Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol
Yi-Xin Guo, Bing Han,Ting Yang, Yu-Si Chen, Yi Yang, Jia-Yao Li, Qin Yang,Ru-Jia Xie
Abstract
Key Words: Hepatocytes; Reticulophagy; Family with sequence similarity 134 member B; Apoptosis;Endoplasmic reticulum stress; Endoplasmic reticulum homeostasis
INTRODUCTION
Endoplasmic reticulum (ER) stress-related hepatocyte apoptosis participates in multiple hepatic diseases, including viral hepatitis[1], hepatic fibrosis[2], fatty liver[3,4] and cirrhosis[5]. Therefore, the alleviation of ER stress-mediated hepatocyte apoptosis is crucial in the treatment of hepatic diseases.Recent findings have indicated that endoplasmic reticulophagy (ER-phagy) promotes degradation of damaged ER fragments during ER stress. Although ER-phagy has a vital role in maintaining ER homeostasis and inhibiting cell apoptosis[6-8], the exact regulatory mechanisms behind this are largely unknown.
Glucose-regulated protein 78 (GRP78) is a prominent ER molecular chaperone, while calnexin (CNX)is a membrane-bound lectin protein in the ER that can increase the protein folding capacity[9,10]. Even though the excessive build-up of misfolded or unfolded proteins can be alleviatedviaER stress,previous studies reported that a selective autophagic mechanism, defined as ER-phagy, can also be activated by ER stress to restore ER homeostasis[11,12]. Family with sequence similarity 134 member B(FAM134B), an ER-resident protein, may interact with CNX in the cytosol or the ER membrane[13].Since FAM134B is not predicted to have an ER lumenal domain, there is an indirect interaction between FAM134B and lumenal proteins through the lumen-resident segment, which has a chaperone activity attributed to CNX. CNX forms transient but relatively stable complexes with unfolded ER proteins until they either become folded or are degraded. Moreover, it has been reported that as with other cargo receptor molecules, FAM134B can interact directly with microtubule-associated protein 1 light chain 3(LC3) when its LIR motif is exposed. The CNX-FAM134B-LC3 complex can mediate the selective isolation of ER fragments containing misfolded proteins, which are subsequently transported to lysosomes for degradation[14-16]. Thus, FAM134B-mediated ER-phagy may play an essential role in maintaining ER homeostasis and promoting cell survival. However, it is unclear whether FAM134Bmediated ER-phagy is involved in the regulation of hepatocyte apoptosis induced by ER stress. In this study, dithiothreitol (DTT) was used to induce ER stress in buffalo rat liver 3A (BRL-3A) hepatocytes,and the expression of ER stress-related and autophagy-related proteins was assessed. In addition, small interfering RNA (siRNA) was used to knockdown the expression ofFAM134Bin hepatocytes and an apoptosis analysis followed. Our study reveals an emerging role of FAM134B-mediated ER-phagy in ER stress-mediated hepatocyte apoptosis, which may provide a novel target for the treatment of hepatic diseases.
MATERIALS AND METHODS
Antibodies and reagents
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco(Grand Island, NY, United States). Trypsin-EDTA solution, trypsin solution without EDTA, and penicillin-streptomycin were purchased from Biological Industries (BioInd, Israel). Bicinchoninic acid(BCA) protein assay kit, DTT, RIPA lysis buffer, and protease inhibitor were obtained from Solarbio(Beijing, China). Annexin V-FITC/PI Apoptosis Detection Kit and Cell Cycle Detection Kit were purchased from KeyGEN BioTECH (Nanjing, China). PVDF membranes were obtained from Merck Millipore. Rabbit polyclonal antibody against FAM134B was purchased from Proteintech (Wuhan,China). Rabbit polyclonal antibodies against ATG12, cytochrome c (cyt c), and cleaved caspase-3 were obtained from Cell Signaling Technology (Danvers, MA, United States). Rabbit polyclonal antibodies against β-actin, LC3, CNX, CHOP and GRP78, and the Ca2+indicator (Rhod-2 AM) were purchased from Abcam (Cambridge, United Kingdom). Dynabeads protein G immunoprecipitation kit and lipofectamine 3000 reagent were purchased from Thermo Fisher Scientific, Inc. HRP-labeled Goat Anti-Rabbit IgG (H + L), Mito-Tracker Green, Lyso-Tracker Green, ER-Tracker Red, and immunofluorescencerelated reagents were purchased from Beyotime Institute of Biotechnology (Nanjing, China).
