Up to seven criteria in selection of systemic therapy for hepatocellular carcinoma
Tarik Silk,Mikhail Silk,Jennifer Wu
Abstract Barcelona clinic liver cancer (BCLC) intermediate stage hepatocellular carcinoma is a heterogenous disease. Transarterial chemoembolization is offered as the first line therapy in this disease stage. Recent advances in systemic therapy have markedly improved outcomes even in advanced stage disease. The use of systemic therapy in BCLC intermediate stage disease may now be of therapeutic benefit in selected patients. We will focus on “the up to seven” criteria and its utility in selecting systemic therapy.
Key Words: Chemoembolization; Hepatocellular carcinoma; Immunotherapy; Drug combinations; Review; Medical oncology
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 80% of primary liver cancers worldwide[1]. It is one of the cancers with the highest mortality rate, with a 5-year survival rate of only 20%[2]. Treatment of HCC depends on the staging according to the Barcelona clinic liver cancer (BCLC) staging system which is determined by tumor characteristics, liver function (assessed by Child-Pugh score) and patient performance status[3]. Using these criteria, patients may be categorized as early, intermediate or advanced stage disease.
“THE UP TO SEVEN” CRITERIA
Candidates for liver transplantation are most often assessed using the Milan Criteria which was published in 1996. It set strict guidelines to identify individuals who are most likely to benefit from transplantation in an effort to minimize cancer recurrence and maximize overall survival (OS)[4].
Recently the authors of the Milan Criteria have purposed an expansion of the guidelines termed “the up to seven” criteria. In a study of 1556 patients who underwent liver transplantation for HCC, the authors developed software that searched for combinations of tumor characteristics exceeding the Milan criteria, but resulted in an estimated 5-year OS of at least 70%. These found characteristics were termed“the up to seven” criteria. Seven being the sum of the size in centimeters and the number of tumors.Examples, as illustrated in the study, one tumor up to 6 cm in size 6 + 1 = 7, to multiple tumors with seven as the sum of the size plus number (i.e., two tumors up to 5 cm in total size , three tumors up to 4 cm in total size,etc.)[5].
A recent retrospective study comparing OS among liver transplant patients based on their selection by the Milan or “the up to seven” criteria found no differences between the two groups[6].
TRANSARTERIAL CHEMOEMBOLIZATION THERAPY IN INTERMEDIATE STAGE DISEASE
Patients with intermediate stage disease, classified by multi-nodular disease, Child-Pugh A-B, with an ECOG performance status of 0, with no extra hepatic spread are candidates for transarterial chemoembolization (TACE).
TACE therapy preferentially targets HCC due to the tumor’s disproportionally higher arterial vascular supply compared to normal liver parenchyma[3]. The success of TACE was demonstrated with two randomized control trials (RCTs) and a meta-analysis[7-9].
TACE therapy can be given in different forms including by conventional TACE (cTACE), by drugeluting beads-TACE (DEB-TACE) and by bland embolization (TAE) which does not use chemotherapy[10].
In cTACE, a cytotoxic drug that has been emulsified in Lipidol is intra-arterially injected followed by the embolic agent. The efficacy of cTACE was recently reaffirmed with an estimated average median OS of 30 mo[3,11-13].
In DEB-TACE, the embolic agent is loaded with cytotoxic medications[14].
In TAE, embolization is performed without a cytotoxic drug[15].
The differences in outcomes between these techniques have been compared. In a phase III trial the Precision Italia Study Group compared DEB-TACE with cTACE and found no difference in response rates, median time to progression, or survival[16]. This finding was also supported by a meta-analysis of 4 RCTs and 8 observational studies which concluded there was a non-superiority of DEB-TACEvscTACE[10]. Similarly a meta-analysis comparing TAEvscTACE found no difference in OS, or objective response to therapy[15].
However despite similar outcomes TAE therapy has its critics who note TAE therapy results in less tumor necrosis compared to other forms of TACE therapy which may prevent its complete adoption[17,18].
