Whole lesion histogram analysis of apparent diffusion coefficient predicts therapy response in locally advanced rectal cancer
Mayra Evelia Jimenez de los Santos, Juan Armando Reyes-Pérez,Victor Dominguez Osorio, YolandaVillasenor-Navarro, Liliana Moreno-Astudillo, Itzel Vela-Sarmiento, Isabel Sollozo-Dupont
Abstract
Key Words: Apparent diffusion coefficient; Diffusion-weighted imaging; Histogram analysis; Magnetic resonance imaging; Locally advanced rectal cancer
INTRODUCTION
Neoadjuvant chemoradiation therapy (nCRT) is the gold standard treatment for patients with locally advanced rectal cancer (LARC), followed by surgical resection and adjuvant chemotherapy[1,2]. After nCRT, the ability to achieve tumor reduction or even a pathological complete response (pCR) is observed in approximately 75% of treated patients, whereas the remainder exhibited no treatment response[3,4]. The ability to predict the response to nCRT is important for patients with potentially curable LARC who wish to explore personalized treatment to expand their therapeutic outcomes[5].
Functional magnetic resonance imaging (MRI) techniques, such as diffusion-weighted imaging(DWI), can provide additional physiological information about a tumor’s cellular environment, offering great potential to evaluate the therapeutic response to nCRT[5]. This is because the apparent diffusion coefficient (ADC), a quantitative parameter used to assess water diffusion through tissue in DWI, shows an inverse relationship with tissue cellularity[6]. Viable tumor cells restrict the mobility of water,whereas necrotic tumor cells allow the increased diffusion of water molecules[7].
The possibility that ADC may be associated with the nCRT response has been amply investigated in LARC[8-12]; however, significant correlations have not been found in any studies to date[10]. Inconsistencies in previous findings may be due to a lack of standardized imaging and acquisition techniques[5,11], but they may also be due to the fact that the ADC measurements were performed using a manually drawn region of interest (ROI) from a single slice of the ADC map, which holds limited ability to reflect the actual whole-tumor characteristics[13-15].
In the case of whole-lesion histogram analysis of the ADC, a volumetric ROI is positioned on the entire lesion over contiguous slices and a histogram of ADC values reflecting voxel frequency is constructed, leading to the improved evaluation of heterogeneity[16]. Based on this method, first-order heterogeneity parameters can be obtained, which assess the spectrum of ADC values gained from all voxels within a volume of interest[17]. A growing number of studies have used ADC histogram parameters, as these analyses provide additional information that can aid in the discrimination between benign and malignant regions, or they can help to better characterize the response to treatment in different tumors, such as ovarian, prostate, and breast cancer[18-21]. The application of whole-volume ADC histogram analysis in rectal tumors is increasing in frequency as well, and the role of this parameter in predicting nCRT is promising but limited[22-25].
The purpose of this study was to investigate the imaging response to nCRT using DWI in patients with LARC. We hypothesized that the ADC histogram-derived parameter might better predict treatment responses to nCRT compared with ADC from the hotspot ROI, as histogram parameters can display the heterogeneous features of tumors.
MATERIALS AND METHODS
Patients
The institutional review board approved this retrospective study, and the requirement to obtain informed consent was waived given the study’s retrospective nature. The study population was selected from LARC patients at our institution between February 2015 and October 2020. According to Enkhbaataret al[23], we defined the inclusion criteria as follows: (1) Proven histopathology of rectal adenocarcinoma; (2) greater than stage T2 on pre-nCRT MR imaging; with or without regional lymph node metastases and no distant metastases; (3) pre- and post-nCRT rectal MRI imaging with diffusionweighted (DW) imaging; (4) long-course nCRT; and (5) surgical resection. Mucinous tumors were excluded from this study.
Forty-eight patients were enrolled in the study (34 men and 14 women; age range: 28-84 years). All patients were further divided into two subgroups based on the pathological response of the primary tumor: responders (R) and non-responders (non-R). Only patients with grade 0 according to the TRGRyan system were regarded as patients with a complete pathological response (R), while patients with TRG 1-3 were non-R.
MRI protocol
All images were obtained on a 3T MRI system (Discovery MR 750w GEM®; General Electric Healthcare,Milwaukee, WI, United States) using a phased-array body coil. Intravenous antispasmodic agents were not administered, and patients received no bowel preparation before the MRI examination. Our study groups comprised patients who underwent pre-treatment MRI for primary tumor staging, and a second restaging MRI for response evaluation 6 wk after the completion of nCRT. The scanning protocol is listed in Table 1[23]. In brief, we obtained standard T2-weighted (T2W) spin-echo sequences in axial,coronal, and sagittal directions. To improve tumor tissue visualization (including the delineation of the muscular layer), these planes were planned perpendicular to the main axis of the tumor. Moreover, a T1W spin-echo sequence in an axial direction, as well as an axial non-enhanced DWI with b = 1200 s/mm2, were acquired. ADC maps were automatically generated using the in-line software provided by the vendor during image acquisition. Additionally, axial, sagittal, and coronal fat-suppressed contrast T1W sequences were acquired and used to suppress the signal from adipose tissue. A gadolinium-based contrast agent (Gd-DTPA, Magnevist; Bayer Schering, Berlin, Germany) was used to enhance the quality of MRI. Representative images of our MRI protocol are provided in Figure 1.
Image analysis
Two radiologists (JARP and MEJ, with 10 years and 5 years of experience in gastrointestinal imaging,respectively) reviewed the imaging studies and performed all tumor measurements on the pre- and post-nCRT images. At the initial review, each radiologist was blinded to the other radiologist’s opinion.Also, they were blinded to the pathology results to assess interobserver and intraobserver variability.After that, the two radiologists would hold a discussion to arrive at a final decision by consensus. If a disagreement occurred, another radiologist with 25 years of experience (YVN) aided in making the final decision.
DWI analysis was performed with a workstation using the GE Advantage Workstation 4.6 software featuring the READYVIWER application (2006-2010; General Electric, Boston, MA, United States). On the pre-nCRT b1200 diffusion images, the tumor was defined as a focal mass with high signal intensity in comparison with the signal of the normal adjacent rectal wall. More precisely, the delineated ROIs covered the edge of each lesion, and the ROIs were drawn along the inner margin of the rectal walls to avoid intraluminal gas, water, and other contents. Further, necrotic areas, cysts, and vessels related to each lesion at the corresponding slice were also avoided, as identified on T2WI images. In addition, the highest and lowest slices of the DWI images were excluded given their partial volume effects[24]. After nCRT, the tumor was defined by focal areas of residual high signal, as identified on the b1200 images within the location of the primary tumor bed and/or corresponding with the residual tumor on T2WI MRI images as a reference standard. To compare and identify the tumor location, the pre-treatment images were at the readers’ disposal when analyzing the post-treatment images.

Table 1 Magnetic resonance imaging sequences and data acquisition parameters

Figure 1 Representative images of magnetic resonance imaging protocol. A-C: Axial T2 (A), apparent diffusion coefficient (ADC) map and T2 fusion ADC map color (B) and images of bulky tumor (C), showing tumor extending more than 5 mm into the mesorectal fat and invading the mesorectal fascia.
It should be noted that, in the first instance, one large ROI was placed to cover most of the largest axial tumor cross-section, which facilitated the calculation of the ADCmean values (ROI ADCmean).Thereafter, a volume of interest (VOI) was manually created on the ADC maps, where ROIs were drawn on all tumor slices (whole-lesion measurement). Within this VOI, the following parameters were calculated: (1) ADCmean, the average ADC value of all voxels within the VOI; (2) ADCn% (10th, 25th,50th, 75th, and 90thpercentiles), the point at which the n% of the voxel values that formed the histogram were found to be at the left; (3) skewness, which measures the asymmetry of the distribution of values about the mean value; and (4) kurtosis, which is a measure of the ‘peakedness’ of the distribution of values in the ROI image. The corresponding frequency table for each lesion was exported, and the histogram parameters were computed by SPSS v. 26.0 (IBM Corporation, Armonk, NY, United States).Figure 2 is a schematic illustration of a representative ROI.

Figure 2 Images of rectal tumor before and after neoadjuvant chemoradiation therapy. A, C: T2-weighted magnetic resonance images obtained in 67-year-old man with a rectal tumor (histopathologic response Ryan 1) to evaluate tumor volume; B, D: Diffusion-weighted images (DWI) that were obtained from the same case. As we can see in the present case, regions of interest were drawn manually slice by slice on DWI images along the edge of the lesion to cover as much tumor area as possible without excluding cystic or necrotic areas. nCRT: Neoadjuvant chemoradiation therapy.
Histopathologic review
Specimens were evaluated according to an established protocol that was previously described by our research team[26]. In brief, fresh surgical specimens were evaluated to determine the quality of the mesorectal excision before being fixed in 4% formaldehyde for 48 h prior to sectioning. After fixation,the specimens were serially sectioned (in slices of 1 cm), and the mesorectal boundary was linked. When the residual tumor was visible, a minimum submission of four blocks was recommended. All mesorectal lymph nodes were histologically examined, as was the involvement of the circumferential resection margin. When no residual tumor cells were identified, each block was cut into 3 level sections, and immuhistochemistry for keratin was done. All hematoxylin and eosin slides were reviewed by an experienced pathologist (EHB, with 15 years of experience examining rectal cancer).
The pathologic response of the primary tumor was estimated using the modified Ryan’s classification as follow[26,27]: TRG0, complete response with no viable cancer cells; TRG1 moderate response with single cancer cells or small groups of cancer cells; TRG2, minimal response with residual cancer outgrown by fibrosis, and TRG3, poor response with minimal or no tumor killing and extensive residual cancer.
Statistical analyses
The following formula was used to calculate changes in all metrics included in the current study:PerC=(Parameter post-treatment - Parameter pre-treatment) / Parameter pre-treatment × 100.
It must be noted that when pre- and post-nCRT kurtosis values were obtained, a result of +3.00 indicated the absence of kurtosis. To simplify the interpretation, we adjusted this result to 0 (i.e.kurtosis of -3 = 0). Thus, any reading other than 0 was referred to as an excess of kurtosis. On the other side, to negate division by 0 when calculating the percentage change in kurtosis, we added 3,i.e.[(Kurtosis posttreatment + 3) - (Kurtosis pre-treatment + 3) / (Kurtosis pre-treatment + 3)] × 100.
In the case of skewness, and to avoid dividing by 0, only change (not the percentage change) was used (i.e.skewness post treatment - skewness pre-treatment)[28]. As skewness did not have a lower bound such as kurtosis, the +/- sign was considered to calculate changes in this parameter. To compare variables among R and non-R, a Mann-WhitneyUtest (MWU) was applied, as the Kolmogorov-Smirnov test confirmed the non-normal distribution of any parameter included here. Accordingly, the data were presented as medians and interquartile ranges (IQR)[29]. When the differences in a variable were significant (P< 0.05) in the MWU test, the cut-off value, sensitivity, specificity, positive predictive value, negative predictive value, area under the receiver operating characteristic (ROC) curve (AUC),and accuracy, were analyzed. The optimal cut-off values of ADCmean from the hot spot ROI and parameters derived from the histogram analysis of DWI were determinedviathe Youden index, while differences in the AUC were analyzed according to the method described by DeLonget al[30].Furthermore, the diagnostic odd ratio (DOR) was designed to provide an additional measure of the performance of our potentially useful biomarkers to predict treatment response in LARC.
Finally, the intraobserver variability and interobserver variability were assessed using the intraclass correlation coefficient (ICC). For the agreement analysis, the outcomes were interpreted as follows, in accordance with Cicchetti (1994): 0.2 or less, poor agreement; 0.21-0.40, fair agreement; 0.41-0.60,moderate agreement; 0.61-0.74, good agreement; and 0.75-1.00, excellent agreement[31]. Statistical analyses were performed using SPSS v. 26.P< 0.05 was considered statistically significant.
RESULTS
Among the 58 patients that were originally included in this study, 10 had severe imaging artifacts. Thus,our final sample included 48 patients whose clinical and pathological characteristics are described in Table 2.
The median values and IQRs for the ROI ADCmean values and parameters derived from the histogram analysis of DWI are described in Table 3. Accordingly, post-nCRT kurtosis, as well as postnCRT skewness, were significantly lower in R than in non-R (bothP< 0.001, respectively). Furthermore,our results showed significant differences in the relative changes of kurtosis (Δ%kurtosis) between R and non-R (P< 0.001), with the largest loss of kurtosis in R. Additionally, median Δskewness displayed lower values in R than in non-R (P< 0.001).
We also found that patients with a favorable response (R) had higher post-nCRT ADC10thvalues than did non-R (P= 0.036). Correspondingly, the median values of Δ%ADC10th, Δ%ADCmean, and ROI Δ%ADCmean were also higher in R than in non-R, (P= 0.020,P= 0.032 andP= 0.020, respectively).
Receiver operating characteristics of those parameters that exhibited significant differences in the MWU test are reported in Table 4. The highest AUC values for predicting the treatment response in LARC were demonstrated by Δ%kurtosis, post-nCRT kurtosis, Δskewness and post-nCRT skewness(AUCs = 0.991, 0.985, 0.885, and 0.815, respectively). Meanwhile, the lowest diagnostic accuracy was observed in post-nCRT ADC10th(AUC = 0.681), Δ%ADCmean (AUC = 0.686), Δ%ADC10th(AUC = 0.589)and ROI Δ%ADCmean (AUC = 0.583).
The ROC curves for Δ%kurtosis, post-nCRT kurtosis, Δskewness, and post-nCRT skewness are displayed in Figure 3, while the comparison of AUC values between all of our potentially useful biomarkers for predicting the treatment response in LARC are presented in Supplementary Table 1.
It is important to mention that according to the DeLong analysis, no significant differences were found in the diagnostic accuracy of Δ%kurtosis and post-nCRT kurtosis. As well, no differences were demonstrated between Δskewness and post-nCRT skewness. However, the latter two parameters had lower accuracy than kurtosis-derivate metrics.
Finally, to verify the diagnostic accuracy of all metrics reported in Table 4, we calculated DORs. The DOR of a test is the ratio of the odds of positivity if a patient has a disease relative to the odds of positivity when a patient does not have a disease. The value of DOR ranges from 0 to infinity, with higher values indicating better discriminatory test performance[32,33]. As demonstrated in Table 5,Δ%kurtosis and post-nCRT kurtosis had the highest power of discrimination for treatment response by using DORs (approximately 376), followed by Δskewness (192.2) and post-nCRT skewness (168.6).Meanwhile, the lowest power of discrimination was observed in post-CRT ADC10th(5.48), Δ%ADCmean(4.26), Δ%ADC10th(3.65), and ROI Δ%ADCmean (3.47).
Regarding interobserver and intraobserver variability, the parameters derived from the histogram analysis of DWI, as well as the ADC values from the hotspot ROI, had an excellent agreement. The ICC measuring intraobserver variability ranges from 0.777-0.931 (Table 6), while the ICC measuring intraobserver variability ranges from 0.889-0.993 (Table 7).
DISCUSSION
Heterogeneity of malignant lesions is a feature that can be determined by characterizing changes in the histogram analysis of ADC values, which is recognized as a promising tool in cancer research when discerning between benign and malignant tumors or to better characterize the response to anti-cancer treatments[34-38].
This study focused on the ADCmean from the hot-spot ROI and a series of parameters corresponding to certain points on the ADC histogram using DWI, which have been proposed to predict treatment response in patients with rectal cancer[39,40]. As our results demonstrated, the parameters that changed significantly in response to nCRT were Δ%kurtosis, post-nCRT kurtosis, Δskewness, post-nCRT skewness, post-nCRT ADC10th, Δ%ADCmean, Δ%ADC10th, and ROI Δ%ADCmean. However, thehighest diagnostic accuracy was obtained for Δ%kurtosis, post-nCRT kurtosis, post-nCRT skewness, and Δskewness, suggesting that these metrics might be useful when selecting responders (TRG 0) for an organ preservation approach with either ‘watch-and-wait’ or local excision[39,40].

Table 2 Clinical and pathological characteristics of the patients’ studies

Table 3 Median and interquartile range of pre- and post-neoadjuvant chemoradiation therapy parameters, as well as of changes between pre- and post-treatment values
The results derivate from parameters with the highest diagnostic accuracy in predicting treatment response to nCRT in the current work are reviewed below.
First, we demonstrated that both post-nCRT kurtosis and post-nCRT skewness were significantly lower in R than in non-R. The overall trends from the histogram studies have shown that, following treatment, the histogram analysis ofDWIand diffusion kurtosis imaging (DKI)shifted to the right upon decreased kurtosis and skewness in rectal cancer[39-43]. For example, in 2017, Huet al[39] reported that the post-treatment mean kurtosis derived from DKI showed reduced values in R when compared with non-R patients, whereas Enkhbaataret al[23] (2019) documented that the histogram of R presented negative changes in skewness following a loss of this parameter after therapy.
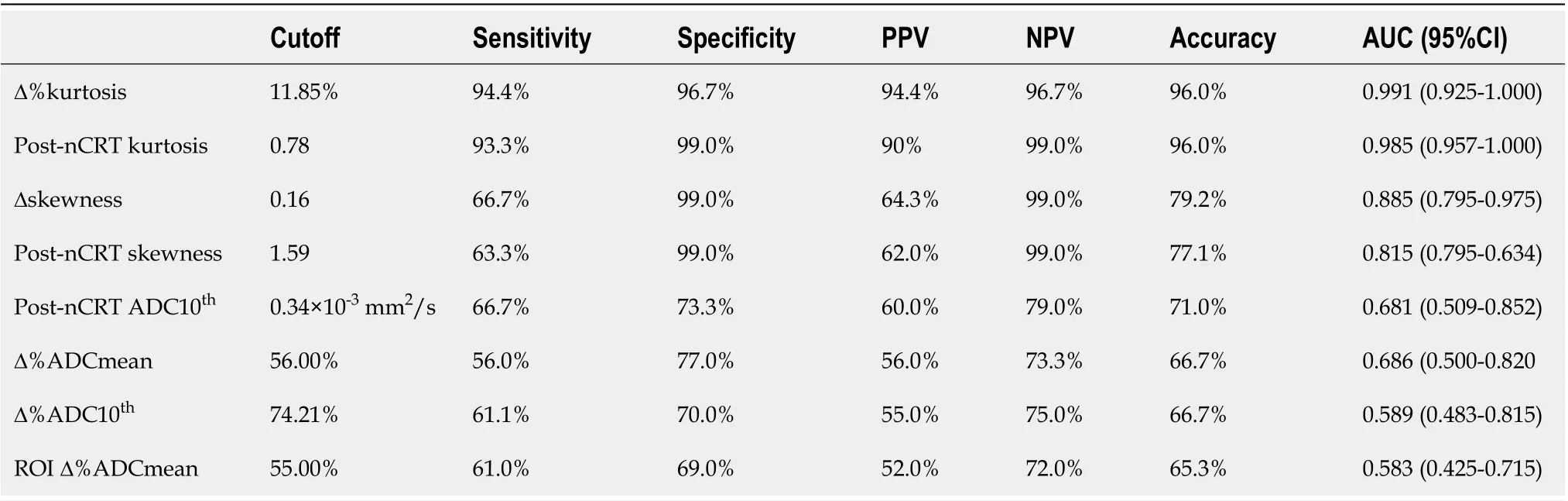
Table 4 Diagnostic performance of the best magnetic resonance imaging histogram derived parameters to detect responder patients

Table 5 Diagnostic odds ratios of magnetic resonance imaging parameters in differentiating respond and non- respond patients in locally advanced rectal cancer
In the same way, kurtosis from R had greater reductions than from non-R, which indicates Gaussian or flatter distributions in patients with a complete response to the therapy. In biological tissues, it is believed that the non-Gaussian behavior (more precisely, a platykurtic curve) of water might occur because of a heterogeneous environment characterized by multiple compartments, organelles, and semipermeable membranes[44]. Thus, when an important reduction in kurtosis is noticed, a higher displacement of water molecules in DWI is assumed.