Cell culture and experiment protocol
BRL-3A cells, bought from Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were cultivated and maintained in DMEM culture media supplemented with 1% penicillin-streptomycin and 10% FBS. BRL-3A cells were seeded at 37 °C and 5% CO2in a constant temperature and humid atmosphere, pre-cultured every 3 d, and further passaged until the density reached approximately 80%.To induce the ER stress, BRL-3A cells were treated with DTT (2.0 mmol/L based on previous studies[17]) for 0, 3, 6, 12, 24, or 48 h.
Apoptosis assessment
Cells were cultured to 80% confluency and treated with 2.0 mmol/L DTT for the specified point-in-time intervals. To determine the efficacy of the different DTT treatments, a cell apoptosis analysis was evaluated with flow cytometry. Each group of cells was trypsinized without EDTA and rinsed thrice with PBS. After centrifugation at 2000 rpm for 5 min, cells were loaded with 500 μL binding buffer and labeled with 5 μL of Annexin V-FITC/PI, according to the manufacturer’s instructions. Labeled cells were detected and analyzed with flow cytometry and NovoExpress®software 1.4.1. The experiments were performed in triplicate.
Cell cycle analysis
To determine the effect of DTT’s 0, 3, 6, 12, 24, and 48 h incubation on the cell cycle progression of BRL-3A, the harvested cells were trypsinized without EDTA and rinsed three times with cold PBS, followed by fixation with 70% ethanol in cold storage. After 24 h incubation at 4 °C, 500 μL PI/RNase was added to each group and maintained at 37 °C for 60 min in a dark place. Stained cells were processed using flow cytometry and further measuredviathe NovoExpress®software 1.4.1. The experiments were performed in triplicate.
Western blot analysis
BRL-3A cells were grown on 10 cm diameter dishes and treated with 2.0 mmol/L DTT for different times. Cells were rinsed three times with pre-cooled PBS after experimentation and collected with cell scrapers in 100 μL RIPA buffer containing 1 mmol/L PMSF. After centrifugation at 12000 rpm for 25 min at 4 °C, the concentrations of total cellular protein extracts were determined using the BCA kit(Solarbio Science, Beijing, China), and known concentrations of BSA were used as standard. The total cellular protein extracts were denatured by boiling at 100 °C using dry bath incubator (Hangzhou Miu Instruments Co., Ltd, Zhejiang, China). Protein samples (30-40 mg) were loaded onto SDS-PAGE and transferred onto PVDF membranes for immunostaining. After blocking with 5% defatted milk for 90 min, membranes were stained overnight with primary antibodies, including β-actin (1:1000), GRP78(1:1000), CNX (1:3000), ATG12 (1:1000), LC3 (1:1000), FAM134B (1:1000), CHOP (1:1000), cleaved caspase-3 (1:1000), cyt c (1:1000) in cold storage, followed by incubation with secondary antibodies(1:4000). The density of protein bands on membranes was exposed and quantifiedviafluorography using Image J software. The images shown are representative of experiments carried out at least three times.
Co-immunoprecipitation analysis
BRL-3A cells, treated with DTT (2.0 mmol/L for 0 h and 24 h), were lysed in RIPA lysis buffer and the lysates were centrifuged at 12000 rpm for 15 min at 4 °C. The supernatant was resuspended in ice-cold PBS to a total volume of 500 μL, and 5 μL of the designated antibody was added overnight at 4 °C. The next day, the Ab-Ag complexes were bound to Dynabeads magnetic beads on a rotary shaker for 10 min. The magnetic bead-Ab-Ag complex was washed and eluted by adding a washing buffer and elution buffer, respectively, according to the manufacturer's protocol. Immunocomplexes were heated for 5 min at 100 °C and prepared for analysis by western blot. The images shown are representative of experiments carried out at least three times.
Calcium imaging and mitochondrial labeling
To observe the effects of DTT treatment at 2.0 mmol/L for specified time points, mitochondrial Ca2+levels were determined using Rhod-2 AM, a specific detection dye for calcium. The treated cells were rinsed with HBSS three times and stained with a mixture of 5 μM Rhod-2 AM and 20 nM Mito-Tracker Green at 37 °C for 30 min in the dark. Finally, live cells were extensively rinsed thrice by adding HBSS without calcium, and images were visualized with Zeiss LSM Image Browser using a Zeiss LSM 900 confocal microscope. The images shown are representative of experiments carried out at least three times.
Live imaging of ER and lysosome
To observe the intracellular localization of the ER and lysosomes, after treatment with 2.0 mmol/L DTT for 0, 3, 6, 12, 24, and 48 h, ER and lysosomes were stained with ER-tracker and Lyso-tracker. Prior to staining, trackers were diluted appropriately in DMEM, on the basis of the manufacturer's instructions.Following dilution, cells were simultaneously incubated with the two trackers listed above, maintained for 30 min at 37 °C, and finally rinsed thrice with HBSS. Stained cells were visualized under the Zeiss LSM 900 confocal microscope. Images shown are representative of experiments carried out at least three times.
SiRNA transfections
Specific siRNA against buffalo ratFAM134Bwas designed and synthesized by OriGene. Product number and targeting sequence: SR510501A-rGrGrArArGrUrGrGrUrUrUrArUrCrArArArUr-UrCrUrGrATA; SR510501B-rArArArUrUrUrGrArCrUrUrArCrArGrUrGrGrArArArCrCAA;SR510501C-rArArGrUrGrGrUrUrUrArUrCrArArArUrUrCrUrGrArUrAGA. Cells were cultured in sixwell dishes until the density of cell fusion reached 60%. Briefly, 75 pmol ofFAM134BsiRNA were added to Lipofectamine 3000 Transfection Reagent and gently mixed for 15 min, then administered to BRL-3A cells, which were resuspended in DMEM. After transfection for 6 h, cells were washed, and then supplemented with fresh medium. Finally, cells were treated with DTT (2.0 mmol/L) for a further 24 h and subjected to western blot assay and apoptosis assessment.
Statistical analysis
GraphPad Prism 7 software was used to perform all the statistical analyses and prepare experimental graphs. Data are expressed as the mean ± SD. Shapiro-Wilk normality test was used to test the normal distribution of the data and all the data were fit to a normal followed by Tukey's post hoc test was performed, and a significant difference was considered asP< 0.05.
RESULTS
DTT-mediated ER stress upregulates ER-phagy-related FAM134B in BRL-3A cells
To assess whether the drug treatments could alter the protein expression of CNX and GRP78, BRL-3A cells were subjected to short-term (3, 6, 12, 24 h) or long-term (48 h) treatment with DTT, and the protein extracts from BRL-3A cells were analyzed by western blot. We found that treatment of BRL-3A cells with 2.0 mmol/L DTT resulted in a prominent increase in CNX and GRP78 levels, both in a timedependent manner (Figure 1A and B). Moreover, CHOP is a specific and stress-responsive transcription factor during ER stress and its protein expression was significantly increased in the 12, 24, and 48 h groups (Figure 1A and B). However, the expression of CHOP in BRL-3A cells treated with DTT for 48 h was lower than that after DTT treatment for 24 h. These alterations in CNX, GRP78, and CHOP confirm that ER stress in BRL-3A was activated.

Figure 1 Impact of the endoplasmic reticulum stressor, dithiothreitol, on endoplasmic reticulophagy mediated by family with sequence similarity 134 member B in buffalo rat liver 3A cells. A and B: Buffalo rat liver 3A (BRL-3A) cells were treated with 2.0 mmol/L dithiothreitol (DTT) for the time intervals (0, 3, 6, 12, 24, 48 h); Western blot showed the effect of endoplasmic reticulum (ER) stressor, DTT, on expression of the ER stress-related proteins glucose-regulated protein 78 (GRP78), calnexin (CNX), and C/EBP homologous protein (CHOP); β-actin was used as a control for normalization; C and D: Analysis of autophagy related gene 12 (ATG12), family with sequence similarity 134 member B (FAM134B), and microtubule-associated protein 1 light chain 3 (LC3) protein expression by western blot. Protein levels were normalized to β-actin; E: BRL-3A cells were treated with 2.0 mmol/L DTT for 0 and 24 h; co-immunoprecipitation analysis detected the presence of CNX-FAM134B-LC3 complex in BRL-3A cells. Values are represented as mean ± SD (n = 3), aP < 0.05 vs 0 h group; bP < 0.05 vs 48 h group.
