Fe,Ni, Zn, Zr, Nb, Mo, Ru, Sn, Sb, Pb, Th) in uranium materials by combining chemical separation with matrix matched external calibration measurement of ICP-MS/MS.Most of the chemical recoveries of impurities were more than 95%, and LODs ranged from ng/g to μg/g level.In the meanwhile, one should not overlook the virtue of the online separation-detection,i.e., coupling those separation techniques directly with mass spectrometry measurement (also referred to as “hyphenated” techniques) [41–45].Zhang and colleagues, on the other hand, designed an installation utilizing microfluidics coupled with ICP-MS for the online measurement of 25 impurities (at sub mg/g U level) in uranium samples[46].In consideration of reducing the amount of radioactive wastes and lowering the risk of exposure to the radioactive materials, the researchers adopted the setup of miniaturization of liquid-liquid extraction in the microchannels as Fig.4 depicted, where microextraction of matrix uranium occurred in parallel two-layer laminar flow (PTL) microfluidics and the target impurities remaining in the aqueous phase are directly and simultaneously analyzed by ICP-MS.
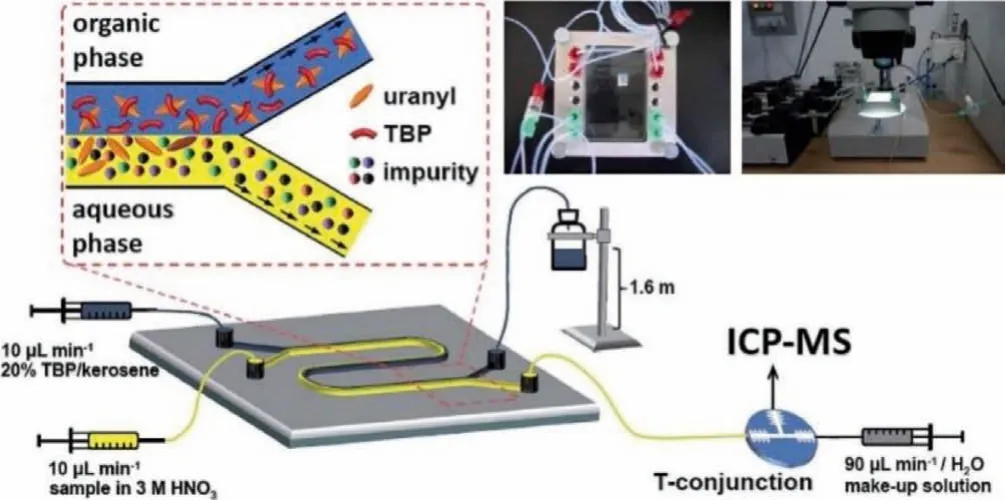
Fig.4.The setup of the PTL-ICP-MS hyphenated technique for online trace impurities determination by Zhang et al. Copied with permission [46].Copyright 2016, the Royal Society of Chemistry.
Special attention was paid to adjust the acidity and flow rate of the aqueous phase in PTL to be compatible with ICP-MS by introducing the make-up solutionviathe T-conjunction.The efficiency of TBP-based PTL micro-extraction systems was proved to be satisfactory (up to 95.5% of the matrix uranium was removed and the target impurities had little loss).The analytical performance of the PTL-ICP-MS device was assessed and a good reproducibility of the measurements with the precision ranged from 1.4% to 4.5% for all the elements was found.This method was further validated with a certified reference material U3O8with known amounts of selected impurities, and the applicability on more radioactive samples such as plutonium and other minor actinides could be postulated.