Another criticism of TACE therapy in general is that as a therapy it is non-standardized[19]. This is especially true of TACE therapy with cytotoxic agents as there are several chemotherapeutic drugs which may be used[20]. Additionally the extent to which stasis of flow is achieve in the target vessel is also physician operator dependent[21]. This lack of standardization and dependency on the skill of the interventionist makes a more uniform approachviasystemic therapy desirable.
THE POTENTIAL OF SYSTEMIC THERAPY IN INTERMEDIATE STAGE DISEASE
Predictive factors of whether to initiate TACE include: Tumor size, vascularity, arterial anatomy, infiltrativevsnodular growth, presence of splenomegaly, Alfa-fetoprotein changes, albumin and bilirubin levels[22]. Furthermore the decision to repeat TACE should depend on the response based on modified RECIST criteria to prior TACE therapy[23,24]. Of note as radiographic assessment is dependent on the reading physician it is important that this be carried out by a radiologist experienced in HCC[25].Patients who have an initial complete response to TACE may undergo a second procedure if warranted as long as they are still candidates for therapy. For patients with a partial response or even stable disease repeat treatment at regular intervals may be offered but that decision should be weighed against liver toxicity from treatment[22,26]. Patients with no objective response to two TACE treatments are unlikely to benefit from further TACE and would likely benefit from alternative therapy[26,27]. Even if clinicians are hesitant to choose systemic therapies as initial treatments in intermediate stage HCC,survival maybe improved by switching to these therapies in TACE refractory disease[28,29]. The 2018 OPTIMIS trial followed 1650 patients with unresectable HCC who were to undergo TACE therapy. 31%of these patients became TACE ineligible during the study but only 9% received sorafenib when deemed ineligible for TACE with the remainder having systemic therapy delayed or not receiving it at all[30]. It is therefore critical to determine which patients would be unlikely to benefit from TACE early as to not delay appropriate care (Table 1).
Although current guidelines recommend TACE as first line treatment in intermediate stage HCC, this disease is characterized by high heterogeneity and its real world management may be as equally diverse[27,31].
HCC exceeding “the up to seven” criteria is less likely to respond to TACE due to higher tumor burden[32,33]. In fact, patients beyond “the up to seven” criteria who undergo TACE had higher rates of liver function deterioration post procedure[34]. This is particularly concerning considering poor liver function may preclude patient’s from promising systemic therapies[35,36].
In a retrospective propensity matched study by Kudoet al[37], patients with BCLC intermediate stage HCC beyond “the up to seven” criteria were treated with lenvatinib systemic therapy or TACE.Whereas TACE treatment led to a decline in liver function, lenvatinib treatment did not result in such a decline. OS was significantly longer in the lenvatinib group 37.9 movs21.3 mo; hazard ratio: 0.48,P<0.01. In the study protocol, after progression on lenvatinib, second line treatment including TACE,hepatic arterial infusion chemotherapy, sorafenib, regorafenib, or investigational therapies were allowed. Of note, about 70% of the patients who received lenvatinib underwent subsequent TACE.Patients who received TACE as initial treatment where allowed to undergo repeat TACE. After becoming TACE refractory, second line treatments were identical to the ones in the levantinib group[37].
Recently results from the phase III IMbrave-150 trail have changed management of locally advanced or metastatic/unresectable HCC who are either not TACE candidates or became refractory to TACE. In this trial, the immunotherapy and vascular endothelial growth factor inhibitor combination atezolizumab + bevacizumab was compared against sorafenib, the old standard of care. Median OS was 19.2 mo with the combination therapyvs13.4 mo with sorafenib [HR, 0.66 (95%CI: 0.52-0.85);P= 0.0009][38,39]. This combination was the first to show clinical benefit over sorafenib since 2007 and is now first line therapy in the treatment of advanced stage liver cancer[40]. Immunotherapy doublet combination treatments have also shown promise. In the Checkmate-40 trial, nivolumab plus ipilimumab in the second line setting (after sorafenib) showed median OS of 22.8 mo with an overall response rate (ORR)of 32%[41]. A similar combination in a phase II study using the anti-programmed death-ligand 1 antibody durvalumab plus tremelimumab (CTLA-4 antibody) for patients who progressed on, were intolerant to, or refused sorafenib showed a median OS of 18.7 mo and an ORR of 22.7%. A trial of this combination in the first line is being tested in the phase III HIMALAYA study[42]. A press releases from the trial stated that the combination significantly improved OS compared to sorafenib with an HR of0.78[43,44].