To determine the effects of ER stress on FAM134B-mediated ER-phagy, alterations in FAM134B,ATG12, and LC3 expression were detected by western blot. As expected, DTT treatment for 3, 6, 12, 24,and 48 h increased the conversion ratio of LC3-I to LC3-II and the FAM134B and ATG12 expression levels compared to those in the 0 h group (Figure 1C and D). Thus, our results revealed that the expression of FAM134B is induced in response to ER stress.
Furthermore, we used an anti-CNX antibody to immunoprecipitate the CNX-FAM134B-LC3 complex,confirming the hypothesis that FAM134B forms a complex with CNX and LC3, exerting a positive influence on ER-phagy (Figure 1E).
Long-term DTT treatment relieved the gradually blocked ER autolysosome delivery in BRL-3A cells
Typically, ER is delivered to lysosomes and finally degraded. To analyze whether ER autolysosomes are formed, we examined the subcellular location of the ER and lysosomes using cell organelle markers. As shown in Figure 2, the treatment groups of 3, 6, 12, 24, and 48 h DTT incubation significantly alleviated the co-localization of the ER with lysosomes, compared to that in the 0 h group. Notably, the colocalization of ER and lysosomes in BRL-3A cells treated with DTT for 48 h was increased compared to those treated for 24 h (Figure 2).
Short-term DTT treatment induces mitochondrial calcium uptake while prolonged DTT treatment reduces it
Calcium in the ER can be released and transferred to the mitochondria owing to an imbalance of ER homeostasis. To explore the altered localization of calcium, collected cells were co-loaded with Rhod-2 AM and Mito-Tracker Green. In response to DTT treatment for 3, 6, 12, 24, and 48 h, the co-localized fluorescence increased considerably (Figure 3). However, the distribution of the co-localized signal was weaker in the 48 h group, compared to that in the 24 h group (Figure 3). These results strongly suggest that mitochondrial calcium accumulation is related to DTT treatment.

Figure 2 Impact of dithiothreitol treatment on the formation of autolysosomes in buffalo rat liver 3A cells. After dithiothreitol treatment for 0, 3, 6,12, 24, and 48 h, the buffalo rat liver 3A cells labeled with endoplasmic reticulum (ER)-Tracker Red and Lyso-Tracker Green were observed and captured under confocal fluorescence microscopy (200 ×) in a live cell imaging experiment. Insets show the magnification of the pictures. Scale bars indicate 100 μm. Arrows head to indicate ER-localized lysosomes. Values are represented as mean ± SD (n = 3), aP < 0.05 vs 0 h group; bP < 0.05 vs 48 h group.

Figure 3 Impact of dithiothreitol treatment on mitochondrial calcium uptake in buffalo rat liver 3A cells. Buffalo rat liver 3A cells were treated for 0, 3, 6, 24, and 48 h with 2.0 mM dithiothreitol, followed by co-incubating with Mitochondria-Tracker Green and Rhod-2 AM, and visualized by confocal microscopy(400 ×). Scale bars indicate 100 μm. Values are represented as mean ± SD (n = 3), aP < 0.05 vs 0 h group; bP < 0.05 vs 48 h group.
DTT treatment induces cell cycle arrest and apoptosis in BRL-3A cells, which is relieved at 48 h
To further validate that DTT treatment leads to apoptosis in BRL-3A cells, we quantitatively measured the number of apoptotic cells using the Annexin V-FITC/PI double staining assay. As shown in Figure 4A and B, the ratio of apoptotic cells treated with DTT for 0, 3, 6, 12, and 24 h exhibited a timedependent increase. Interestingly, the apoptotic percentage in the 48 h group was significantly lower than that in the 24 h group (Figure 4A and B). Subsequently, we sought to use flow cytometry to determine the impact of DTT treatment on the cell cycle progression, and the data suggests that the proportion of BRL-3A cells in G1 phase after DTT treatment was noticeably higher than that of the 0 h group (Figure 4C and D and Table 1). Moreover, the number of cells in G1 phase in the 48 h group was smaller than that of the 24 h group.
BRL-3A cells undergo apoptosis upon FAM134B knockdown
We further verified whetherFAM134Bknockdown could alter DTT-induced apoptosis. We first investigated the transfection efficiency of siRNA with three different siRNAs targetingFAM134B(siRNA 1, 2,and 3) and found that theFAM134BsiRNA2 was the most effective (Figure 5A and B). Next, we investigated FAM134B protein levels by performing a western blot on already transfected samples, which were treated with DTT for 24 h. As shown in Figure 5C and D, FAM134B and β-actin expression levels were determined, and it was found that FAM134B protein levels were down-regulated compared with the control and control siRNA groups.
It has been reported that cyt c and cleaved caspase-3 are apoptosis-related proteins and important hallmarks of apoptosis activation involved in mitochondrial dysfunction. Consequently, siRNAmediated silencing ofFAM134Bcaused a high level of cleaved caspase-3 and cyt c in BRL-3A cells treated with DTT for 24 h (Figure 5E and F). We examined the rates of apoptotic cells using Annexin-VFITC/PI staining assays, which revealed that the apoptotic rates also increased in theFAM134BsiRNAgroup, compared with those in the control and control siRNA groups (Figure 5G and H). These results suggest that ER-phagy mediated by FAM134B is likely to serve a cytoprotective function in response to DTT treatment in BRL-3A cells.
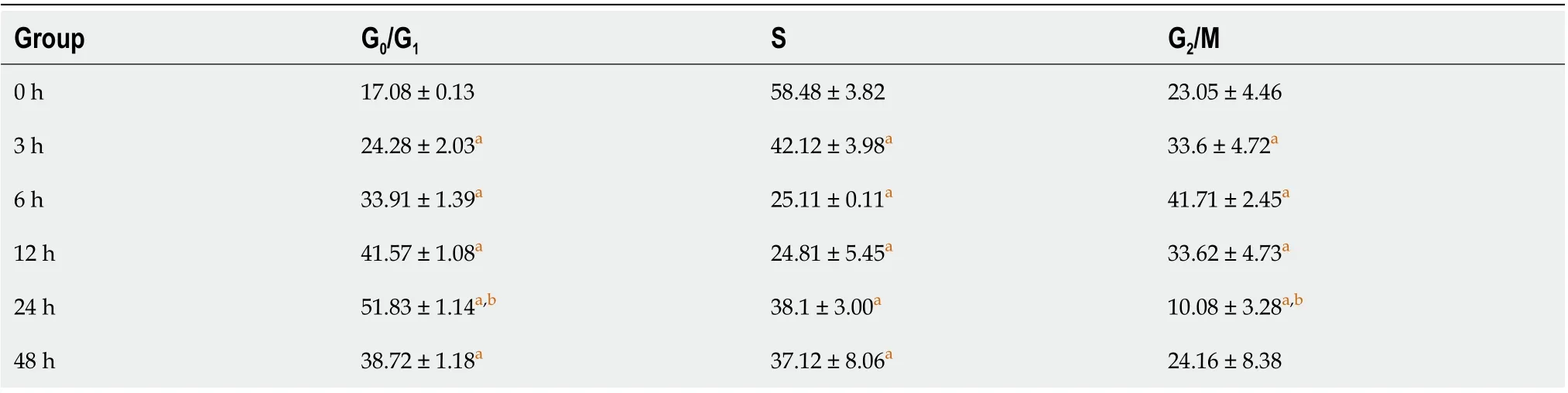
Table 1 The cell cycle distribution of buffalo rat liver 3A cells treated with dithiothreitol for different times was detected by flow cytometry
DISCUSSION
Hepatic injury caused by multiple harmful factors is closely associated with ER stress-induced hepatocyte apoptosis[18-20]. The ER is responsible for proper protein folding, intracellular calcium storage, and lipid biosynthesis[21,22]. Various stressors, including unfolded protein aggregation in the ER, intracellular Ca2+disturbance, and pharmacological inducers, such as DTT, can disrupt ER homeostasis and lead to ER stress in hepatocytes. If the ER stress cannot be alleviated, aberrant ER stress can trigger cell apoptosis[23]. In the present study, we found that the protein levels of GRP78 and CNX,which are ER stress biomarkers, were upregulated in BRL-3A cells during ER stress. GRP78 and CNX are ER chaperone proteins and accelerate the proper folding of the accumulated unfolded proteins in the ER, which engages effector mechanisms to rebalance ER homeostasis[24,25]. A series of studies have revealed that ER-phagy is an ER selective autophagy mechanism that can promote the clearance of damaged ER lumens containing the unfolded proteins, and helps restore ER homeostasis[26-28]. ERphagy is a critical quality control mechanism for the ER in multiple cell types. Defects in ER-phagy pathways are associated with multiple human pathologies, including infectious and neurodegenerative diseases, aging and cancer. However, whether ER-phagy is involved in the regulation of ER homeostasis in hepatocytes under ER stress remains elusive. In this study, we assessed the levels of reticulophagyrelated proteins in BRL-3A cells treated with DTT. We found that the levels of FAM134B and ATG12 were markedly elevated, and the ratio of LC3II/LC3I also increased. These data indicate that DTTinduced ER stress increases the level of reticulophagy-associated proteins.
Recent findings have indicated that receptor proteins of ER-phagy play crucial roles in driving the sequestration of isolated ER fragments into autophagosomes[29]. FAM134B, an ER-anchored protein,was recently proposed as a major mammalian receptor for reticulophagy[30,31]. FAM134B contains an LC3-interacting region that can interact with LC3 protein to form autophagosomal membranes, leading to efficient ER sequestration into an autophagosomal lumen[32-34]. In a previous report, the authors found that CNX serves as a co-receptor that recognizes misfolded proteins within the ER lumen and interacts with FAM134B[35,36]. In turn, the CNX-FAM134B complex binds with LC3, the autophagosome membrane-related protein, which delivers ER lumens containing misfolded proteins to the lysosome for degradation. To investigate how FAM134B modulates ER-phagy in BRL-3A cells, immunoprecipitation was performed to detect the interaction between CNX, FAM134B, and LC3. The results confirmed that CNX interacted with FAM134B, and FAM134B interacted with LC3 after DTT treatment.Thus, the formation of the CNX-FAM134B-LC3 complex allows for the selective delivery of ER lumens containing misfolded proteins to the lysosome for eventual degradation. Complete ER-phagy indicates that autophagosomes fuse to form autolysosomes[37,38], hence, we detected the number of autolysosomes in BRL-3A cells treated with DTT. We found that the formation of autolysosomes decreased in the early stages of ER stress, whereas autolysosomes were elevated in later stages. As it has been reported that CHOP can suppress autolysosome formation[39], we speculated that decreased autolysosomes in the early stages of ER stress were associated with increased CHOP expression.

Figure 4 Impact of dithiothreitol treatment on cell cycle and apoptosis of buffalo rat liver 3A cells. A and B: Buffalo rat liver 3A (BRL-3A) cells were treated with 2.0 mmol/L dithiothreitol (DTT) for 0, 3, 6, 12, 24 and 48 h. The population of apoptotic cells was detected by flow cytometry. The lower right quadrant represents the early apoptotic cells, and the upper right quadrant represents the late apoptotic cells; C and D: BRL-3A cells were treated with 2.0 mmol/L DTT for 0, 3, 6, 12, 24 and 48 h. The analysis of the cell cycle was assessed by flow cytometry. aP < 0.05 vs 0 h group; bP < 0.05 vs 48 h group.
The ER is the main pool for Ca2+storage, and ER dysfunction leads to Ca2+efflux from the ER[40,41].In the early stages of ER stress, the suppression of the autophagosomes’ fusion with lysosomes may lead to calcium release and subsequent Ca2+overload in mitochondria[42-44]. As expected, we found that DTT treatment dramatically elevated the levels of mitochondrial Ca2+, the apoptotic rate, and G1 arrest in BRL-3A cells. Nevertheless, these trends were relieved after treatment with DTT for 48 h. Our results reveal that hepatocytes initiate adaptive mechanisms in response to DTT-induced ER stress;consequently, apoptosis in BRL-3A cells treated with DTT for 48 h was lower than that in cells treated with DTT for 24 h.
To clarify whether FAM134B is involved in the regulation of cellular homeostasis during ER stress,we used a small interference RNA technique to knockdownFAM134Bexpression in hepatocytes. We found thatFAM134Bsilencing not only significantly attenuated the DTT-upregulated FAM134B expression, but also accelerated the activation of the mitochondrial apoptotic pathway and aggravated DTT-triggered hepatocyte apoptosis.