3.3.Age determination of nuclear materials
Uranium age has been used to deduce the mineralization time of ore long time ago in geology [47], and its age is thousands or even millions of years.However, the age of uranium in nuclear field is used to characterize the production/purification time of uranium materials, hence relatively “young”.Given the long halflife of uranium isotopes, “young” uranium material samples have a rather high parent/daughter ratio, so analyzing the age of uranium samples by radiochronometers is of no ease.The accuracy of“model ages” determined by ratio of chronometers relies heavily, if not solely, upon precise isotopes measurements in the developed isotopic dilution mass spectrometric methods, therefore the concentration of the specific spike (isotope tracer) needs to be accurately measured.The calibration methods for isotope spike229Th,which could be used for uranium age dating by234U/230Th radiochronometer, was studied by Chen and colleagues [48].The primary229Th solution, prepared by separating Th from an old233U solution, was calibrated by reverse isotope dilution ICP-MS measurement with other isotope standards,230Th and232Th.The authors found the combined uncertainty of average spike concentration calibrated by230Th was larger than that calibrated by232Th.So rather than uncommon230Th standard material, easily available232Th standard material was preferable for calibration of229Th spike.Uranium oxide reference material CRM 850 (recorded purification date as 1957–12–31) [49] was employed to carry out the age determination using the229Th spike calibrated by230Th or232Th.The calculated results (1956–2–18 ± 301 days for spike calibrated by230Th; 1957–4–15 ± 209 days for spike calibrated by232Th)again supported the analysis of methodology uncertainty.
Among all the common radiochronometer pairs,235U/231Pa is an interesting one as the actual decay chain is235U–231Th–231Pa,a parent-daughter-granddaughter decay with the half-life of the daughter231Th (25.5 h) much smaller than those of the parent235U (7.04 × 108a) and granddaughter231Pa (3.28 × 104a) [50].Accordingly,231Pa can accumulate to a detectable level in uranium samples aged several years with a precondition that measuring instrument has both wide dynamic range and high sensitivity (235U/231Pa ratio 107~109,235U and231Pa must be measured individually).Another difficulty of this method lies in the quest of a suitable Pa isotopic spike since the longest living Pa isotope other than231Pa is233Pa with a half-life of 26.97 days [50,51]and no commercially available233Pa isotopic spike/reference materials could be obtained.Huanget al.separated the spike of233Pa from237Np after twice TTA extraction and back-extraction, before calculating the235U/231Pa ratio with isotope dilution mass spectrometry method [52].The relative error of the determined age of a high enriched uranium (HEU) sample is 1.97%.Chenet al.also reported a chemical purification procedure of recovering233Pa from an aged237Np stock solution [53], using TRU columns and silica gel columns several times with a Pa/Np separation factor reaching 107and an overall yield of 92%.The model ages of two uranium standard samples, CRM U010 and IRMM-1000b, were determined to verify the effectiveness of the method.For CRM U010, the calculated235U/231Pa model date agreed well with the known purification date and another model date calculated by234U/230Th.However, an older model date outside of the uncertainty range was found for IRMM-1000b, indicating an incomplete of Pa removal in the production process of the uranium standard sample.This again reminds us that nuclear forensics investigators should remain highly cautious and better not use a single model age to judge the production date when the production process is not clear.And this is also the motivation that Xu and coworkers conducted comparative diagnosis of the plutonium age with radiochronometers from four decay chains (238Pu/234U,239Pu/235U,240Pu/236U,241Pu/241Am) [54].
Besides mass spectrometry, radiometric measurement is proved to be useful and complementary to conduct age determination of nuclear materials.For instance, Zhanget al.developed a method [55] combining alpha spectrometry with thermal ionization mass spectrometry to the age dating of plutonium material using241Pu/241Am as the radiochronometer.In this method, the original sample solution was adjusted to a suitable concentration for the measurement of alpha activity ratio (241Am +238Pu)/(239Pu+240Pu).241Pu and241Am would interfere each other during the mass spectrometry analysis, so the completely separation of Pu and Am was the key step, where Dowex 1×2 ion-exchange resin was used with a decontamination factor of241Am reaching 4.57×103.The purified solution was taken out for the measurement of activity ratio238Pu/(239Pu +240Pu) by alpha spectrometry,isotopic ratios241Pu/239Pu and240Pu/239Pu by TIMS respectively.The age of a plutonium material could thus be obtained by correlating these four relative ratios with decay constants of241Pu and241Am.The advantage of this method is that the atomic numbers of241Am and241Pu do not need to be quantified by isotope dilution method, hence omitting tedious calculation and lowering the uncertainty associated.The method was validated using three plutonium sources of known age and the results obtained were all in good agreement with the last purification date, with an ideal accuracy ~0.4% for the oldest sample.The researchers also performed an evaluation of the ‘minimal’age reachable by this method with only 500 ng samples, and proved its ability to distinguish freshly prepared plutonium materials from the ‘old’ones, in sharp contrast to the non-destructive gamma spectrometry method which typically needs at least 100 mg plutonium for measurement [56].