Table 1 Considerations in initiating systemic therapy over transarterial chemoembolization[26,44,53-55]
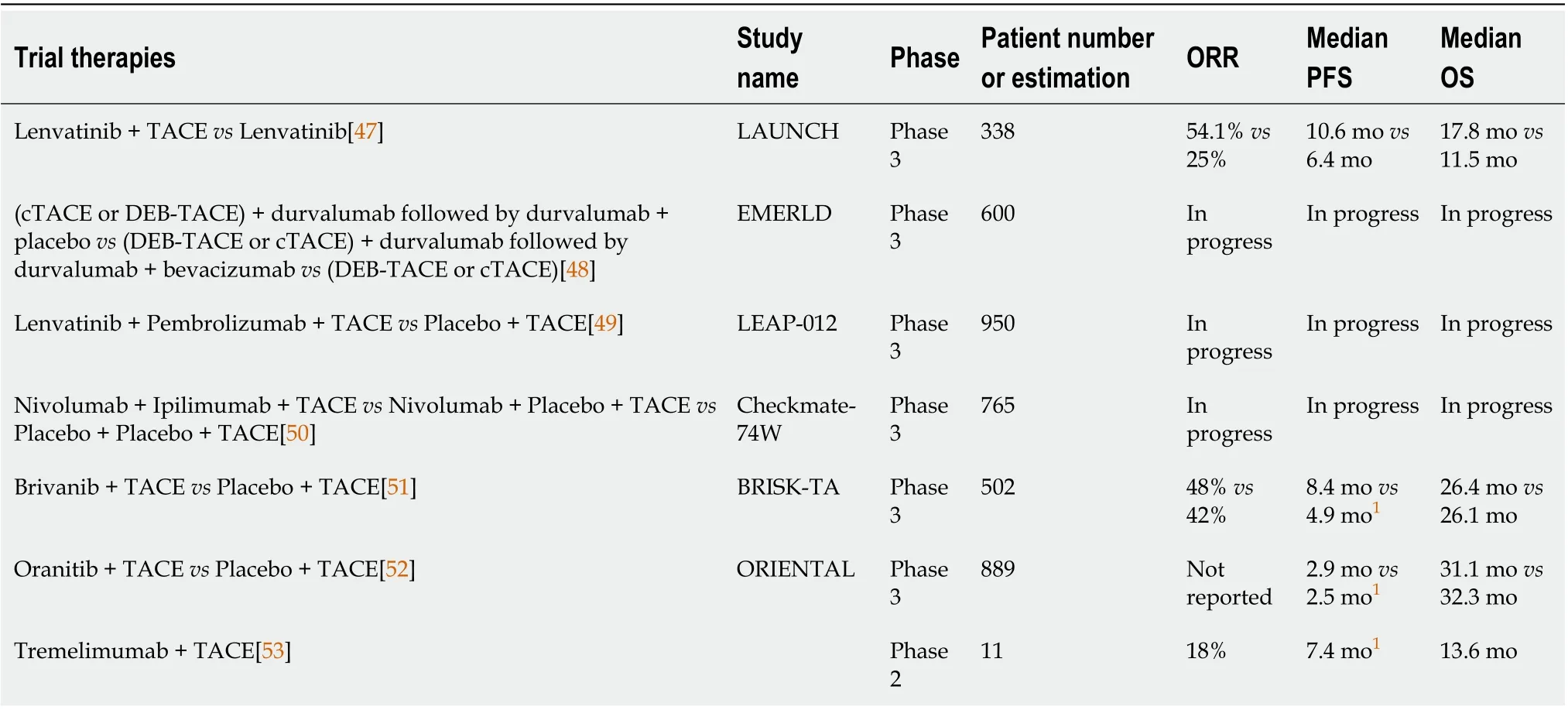
Table 2 Combination therapy trials
In select BCLC intermediate stage disease systemic therapy should be considered in the frontline setting, especially for patients who have been refractory to TACE or in whom TACE is unlikely to be effective. Patient’s unlikely to respond well to TACE include patients who exceed “the up to seven”criteria, as well as those who have tumors without a clear boundary, multifocal tumors, or poorly differentiated HCC[33,34,36,45,46].