CONCLUSION
In conclusion, DTT treatment significantly upregulated the protein levels of GRP78, CNX, FAM134B,and ATG12, and also increased the ratio of LC3II/LC3I in BRL-3A cells. Moreover, FAM134B-mediated reticulophagy ameliorates DTT-induced hepatocyte apoptosisviaselective clearance of damaged ER lumens. Accordingly, knockdown of FAM134B enhanced ER stress-mediated apoptosis in BRL-3A cells.Our data show that FAM134B-mediated reticulophagy plays a key role in rebalancing ER homeostasis in hepatocytes undergoing ER stress. Therefore, FAM134B-mediated reticulophagy may be a novel therapeutic target, and our findings may provide emerging evidence to demonstrate the prominence of ER-phagy in ER stress-related hepatocyte apoptosis. Alleviation of ER stress-mediated hepatocyte apoptosisviarestoring ER homeostasis is critical in the treatment of liver diseases.
ARTICLE HIGHLIGHTS

Research results
DTT treatment upregulated glucose-regulated protein 78 (GRP78), CNX, FAM134B, and autophagy related gene 12 (ATG12) protein levels and increased the ratio of LC3II/LC3I in BRL-3A cells.FAM134B-mediated reticulophagy maintains ER homeostasis in ER-stressed hepatocytesviathe clearance of damaged ER fragments. FAM134B-mediated reticulophagy ameliorates DTT-induced hepatocyte apoptosis. Knockdown ofFAM134Benhanced ER stress-mediated apoptosis in BRL-3A cells.
Research conclusions
FAM134B-mediated ER-phagy attenuates hepatocyte apoptosis by suppressing the mitochondrial apoptotic pathway.
Research perspectives
FAM134B-mediated reticulophagy may be a novel therapeutic target, and our findings provide emerging evidence demonstrating the prominence of ER-phagy in ER stress-related hepatocyte apoptosis. Alleviation of the ER stress-mediated hepatocyte apoptosisviarestoring ER homeostasis is critical in the treatment of liver diseases.
ACKNOWLEDGEMENTS
We thank the Basic Medical Science Research Center of Guizhou Medical University for their technical advice in using the microscope.
FOOTNOTES
Author contributions:Yang Q and Xie RJ designed and coordinated the study; Guo YX, Han B and Yang T performed the experiments and acquired data; Chen YS, Yang Y and Li JY analyzed and interpreted data; Guo YX and Xie RJ drafted the manuscript; all authors approved the final version of the article.
Supported byNational Natural Science Foundation of China, No. 81560105; Science and Technology Foundation of Guizhou Province, No. Qiankehe Jichu-ZK[2021]365, and No. Qiankehe Pingtai Rencai[2019]5801; and National Natural Science Foundation Cultivation Project of Guizhou Medical University, No. 20NSP016.
Institutional review board statement:This study did not involve human subjects or living animals.
Institutional animal care and use committee statement:This study did not involve human subjects or living animals.
Conflict-of-interest statement:The authors declare no conflicts of interest.
Data sharing statement:The data used to support the findings of this study are available from the corresponding author at 592153968@qq.com upon request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yi-Xin Guo 0000-0001-6789-8669; Bing Han 0000-0002-9577-293X; Ting Yang 0000-0001-5174-7575; Yu-Si Chen 0000-0003-2566-8878; Yi Yang 0000-0003-2756-6955; Jia-Yao Li 0000-0003-2880-4978; Qin Yang 0000-0003-1479-6700; Ru-Jia Xie 0000-0001-5991-2678.
S-Editor:Fan JR
L-Editor:A
P-Editor:Qi WW
 World Journal of Gastroenterology2022年23期
World Journal of Gastroenterology2022年23期
- World Journal of Gastroenterology的其它文章
- Reconstructing the puzzle of the role of therapeutic endoscopy in the management of post-bariatric surgery complications
- Primary gastric dedifferentiated liposarcoma resected endoscopically: A case report
- Whole lesion histogram analysis of apparent diffusion coefficient predicts therapy response in locally advanced rectal cancer
- Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn’s disease
- Infliximab trough level combined with inflammatory biomarkers predict long-term endoscopic outcomes in Crohn’s disease under infliximab therapy
- Up to seven criteria in selection of systemic therapy for hepatocellular carcinoma