Since236U and241Am in plutonium samples are much less affected by the natural background than234U and235U, the atomic ratios of240Pu/236U and241Pu/241Am are more frequently used to determine the age of plutonium.Different measuring methods of these two pairs for the age determination of nanogram-sized plutonium samples were tested and compared by Chen and coworkers[57].The separation of U, Pu and Am was achieved by TEVA and TRU chromatography columns, with the addition of233U,242Pu and243Am as spikes.The decontamination factor was>250 for Pu in Am fraction and>1000 for Am in Pu fraction and were both adequate for the subsequent quantitative analysis.A plutonium sample with known age was determined by three methods, including isotope dilution multicollector inductive coupled plasma mass spectrometry (ID-MC-ICP-MS), ID-α-spectrometry combined with MCICP-MS (α-ICP-MS) and ID-α-spectrometry combined with liquid scintillator counting (α-LSC), all in good agreement with the reference value.To take a closer look, the second method gave the most accurate result (~3%), which could be explained as the assessment of241Am was done byα-spectrometry, therefore the interference of241Pu on241Am and the correction of Am by uranium standard during the MC-ICP-MS measurement were avoided.The third method, though with a ~10% negative deviation from the reference age which was considered to be mainly from the241Pu measurement of LSC, could assist in a quick and low-cost measurement in labs not equipped with ICP-MS.
Delayed neutron counting (DNC) can serve as a powerful tool to determine fissile nuclides such as233U,235U and239Pu hence feasible for certain nuclear forensics application.Researchers in CIAE applied this method for quantitative determination of235U/238U,235U,239Pu in samples of natural uranium, enriched uranium, plutonium and235U-239Pu mixture after sample irradiation in Miniature Neutron Source Reactor (MNSR) [58,59].It was found that DNC combined with neutron activation analysis (NAA) was suitable for a fast determination of235U/238U ratio with a relative deviation ~5% since the half-lives of delayed neutrons and239U were both short.The established quantification method by standard decay curves of delayed neutrons from235U and239Pu fission products could reduce the uncertainty compared with the traditional delayed neutron determination and DNC is capable of simultaneously determination of235U and239Pu in U-Pu mixture samples with a precision of about 10% in MNSR.It was expected that higher irradiation neutron flux, as new DNC facilities at China Advance Research Reactor (CARR), could further increase the accuracy for fissile nuclides determination and eventually aid in the age dating of nuclear materials in nuclear forensics [59].
4.Application of methods for various nuclear material related samples
At present, the applicable objects of the developed methods within nuclear forensics field are basically nuclear materials at different stages of the nuclear fuel cycle, mainly including uranium ore (UO), uranium ore concentrate (UOC), nuclear fuel and spent nuclear fuel,etc.
4.1.UO and UOC
The uranium ore at the forefront of the nuclear fuel cycle carries a lot of geological and geographical characteristic information.After entering the cycle, some of the geological and geographical characteristic attributes in the original ore are gradually reduced or removed, in the meantime, the technological characteristic attributes introduced in the process are increasing, and these two kinds of attributes are mixed to form new features.Therefore, the geographical traceability research of nuclear forensics mainly focuses on the front end of the nuclear fuel cycle.UOC is transformed from mined uranium ore which is crushed and extracted by leaching, then separated and purified by extraction or ion exchange.Modern UOC contains approximately 60%-80% uranium,whose chemical forms are various, mainly including ammonium diuranate, sodium diuranate, uranyl hydroxide, uranyl peroxide and triuranium octaoxide (U3O8) [60].Compared with the nuclear materials in the middle and later stages of the nuclear fuel cycle,UOC is relatively insensitive, and commonly traded on the international market due to the high uranium content and convenient transportation.If by any chance obtained by terrorists, UOC can be converted into various levels of nuclear materials through purification and enrichment, and even used for terrorist attacks or to create social panic, bringing potential risks to international nuclear security and nuclear safeguards.UOC is the material that carries the most geographic characteristics besides uranium ore, so it has become a hot sample for nuclear forensics research [61].In theory, UOC has many geographic attributes that can be used to trace the origin of its uranium ore, but many attributes cannot function in the absence of a complete database.At present, the characteristic attributes that can be used for origin assessment of UOC samples mainly include uranium isotopes, REE distribution patterns,contents and isotopes of other characteristic impurity.Based on the research achievements in recent years, Jianget al.summarized the developments of analytical technology and data processing in UOC’s nuclear forensic geographic traceability analysis [62].Zhao and colleagues [63] used AMS to analyze 307 UOC samples produced by 20 manufacturers and UOC standard samples under development, and studied the nuclear forensic fingerprint nuclides236U/238U,187Os/188Os, of which187Os/188Os was proposed as a new fingerprint feature for the first time.