Furthermore, as mentioned above, negative changes of skewness after nCRT were seen in R, while non-R exhibited positive changes in this parameter. Negatively skewed curves show the majority of scores above the mean, and positively skewed curves are just the opposite[44]. In physiology, the association between changes in skewness and responses to antineoplastic therapy have not been fleshed out, but a curve negatively skewed suggests a loss of cellular structure[23]. Therefore, favorable treatment response is suspected.
Our MWU analysis also found differences between R and non-R across other parameters, such as ADC10th, Δ%ADCth, Δ%ADCmean and ROIΔ%ADCmean, as stated in our results section. However,both the ROC curve analysis and the DOR calculation indicated that only Δ%kurtosis, post-nCRT kurtosis, Δskewness, and post-nCRT skewness appear to predict a favorable response to the therapy,whereas the other metrics did not possess that predictive property.
Briefly, the Youden index calculation indicated that post-nCRT kurtosis, post-nCRT skewness, and Δskewness values below 0.78 × 10-3mm2/s, 1.59 × 10-3mm2/s, and 0.16, respectively, might be significant indicators of the occurrence of pCR. Meanwhile, Δ% changes above 11.85% also indicated a positive treatment effect with high accuracy. It is important to remember that, according to the DeLong analysis, the kurtosis-related parameters exhibit a better diagnostic performance than do skewnessrelated parameters.

Table 6 Intraobserver variability

Test1 and test2, reader290th percentile 0.9010.850-0.975 Basal Test1 and test2, reader1 Skewness 0.9200.900-0.940 Test1 and test2, reader2 Skewness 0.9010.880-0.923 After treatment Test1 and test2, reader1 Skewness 0.9310.920-0.950 Test1 and test2, reader2 Skewness 0.8890.877-0.910 Basal Test1 and test2, reader1 Kurtosis 0.9200.890-0.950 Test1 and test2, reader2 Kurtosis 0.9100.850-0.960 After treatment Test1 and test2, reader1 Kurtosis 0.8900.850-0.960 Test1 and test2, reader2 Kurtosis 0.8800.840-0.982 ADC: Apparent diffusion coefficient; ICC: Intraclass correlation coefficient; ROI: Region of interest.

Table 7 Interobserver variability (intraclass correlation coefficient and 95% confidence intervals)
Aligned with this finding, numerous authors have documented that kurtosis is more directly correlated to the underlying structural, physiological, molecular, and metabolic changes that occur during tumor progression than skewness[45]. This may be the reason why the kurtosis of ADC values has been used to indicate deviations from Gaussianity, even in the most challenging mathematical designs that predict the response to chemotherapy, such as radiomics analysis[46-48].
The results obtained from the ROC curved are partially supported by the estimated DORs, which were approximately 376 for both Δ%kurtosis and post-nCRT kurtosis. This means that for the cut-off points of Δ%kurtosis and post-nCRT kurtosis calculated here, the odds for positivity among subjects with a non-pCR was 376 times higher than the odds for positivity among subjects with a pCR. In the same way, Δskewness and post-nCRT skewness demonstrated respectable diagnostic performances with DOR values of 192.17 and 168.56, respectively. Although these values appear to be lower than DORs of Δ%kurtosis and post-nCRT kurtosis, the confidence intervals for these metrics clearly overlap,so we cannot conclude that the kurtosis-related parameters were statistically better than the skewnessrelated parameters using DOR.
Finally, this study confirm that ADC histogram analysis is a reproducible technique. Similarly, van Heeswijket al[49] demonstrated that histogram-derived parameters had good interobserver agreement,with ICC values ranging from 0.80-0.98. This result supports the method’s validity and suggests that it can be used in clinical practice. Furthermore, we utilized non-precise tumor delineation, which was quicker and produced comparable findings to those obtained by an expert radiologist's measurement,suggesting that this technique could be performed semiautomatically with an excellent interobserver agreement. This finding is very important when considering the implementation of histogram analysis in routine clinical practice.

Figure 3 Receiver operating characteristic curves displaying the diagnostic performances of the four histogram parameters derived from apparent diffusion coefficient values with the highest accuracy. A: Δ%kurtosis; B: Post-neoadjuvant chemoradiation therapy (nCRT) kurtosis; C:Δskewness; D: Post-nCRT skewness. AUC: Area under the receiver operating characteristic curve.
Our study had important limitations. First, this was a retrospective, single-center evaluation. We believe that the present study might serve as a foundation for larger prospective studies in the future.Second, we included only a small number of patients (n= 48), while no validation group was included(both restricting the conception of a predictive model by using a multivariate logistic regression analysis). Third, the patient numbers among the different histopathologic TRGs were not well balanced.Only 18 patients (38%) achieved a histopathologic complete response, which may have introduced an element of statistical bias. However, these patients achieved a strict pCR, underlying the high degree of accuracy of our metrics. Fourth, the parameters obtained from the hotspot ROI were not conclusive enough to predict treatment response in the present study. This result is still in significant disagreement with our prior work where we demonstrated a high diagnostic accuracy of the Δ%ADCmean when distinguishing a pCR in rectal cancer by choosing a cutoff value of 55%[26]. Differences in research methods might explain this discrepancy, but we sustain that it is more reliable to use volumetric ROIs than one slice ROIs.
In summary, although further studies are needed to address the limitations of the current work, we demonstrated the benefits of considering measures other than the ROI ADCmean to evaluate the response to therapy in patients with LARC. Moreover, kurtosis and skewness have been selected by many radiomics studies of rectal cancer, emphasizing the importance of first-order statistics features for the assessment of therapy response[47,50]. Our results support the importance of these parameters, but they also helped us to standardize both the extraction and analysis of the data collected, which is a crucial step when developing and validating our own multiparametric model to predict treatment outcomes.
CONCLUSION
Based on the DWI technique, some whole-lesion histogram parameters could provide valuable information when diagnosing rectal cancer. In particular, kurtosis and skewness might be a useful indicator in the preoperative evaluation of a pCR in rectal cancer. Understanding skewness and kurtosis of the ADC parameters is the simplest way to recognize the deviation of Gaussianity, which indicates tumor heterogeneity. Moreover, we demonstrated high interobserver reliability for measurements of all of the histogram-derived parameters analyzed in the current work, addressing the challenges associated with replication that are well-known among more complex predictive models. Further long-term studies are needed to determine the ultimate clinical utility of our results.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions: Sollozo-Dupont I designed the study; Sollozo-Dupont I and Domínguez Osorio V analyzed the data; Domínguez Osorio V and Vela-Sarmiento I collected the data; Sollozo-Dupont I, Jiménez de los Santos ME, and Reyes-Pérez JA wrote the paper; Villaseñor-Navarro Y and Moreno-Astudillo L reviewed the study; Jiménez de los Santos ME and Reyes-Pérez JA contributed equally to this work; All authors contributed to the manuscript for important intellectual content and approved the submission.
Institutional review board statement:The study protocol was approved by the Institutional Review Board of the National Cancer Institute from México, city, and was in accordance with the Declaration of Helsinki (Approval No.2021/026).
Informed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Data sharing statement:The raw data supporting the conclusions of this article will be made available by the authors.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Mexico
ORCID number:Mayra Evelia Jiménez de Los Santos 0000-0003-1350-4761; Juan Armando Reyes-Pérez 0000-0002-1519-3613; Victor Domínguez Osorio 0000-0001-8660-7994; Yolanda Villaseñor-Navarro 0000-0001-5065-7922; Liliana Moreno-Astudillo 0000-0003-3125-708X; Itzel Vela-Sarmiento 0000-0002-6516-4212; Isabel Sollozo-Dupont 0000-0002-4569-3643.
S-Editor:Wang JL
L-Editor:Filipodia
P-Editor:Wang JL
 World Journal of Gastroenterology2022年23期
World Journal of Gastroenterology2022年23期
- World Journal of Gastroenterology的其它文章
- Reconstructing the puzzle of the role of therapeutic endoscopy in the management of post-bariatric surgery complications
- Primary gastric dedifferentiated liposarcoma resected endoscopically: A case report
- Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn’s disease
- Infliximab trough level combined with inflammatory biomarkers predict long-term endoscopic outcomes in Crohn’s disease under infliximab therapy
- Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol
- Up to seven criteria in selection of systemic therapy for hepatocellular carcinoma
