Primary gastric dedifferentiated liposarcoma resected endoscopically: A case report
Joon Hyun Cho, Jun Hyeon Byeon, Si Hyung Lee
Abstract
Key Words: Gastric liposarcoma; Dedifferentiated liposarcoma; Submucosal tumor; Endoscopic resection;Case report
INTRODUCTION
Liposarcoma is one of the most common adult soft tissue sarcomas and has a peak incidence between the ages 50 and 65 and a prevalence of 15%-20% among all sarcoma patients[1]. There are four histological subtypes of liposarcomas[2]. Atypical lipomatous tumor/well-differentiated liposarcoma(WDL) is the most common subtype followed by dedifferentiated liposarcoma (DL), which frequently occurs in retroperitoneum[3,4]. The myxoid and pleomorphic types usually present in the extremities[5]. Thus, liposarcoma usually arises in deep soft tissues of the proximal extremities, retroperitoneum, or trunk[6], and is encountered in the gastrointestinal tract in only 2% of cases[7].
The majority of liposarcomas of the alimentary tract arise in the esophagus[8], and liposarcomas originating at more distal sites, such as stomach, small intestine, and large intestine, are rare[9]. Less than 40 cases of primary liposarcoma of the stomach have been described in the medical literature, and the most reported histological subtypes of gastric liposarcoma are WDL and myxoid liposarcoma[10]. In fact, no report on DL of the stomach has been published in the English literature, though one case report on DL of the gastroesophageal junction was issued in 2018[11]. Here, we describe the first case of a small primary gastric DL resected endoscopically and provide a review of the literature.
CASE PRESENTATION
Chief complaints
A 67-year-old female Korean patient was referred to our institution for further evaluation and treatment of a gastric submucosal tumor (SMT) incidentally discovered by esophagogastroduodenoscopy (EGD)during a routine medical check-up.
History of present illness
The patient had experienced no abdominal pain or discomfort.
History of past illness
She had a history of breast conserving surgery due to breast cancer 6 years previously.
Personal and family history
The patient had diabetes and was being treated with oral hypoglycemic agents. She was a non-smoker and non-alcohol drinker and had no significant family history.
Physical examination
Physical examination was unremarkable, and her abdomen was soft, nontender, and nondistended with no palpable mass.
Laboratory examinations
Laboratory tests, which included common serum tumor markers such as carcinoembryonic antigen(CEA) and carbohydrate antigen 19-9 (CA 19-9), were normal.
Imaging examinations
EGD revealed a SMT-like protruding lesion of approximately 15 mm diameter located in the posterior wall of the lesser curvature of the distal part of the gastric body (Figure 1A). The lesion was covered with normally appearing mucosa except for focal mucosal erythema and depression over the lesion.Gastric endoscopic ultrasonography (EUS) examination demonstrated a well-demarcated mass, which measured 17 mm × 10 mm, located in the third layer of the gastric wall. The echo pattern of the mass was slightly heterogeneous and isoechoic (Figure 1B), but its EUS appearance was insufficient for diagnosis. The differential diagnosis included lipoma, neuroendocrine tumor, and ectopic pancreas.Initial biopsy specimens obtained at the site of the focal mucosal erythema and depression were negative for a neoplasm. Computed tomography (CT) of the abdomen showed a well-enhanced,intraluminal protruding polypoid lesion arising from the gastric body but no evidence of lymph node enlargement or distant metastasis (Figure 2).
FINAL DIAGNOSIS
After initial work-up, endoscopic resection was conducted using the snare resection technique after mucosal precutting for a definitive histopathologic result (Figure 3). At gross examination, the tumor was whitish yellow, well-shaped, and solid. Histopathologic examination showed the tumor was located submucosally and primarily composed of spindle-shaped atypical cells with pleomorphic and hyperchromatic nuclei and indistinct pale cytoplasm (Figure 4A-D). Deep resection margins were positive for tumor. Immunohistochemical staining performed for the differential diagnosis of gastrointestinal stromal tumor, schwannoma, leiomyosarcoma, malignant melanoma, and any poorly or undifferentiated sarcoma, revealed tumor cells were negative for CD117, DOG1, CD34, SMA, S-100,Desmin, HMB45, and SOX10, but positive for MDM2 and CDK4 nuclear staining (Figure 4E and F).Based on these findings, the histopathological diagnosis was consistent with DL.
TREATMENT
The patient was planned for surgery because the deep resection margin was positive for DL, but declined any invasive procedure or adjuvant treatment.
OUTCOME AND FOLLOW-UP
She remains under close follow-up, which includes biannual CT scanning and EGD, and at one year after endoscopic resection remained disease free.
DISCUSSION
Although they are the most common mesenchymal neoplasms, liposarcomas are rarely found in the gastrointestinal tract. Since the disease was first described by Abramset al[12] in 1941, fewer than 40 cases of gastric liposarcoma have been reported in the literature worldwide[10]. The etiology of gastric liposarcoma remains uncertain, though some patients have a family history of soft tissue neoplasms,which suggests the involvement of genetic factors[13]. Gastric liposarcomas originate due to the proliferation of undifferentiated mesenchymal cells within submucosa and the tunica muscularis layer of stomach, and an exophytic growth is typical[10,14]. Most gastric liposarcomas are located in the antrum or lesser curvature.
As is the case for other soft tissue sarcomas, there are no characteristic clinical findings[15], and patients may remain asymptomatic for years. Symptoms depend primarily on tumor location and size and the presence or absence of ulceration. The most common symptoms are a palpable mass,mechanical obstruction, and gastrointestinal bleeding. In general, nonspecific symptoms are the main cause of delayed diagnoses, and thus, most liposarcomas are large at diagnosis. However, our patient underwent regular biennial EGD under a national cancer screening program, and the small SMT was detected early. Furthermore, endoscopic resection performed for a definite diagnosis resulted in the diagnosis of a small, asymptomatic gastric liposarcoma.