Due to the strict control of UOC in China, most domestic studies choose uranium ore instead since the REE pattern hardly changes during the milling processes [27,64], Dinget al.[30] also utilized their developed method (Refer to Part 3.1 of this review) to investigate a series of uranium ore samples and found the REE distribution patterns in the same type of uranium ore sample were basically consistent, while those in different types varied significantly(Fig.5), further validating that REE distribution patterns could be used as fingerprints for source identification of related nuclear materials for nuclear forensics purposes.
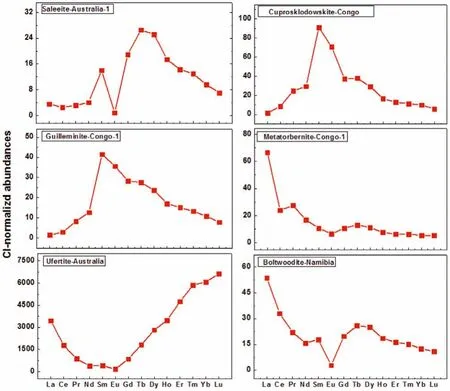
Fig.5.The chondrite-normalized abundances of REEs in uranium ore samples from different mines.Redrawn with permission [30].Copyright 2021, Royal Society of Chemistry.
Linet al.constructed a comparison database on REE signatures by gathering published REE data of 116 uranium ores from 22 deposits in 10 countries [65,66], then transformed the data by a two-step pretreatment so that they could show stronger geographical and geological indications.The researchers tried several multidimensional statistical techniques and found iterative orthogonal partial least squares discriminant analysis (OPLS-DA) worked best in pattern recognition, and the discrimination orders formed a decision tree (an attribution strategy) by which samples from 10 different countries were totally distinguished within several iterations[66].Deposit or uranium deposit type of an UOC unknown sample might also be identified by analyzing samples from that particular country with the help of iterative OPLS-DA.
Besides REE pattern, the isotopic compositions of REEs are recently recognized to be a potential signature for tracing the origin of nuclear-related materials as they are less prone to be affected by metallurgical processes, especially for some primary products of the nuclear fuel cycle [64,67,68].Zhou [69] measured the Nd isotope in seven actual UOC samples by multiple collectors inductively coupled plasma mass spectrometry (MC-ICP-MS), and traced the origin of the UOC samples preliminarily.The results verify that Nd isotope can provide an effective supplementary information in nuclear forensics.
Shaoet al.[33,34] applied their developed measurement method for Nd and Ce isotopic ratios in uranium ore samples of world-wide origin.The results revealed that the obtained analytical precision successfully met the requirements for sample source identification.Based on the obtained REE contents and Nd, Ce isotope information in the uranium ore samples, the researchers[70] utilized multidimensional statistical methods such as cluster analysis (CA), principal component analysis (PCA), principal component-cluster analysis (PCA-CA) and linear discriminant analysis (LDA) to carry out geographical traceability research on uranium ore samples from different sources.The results show that these statistical methods behaved well in accuracy and reliability.Furthermore, compared to using only REE contents as the input,the sourcing efficiency significantly improved when combining the REE contents with Nd-Ce isotope composition.
4.2.Nuclear fuel
With the development of nuclear power industry, uranium pellets, being used as fuel for nuclear reactors, have become one of the potential objects of illegal smuggling.The manufacture of uranium pellets has to go through multiple production processes, such as uranium extraction, conversion and enrichment, and most of the characteristic attributes inherited from uranium ore have disappeared [71].However, subsequent pellet production introduces new impurity elements, including those impurities from chemical reagents, containers and utensils used, and additives added according to process requirements, such as pore formers, lubricants [72].These can be used as the new characteristic fingerprint information of the uranium pellets in nuclear forensics; therefore, the focus of uranium pellet forensics lies on the traceability of the production process.
Theoretically, the uranium pellet production process can be traced based on the impurity content or composition, but the actual implementation is very difficult.The sources of impurity elements are diverse and complex, and their contents vary with the process used, and even the impurity contents in different batches of pellets can be different [73].In order to use fingerprint information of impurity elements in uranium pellets, it is necessary to find the characteristic impurity elements that are related to the process the most closely and compare with the pellet database established in advance.
Jianget al.[71] made an important attempt to trace the source and process conditions of real uranium pellets.During the investigation, non-destructive and destructive analysis methods and the sequence were gradually adjusted according to the results of the previous analysis.The attributes were divided into characteristic fingerprint information and supplementary information.They believed that it was necessary to comprehensively analyze multiple characteristic fingerprint information to complete the sourcing,rather than relying on a single characteristic fingerprint.In their process of traceability analysis, pellet diameter,235U abundance and uranium age were recognized as important characteristic fingerprint information.The traceability result will be more reliable with more supplementary information.
In the process of nuclear fuel cycle, oxygen isotopes are involved in each stage.According to geological research, the content of natural oxygen isotopes in different regions varies by about 1%to 5% [74,75], hence oxygen isotopes can be regarded as an important characteristic fingerprint information to track the source of primary uranium products or raw uranium mineral products.The classic method for measuring oxygen isotope is the oxidation method, which requires a large sample volume and a complicated analysis process.Wanget al.[76] established a TIMS method for direct determination of the oxygen isotopic ratio in uranium oxide powders (1 mg), and the precision of18O/16O ratio was improved to 0.28% by eliminating the influence of oxygen in the environment and optimizing the measurement parameters of mass spectrometer.SIMS has the advantages of small sample volume, simple process, fast measurement speed, and microanalysis.Therefore, related researches have been carried out in many laboratories.Wanget al.[77] developed the method to analyze the oxygen isotopic ratio in uranium oxide by SIMS, and the relative standard deviation (RSD)is better than 1.6%, which could fulfill the traceability requirements for uranium oxide in nuclear forensics.
4.3.Spent nuclear fuel
A main threat to nuclear and radiological security is “dirty bomb”: a conventional explosive device filled with radioactive substances, and the most potential filled material is spent nuclear fuel[78,79].Suet al.[80] did the scene analysis of post-detonation nuclear forensics, mainly for the damage effects analysis and risk evaluation of nuclear or radioactive explosion devices, hoping to provide support to post-detonation nuclear forensics events that might happen.
Many of the previous studies have been trying to find suitable signatures of spent nuclear fuel nuclides or nuclide ratios to characterize the source of the unknown spent nuclear fuel samples, including the reactor type, initial enrichment of fuel, burn-up and cooling time.Unluckily there seems to be no notable success because the difference is hard to be measured in real situations to give a high confidence conclusion.However, with the promotion of the establishment of nuclear forensic database by IAEA, the research on how to characterize the source information of unknown nuclear materials by multivariate analysis based on database has become increasingly active, including research on spent nuclear fuel [81–83].Zhanget al.[84] developed a numerical method to simulate the nuclide components of spent fuel, and improved capabilities of the MCCOOR code for simulation of the nuclide componentsvs.the cooling-time.Suet al.[80] studied the application of multivariate statistical analysis on the identification of spent nuclear fuel.They selected and analyzed three different multivariate methods (factor analysis, discriminant analysis and regression analysis) for different identification needs.They also found that different groups of nuclides could be selected for different identification needs, so as to optimize the identification scheme of spent nuclear fuel.Based on the multivariate analysis of the real spent nuclear fuel database SFCOMPO 2.0 with the combination of the data from numerical simulation, it can meet the requirements of the current nuclear forensics scene.Finally, Suet al.[85] gave an evaluation on the feasibility of the identification of spent nuclear fuel with multivariate analysis.
4.4.Wipe samples (particles)
During the operation of nuclear facilities, some μm-level particles are released inevitably, and settle on the surface of facilities and equipment in a large area.These particles are collected through surface wiping sampling and analyzed by highly sensitive analytical equipment to obtain their morphologies, U/Pu isotopic ratios, ages, and so forth.These data are of importance in analyzing the technological process of nuclear facilities and the properties of nuclear materials, further to determine whether there are undeclared nuclear activities and nuclear materials.Therefore, IAEA has adopted wipe sample particle analysis as a powerful technical means in international nuclear safeguards verification [86,87].
HEU and Pu are directly used materials defined by IAEA, which are strictly controlled for nuclear safeguards, and also the main objects of particle analysis in nuclear forensics.The difference between particle analysis and other analytical methods is that there is a large amount of dust in the wipe sample (particles).How to identify and locate HEU or Pu particles from abundant dust particles is the first problem to be solved in particle analysis.Luoet al.[88] established a combined method of ultrasonic oscillation and ashing to recover particles, whose recovery rate could be more than 90% while agglomerations were significantly reduced.Shenet al.[89] described a method of making reference marks on 25 mm diameter graphite planchet using nuclear track membrane and the average deviation of relocation is 5.8±2.6 μm with the new method.An advanced method of identifying uranium-bearing particles through fission track was developed by Yang and coworkers [90].This method can result in the one-to-one correspondence between the fission track and uranium-bearing particles, helpful in reducing the loss of particles for analysis.
At present, there are four methods that can be used for particle identification: fission track method (FT), scanning electron microscopy (SEM), secondary ion mass spectrometry (SIMS) andαtrack method.Through comparison, it can be seen that SIMS can not only identify particles, but also roughly measure the abundance of the elements of interest in the particles, providing a reference for subsequent particle analysis to accurately analyze the particles of interest.SEM can also quickly identify particles and give the element composition of the particles, but it cannot give the abundance of the elements of interest in the particles.Moreover, the particles need to be transferred with a micromanipulator to facilitate subsequent analysis, but there is a risk of particle loss.FT made use of thermal neutron fission to find particles, so reactor irradiation is required, however, the disadvantage of this method is that it cannot distinguish between U-containing particles and Pu-containing particles, and the operation process is relatively complicated.Theα-track method needs no reactor irradiation, and the process is simple, but it can only distinguish between HEU and Pu particles.The combination of FT andα-track method can not only classify particles, but also has the possibility of identifying weapon-grade particles [91–95].
Variation of the isotope signatures in particles would help in identification and source attribution of different uranium materials.Wanget al.[96] established an analytical method for direct analysis of235U/238U ratio in uranium particles by laser ablation multiple-collector inductively coupled plasma mass spectrometry(LA-MC-ICP-MS), and performed analysis of particles smaller than 10 μm with the new method.The isotopic fingerprints of uranium are of particular interest for nuclear forensics and nuclear safeguards, especially the need for lower detection uncertainties of minor isotopes, including234U and236U.Wanget al.[97] developed a faraday-ground-connect method to measure the minor isotopic ratios, such as234U/235U,233U/235U and233U/234U by faraday cups.The electric current produced by238U+in the faraday cup was conducted to the ground potential before arriving the preamplifier.The relative uncertainty for234U/235U could be smaller than 0.020% for the uranium sample solution (21 μg/g) under magnet-scan mode.The researchers also developed an analytical method for direct determination of240Pu/239Pu ratio in plutoniumcontaining particles by LA-MC-ICP-MS [98].The time for determining239Pu/240Pu ratio in single plutonium particle was shorted to 0.5 h, the measured value deviated by less than 4.7% from the reference value, and the relative uncertainties for239Pu/240Pu was less than 1.4% (n=6), The results demonstrated that this technique was rapid, precise and accurate for determination of239Pu/240Pu in plutonium-containing particles.
High-sensitivity uranium isotope analysis method can effectively reduce the amount of samples used, so it is conducive to the preservation of evidence and technical comparison, and is expected to provide technical support for domestic nuclear safeguards and nuclear forensics.Wanget al.[99] determined uranium isotopic ratios and230Th/234U in single uranium particle without chemical separation procedure by MC-ICP-MS.The relative expanded uncertainties for235U/238U,234U/235U,236U/235U and230Th/234U in CRM124–4, GBW04234 and GBW04238 could be better than 3.6%,3.5%, 3.8% and 15% (k=2), respectively.The results agreed with the certificated values within uncertainty range.As a rapidin-situanalytical technique, laser ionization mass spectrometry (LIMS) is applicable in uranium isotopic ratio measurement of both bulk material and individual particles.Wanget al.[100] determined the uranium isotopic ratio of single particles with diameters of about 50 μm by SEM combined with LIMS.According to their research,LIMS has demonstrated promising application in uranium isotope analysis in nuclear forensics and nuclear safeguards.
4.5.Biological and environmental samples
In the presence of illegal possessing and smuggling of nuclear and/or radioactive materials, it is necessary to find evidence that can link the perpetrator to the crime.As a non-invasive biological test material, hair has a certain accumulation effect on trace elements [101].The human body exposure can be inferred by analyzing the distribution of elements in the hair.Huet al.[102] studied the correlation between the uranium content, isotopic ratio in rat hair and the intake of uranium dose by MC-ICP-MS.The results showed that the uranium content in rat hair was positively correlated with the uranium intake of rats, and the235U/238U value in rat hair was basically consistent with that value in the ingested depleted uranium solution.Zhanget al.[18] also carried out measurements of hair samples from two workers in their institute to extend the nuclear forensics applications to biological samples.Besides, in order to evaluate the contamination of U, Pu isotopes in the environment from the possible nuclear accident, the background distribution levels of U and Pu have to be known.Recently,many domestic researchers have made plenty of related efforts,and the data obtained could not only serve as background data for possible contamination from nuclear reactor, but also provide some indications for dealing with nuclear accidents in the future[103–108].
5.Nuclear forensics exercises
5.1.International cooperation
The Nuclear Forensics International Technical Working Group(ITWG) is an informal forum for collaboration among nuclear forensics practitioners.ITWG has organized a series of Collaborative Materials Exercises (CMXs) and Table top Exercises (Galaxy Serpent Series) to achieve the goal of improving international technical capabilities in nuclear forensics, cooperation and preventing illicit trafficking of nuclear and radioactive materials [109–113].Each exercise distributes materials from the nuclear fuel cycle,rather than certified reference materials, to participating laboratories for nuclear forensic analysis, which is designed to be a learning experience, not a performance test [109–111].Following recommendations by IAEA, participating laboratories, including Chemical Analysis and Test Center of CIAE, are required to submit reports after 24 h, one week and two months following the start of the exercise, respectively [109].
In order to ensure the effectiveness of the Nuclear Non-Proliferation Treaty and meet the needs of trace characteristic isotope analysis in environmental samples involved in the nuclear fuel cycle, the Institute of Reference Materials and Measurements (IRMM) of EU Joint Research Center (JRC) launched the Nuclear Signatures Interlaboratory Measurement Evaluation Programme (NUSIMEP) in 1996 [109,114].IRMM organizes international analysis comparisons regularly to verify the trace sample analysis level of the participating laboratories.IAEA has attached great importance to this event and recommends its Network Laboratory (NWAL) to participate.In addition, “expert laboratories” in other related fields (such as environmental science, geochemistry,etc.) has also been invited.The Particle Analysis Laboratory of CIAE was invited to partake in NUSIMEP-6 and NUSIMEP-7 as an “expert laboratory” [114].In these particle analysis comparison programs,the participating laboratories measured the uranium isotopes ratio in uranium-containing particles and received positive responses and good feedback.
In 2014, the United States Department of Energy (DOE) and CIAE collaborated in a study measuring the model ages of uranium certified reference materials.Lawrence Livermore National Laboratory (LLNL), Los Alamos National Laboratory (LANL) and CIAE determined234U/230Th model ages for uranium certified reference materials U010 and U850.All three laboratories used independent methods to calculate the model ages of uranium standards that agreed with known production ages and with previously reported results [115].
5.2.Domestic comparison program
In 2014, with the aim of tracking the world’s advanced technology in nuclear forensics and developing domestic radiochemical analysis capabilities, six professional laboratories in China were organized to carry out a comparison program of uraniumcontaining sample measurements and traceability in nuclear forensics.The samples included UF6hydrolysate, U3O8powder and uranium pellets.During the comparisons, uranium isotopes and impurity analysis were the must-test items.The participating laboratories used different instruments and analytical methods, and the results showed no significant differences, indicating that all these laboratories have got rid of the limitations of the instrument and demonstrated excellent analytical capabilities.For the comparison of uranium pellet samples, in addition to the required assays, some laboratories also gave a number of other characteristic parameters of the samples according to the traceability procedures of nuclear forensics.On this basis, attempts to trace the source of the uranium pellet samples were made, and the results were relatively reliable [116].With the performing of the comparison program, the nuclear forensics technical capabilities, cooperation, and communication between domestic practitioners has been improved significantly.
6.Conclusion and outlook
In the world-wide battle against illicit activities involving nuclear or radioactive materials, a relatedly novel and evolving discipline, nuclear forensics plays a vital role, whose foundation is firmly supported by principles and methods of radioanalytical chemistry.In China, many scientific institutes and university laboratories are committed to nuclear forensics research, and significant progress has been achieved in a relatively short time in this field, especially in the methods development and application of REE isotope separation and measurement, trace impurity analysis, data processing, which has gained wide interest from nuclear forensics investigators all around the world.Future improvement of this methodology come from more complicated scenarios with nuclear smuggling that only trace or ultra-trace amount of suspicious material may be acquirable and need to be analyzed and identified in a short time, hence calling for a faster detection and accurate identification and attribution.To achieve this goal, several issues should be addressed for the next steps: (1) the relevant analysis technology still relies to a large extent on nuclear chemistry, material science, instrumental science, and other related fields.It is necessary to form a comprehensive technical capability under the background of nuclear forensics,e.g., modern microanalytical techniques, such as scanning transmission X-ray microscopy(STXM), μXAFS could assist in the micro-analysis of actinide particles at a nanoscale [117,118]; (2) standard samples are the guarantee for quality control of the analysis process, while databases for comparison are the basis for the traceability of nuclear materials.Therefore, there is an urgent need for more standard materials and construction of databases of nuclear materials as complete as possible to greatly improve the source attribution capabilities; (3) in response to the evolving demands for the rapid determination of ultra-trace level radionuclides under some urgent circumstances,the development of rapid and automatic separation and/or quantification techniques are of necessity, which hopefully could free the nuclear forensics investigators from tedious handwork and potential radiation risk; (4) since the legislative and practical control measures of nuclear materials in China is rather strict, rarely could an illicit nuclear or radioactive substance that is out of regulatory control be intercepted and hence the chance for a real-case nuclear forensics study is extremely limited.It should be encouraged for domestic laboratories to actively participate in international nuclear forensics exercises as well as carry out more comparison programs among domestic research labs, in the purpose of enhancing the analytical skills and experiences of Chinese nuclear forensics practitioners through engagement of more practices.In the meanwhile, more pioneering work in nuclear forensics could foster the radiochemistry research of China and cultivate more young scientists devoting to this field of high importance.We believe these continued developments and efforts could bring about more practical analytical and sourcing methods over a wide range of analytes, broaden the scope of nuclear forensics, and finally benefit global nonproliferation.
Declaration of competing interest
The authors declare no competing interest.
Acknowledgments
We gratefully acknowledge the financial support from the Science Challenge Project (No.TZ2016004), National Natural Science Foundation of China (No.21906153) and the Presidential Foundation of CAEP (No.YZJJLX2020002).
 Chinese Chemical Letters2022年7期
Chinese Chemical Letters2022年7期
- Chinese Chemical Letters的其它文章
- Professor Zhifang Chai: Scientific contributions and achievements
- Stable isotope labeling of nanomaterials for biosafety evaluation and drug development
- Emerging nanozymes for potentiating radiotherapy and radiation protection
- Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals
- Recent development in selective Tau tracers for PET imaging in the brain
- 64Cu radiolabeled nanomaterials for positron emission tomography(PET) imaging