As a heterogenous disease BCLC intermediate stage HCC maybe best treated with combination therapy. In fact, the success of combination therapy in advanced disease is now being tested in BCLC intermediate stage disease. Current investigations that combine TACE with systemic therapy include the phase III LAUNCH study in which patients with BCLC stage C disease was treated with lenvatinib+ TACEvslenvatinib alone. The combination group saw an improved OS from 11.5 to 17.8 mo.Additional the combination had higher ORR, 54.1%vs25%, and higher disease control rate (DCR),94.1%vs73.2%, as well as a longer progression free survival, 10.6 movs6.4 mo[47]. Other upcoming TACE and systemic therapy combination treatments include the studies EMERLD-1, LEAP-012, and Checkmate-74W. EMERLD-1 will assess efficacy and safety for durvalumab monotherapy with DEBTACE or cTACE followed by durvalumab with or without bevacizumab therapy in patients with HCC not amenable to curative therapy. LEAP-012 will test lenvatinib plus pembrolizumabvsplacebo in combination with TACE in patients with intermediate HCC. Checkmate-74W will analyze the combination of dual immune checkpoint blockade and TACEvsmono-therapy immune checkpoint blockade and TACE for patients with HCC exceeding the up to seven criteria[48-50].
Although these ongoing trials are exciting, it is worth noting that several studies which combined TACE and systemic therapy have failed to show desired efficacy. These include BRISK-TA and ORIENTAL which both compared targeted therapy and TACE to TACE alone. In both trials there was no improvement in OS compared to TACE alone[51,52]. Finally in a 2017 study by Duffyet al[53] the addition of anti CTLA-4 immunotherapy in 11 patients previously treated with TACE showed a OS of 13.6 mo which is comparable to systemic therapy alone[53] (Table 2).
CONCLUSION
BCLC intermediate stage disease that exceeds “the up to seven” criteria, especially with lesions larger than 5 cm, is less likely to respond to TACE alone and is therefore a disease that may respond better to systemic therapy[32,33,37,54]. The use of “the up to seven” criteria can be a helpful guidepost for when to consider systemic therapy alone or in addition to TACE. With the recent breakthroughs in immunotherapy for advanced HCC which clearly demonstrated OS advantage over single agent tyrosine kinase inhibitors sorafenib, it is promising that the use of immunotherapy would likely lead to better outcome when used in intermediate disease. However, this conjecture requires validation from prospective phase III studies.
Improvements in the treatment of liver cancer have the ability to change the lives of the nearly 800000 patients diagnosed with liver cancer annually. The use of TACE therapy rightfully remains a cornerstone of treatment. However for patients who are unlikely to benefit from TACE therapy alone such as patients exceeding “the up to seven” criteria, alternative treatments including systemic therapies warrant consideration especially with recent advancements in the field.
FOOTNOTES
Author contributions:Silk T drafted the manuscript, coordinated all the author's efforts and provided the final revisions; Silk M edited the section related to interventional radiology and TACE; Wu J provided the concept of the manuscript, established the structure of the manuscript, offered the references and revised the drafts.
Conflict-of-interest statement:Dr. Silk has nothing to disclose.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Tarik Silk 0000-0003-2291-2417; Mikhail Silk 0000-0002-4616-7485; Jennifer Wu 0000-0002-1714-0021.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年23期
World Journal of Gastroenterology2022年23期
- World Journal of Gastroenterology的其它文章
- Reconstructing the puzzle of the role of therapeutic endoscopy in the management of post-bariatric surgery complications
- Primary gastric dedifferentiated liposarcoma resected endoscopically: A case report
- Whole lesion histogram analysis of apparent diffusion coefficient predicts therapy response in locally advanced rectal cancer
- Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn’s disease
- Infliximab trough level combined with inflammatory biomarkers predict long-term endoscopic outcomes in Crohn’s disease under infliximab therapy
- Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol