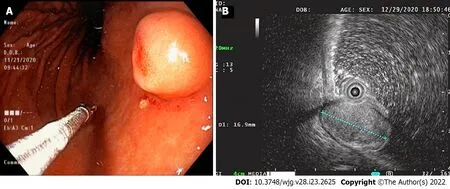
Figure 1 Endoscopy and endoscopic ultrasound images. A: Endoscopic image showing a 15-mm-sized, submucosal tumor-like, protruding lesion with focal mucosal erythema and depression of overlying mucosa; B: Endoscopic ultrasound image showing a well-circumscribed, slightly heterogeneous, 17 mm × 10 mm sized, isoechoic mass originating from the third sonographic layer.
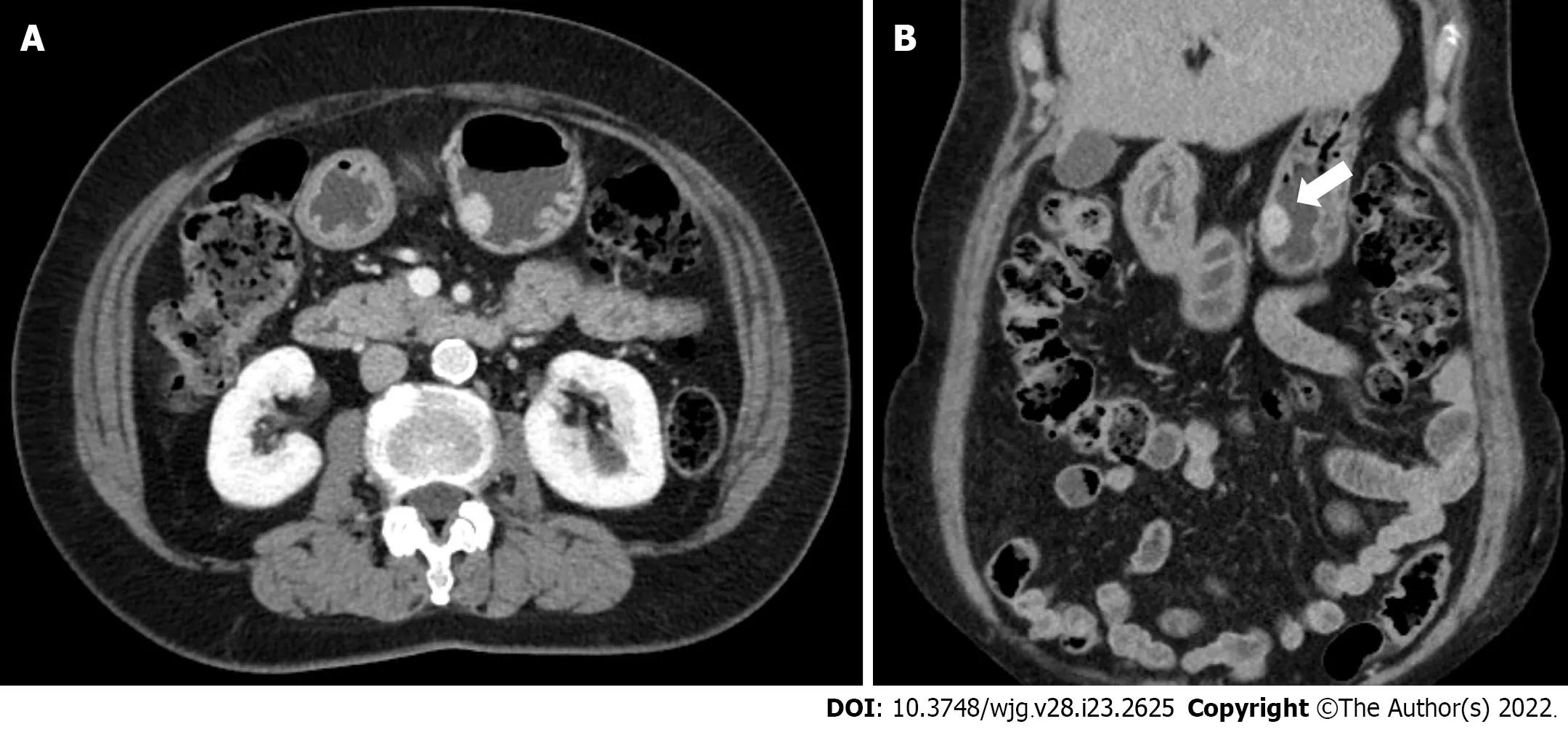
Figure 2 Abdominal computed tomography images. A and B: Axial and coronal computed tomography images showing a well-enhanced and protruding intraluminal mass in the gastric body.
The characteristic histological features of liposarcoma are the presence of immature fat cells and lipoblasts[14]. Liposarcomas are classified histologically as WDL, DL, myxoid/round cell liposarcoma,or pleomorphic liposarcoma[2], and considerations of histological subtype are important during the disease course. While DL, round-cell, and pleomorphic liposarcomas are high-grade aggressive tumors with the ability to metastasize, WDL and myxoid liposarcomas are low-grade tumors that progress more slowly[3,5]. Most of the gastric liposarcomas reported have been of the well-differentiated or myxoid histologic subtypes[10], and DL arising in the stomach has not been previously reported in the English literature; although a case of DL of heart and stomach was reported in 2017[16]. Notably, in this report, histopathological examination failed to determine that the primary lesion was located in the stomach. In addition, one case of primary DL of the gastroesophageal junction has been reported[11].Thus, to the best of our knowledge, this is the first reported case of primary gastric DL.
DL is defined as a combination of WDL and high-grade sarcoma that displays evidence of nonlipogenic differentiation like undifferentiated high-grade pleomorphic sarcoma, fibrosarcoma, or myxofibrosarcoma[17,18]. DL can occurde novo(90% of cases) or as recurrence from a preexisting WDL(10% of cases)[19]. If DL develops from the transformation of a preexisting WDL into non-lipogenic sarcoma, dedifferentiation develops in 20% of the first and 44% of the second local recurrences and has been shown to be associated with poor progression and metastasis[5]. The histologic diagnosis of DL is generally based on the identification of WDL areas, which were scarce in our patient. In these cases, the differential diagnosis of any poorly-differentiated or undifferentiated sarcoma is important, and MDM2 and CDK4 immunohistochemical staining or FISH testing for amplification of the MDM2 and CDK4 genes is diagnostically helpful[20]. In our patient, DL was confirmed by CDK4 and MDM2 immunostaining.

Figure 3 Process of the endoscopic resection. A: Marking outside the lesion; B: Injection of saline-epinephrine for submucosal lifting; C: Circumferential mucosal incision and partial submucosal dissection; D and E: Resection using a snare; F: Resected tumor.
DL often exhibits abdominal cavity involvement usually of retroperitoneum, and an intraperitoneal origin is extremely rare[3,21,22]. When DL occurs inside the abdominal cavity it presents as a spaceoccupying mass lesion. The pathognomonic findings of DL in CT and magnetic resonance images are a heterogeneous, non-lipogenic, encapsulated mass[23], which are sufficient for diagnosis and obviate the need for needle biopsy. In our patient, because DL arising from the gastric wall presented as a small gastric SMT, CT was not diagnostically useful. Although EUS is considered the most useful modality in terms of defining the presumptive nature of SMT, our case demonstrates the difficulty of differentiating benign and malignant SMT by EUS alone, particularly when a lesion is small. Lesion echogenicity is also worth mentioning. Unlike the hyperechogenic EUS feature of other pathologic types[10,24,25], our case showed isoechoic EUS features, which appeared to be related to a sparsity of immature lipocytes or lipoblasts as the WDL area was extremely limited in our patient. DL seems to have a better prognosis than other high-grade sarcomas, especially in terms of its metastatic potential. Yet, careful long-term follow-up is essential because approximately 40% of DLs recur locally, 17% metastasize, and 28% of patients eventually die of the disease[1]. Surgery remains the treatment of choice for DL, and it is crucial that the tumor be completely removed[4], though the efficacies of targeted chemotherapy and radiotherapy are being investigated[26].
CONCLUSION
Liposarcoma is uncommon in the gastrointestinal tract and rare in the stomach, and primary DL in the stomach is extremely rare but should be considered in the differential diagnosis of any poorly-differentiated or undifferentiated sarcoma. The reasons why this case report is valuable are: (1) It presents the first reported case of a small primary gastric DL, resected endoscopically, and followed up; and (2) it cautions that if a diagnosis of SMT is uncertain after initial examination by EGD, EUS, and/or CT, the use of aggressive techniques, including endoscopic resection, should be considered.
FOOTNOTES
Author contributions:Cho JH, Byeon JH, and Lee SH were responsible for acquiring clinical data and for manuscript writing and revision.
Informed consent statement:Informed written consent was obtained from the patients for the publication of this report and any accompanying images.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:South Korea
ORCID number:Joon Hyun Cho 0000-0002-3584-6300; Jun Hyeon Byeon 0000-0001-9857-1810; Si Hyung Lee 0000-0001-7221-7506.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
 World Journal of Gastroenterology2022年23期
World Journal of Gastroenterology2022年23期
- World Journal of Gastroenterology的其它文章
- Reconstructing the puzzle of the role of therapeutic endoscopy in the management of post-bariatric surgery complications
- Whole lesion histogram analysis of apparent diffusion coefficient predicts therapy response in locally advanced rectal cancer
- Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn’s disease
- Infliximab trough level combined with inflammatory biomarkers predict long-term endoscopic outcomes in Crohn’s disease under infliximab therapy
- Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol
- Up to seven criteria in selection of systemic therapy for hepatocellular carcinoma
