Toxicological advances of traditional medicine in 2021
Fu-Rong Liu,Ling-Ru Gong,Hao-Yan Sun,Long-Zhang Song,Wen-Yuan Gao,Shu-Li Man*
1State Key Laboratory of Food Nutrition and Safety, Key Laboratory of Industrial Microbiology, Ministry of Education, Tianjin Key Laboratory of Industry Microbiology, China International Science and Technology Cooperation Base of Food Nutrition/Safety and Medicinal Chemistry, College of Biotechnology,Tianjin University of Science & Technology, Tianjin 300457, China.2Tianjin Key Laboratory for Modern Drug Delivery and High Efficiency, School of Pharmaceutical Science and Technology,Tianjin University,Tianjin 300072,China.
Abstract In 2021, there have been many studies on the toxicological evaluation of traditional medicine, as well as natural active ingredients.Among them, the toxicity evaluation of evodiamine, Gardenia Jasminoides J.Ellis, and anthraquinone in the liver, kidneys, heart,and other organs has become a popular topic.Their toxic mechanisms include oxidative stress, inflammatory response, apoptosis, mitochondrial damage, and disorders of lipid and amino acid metabolism.In response to the drawbacks of time-consuming, expensive, and ethical restrictions of animal models, a variety of techniques, such as 3D organoid models,metabolomics, toxicokinetics, bioprinting methods, and network toxicology methods, have been gradually employed in the toxicity evaluation of traditional medicine.This review summarizes the drug toxicity and safety assessment of traditional medicine in 2021.In the future, attention should be paid to preventing traditional medicine toxicity.
Keywords: traditional medicine; natural product; toxicity; toxic target organs; risk assessment; safety evaluation
Background
Traditional medicine (TM), with its long history, plays an important role in maintaining national health and economic development.As TM treatment becomes widely accepted, its toxicity in human organs has become an inevitable point of research.In 2021, numerous articles evaluated the toxicity of TM inTetrastigma hemsleyanum,Metagentiana,Radix Astragali,Aconiti Radix,Akebiae Caulis,Kochia Scoparia(L.)Schrad,Semen Strychni,Ganoderma Lucidum,Gynura Segetum(Lour.)Merr.,Galla Chinensis, andForsythia Suspensa[1–11].In addition,common ingredients, such asZingiber Officinale Roscoe,Lentinula Edodes,Kaempferia GalangaL., andArtemisia ArgyiLevl.et Vant.have also been extensively studied [12–15].
In 2021, various theories and detection techniques were applied to the toxicity evaluation of TM.For example, ultra-performance liquid chromatography electrospray mass spectrometry was used to analyze the toxicity ofTetrastigma hemsleyanum[16].Imaging techniques for cardiovascular magnetic resonance have been used to evaluate cardiotoxicity induced by anthracyclines [17].Furthermore,numerous countries have focused on the progress of TM toxicology research in 2021 (Figure 1).Among these countries, China, the USA,and India are ranked from the first to the third most important countries researching the subject.At the same time, toxicological evaluation of TM plays an important role in determining the clinical safety of TM.
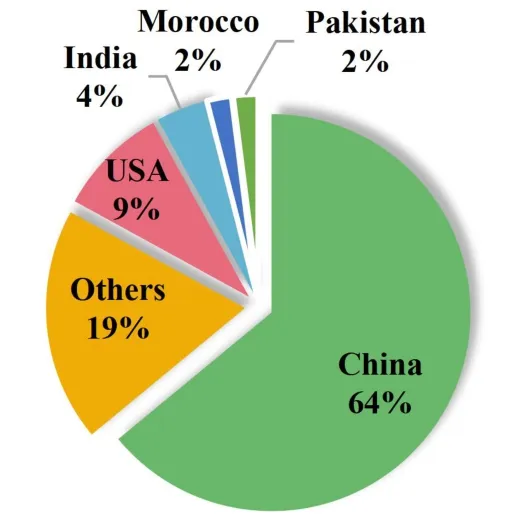
Figure 1 2021 Distribution of annual publications of TM toxicological studies by country.TM, traditional medicine.
Toxic target organs
As shown in Figure 2, the target organs of TM toxicity were categorized into liver, kidney, heart, and other target organs in 2021 publications.Their characteristics are summarized in Table 1.
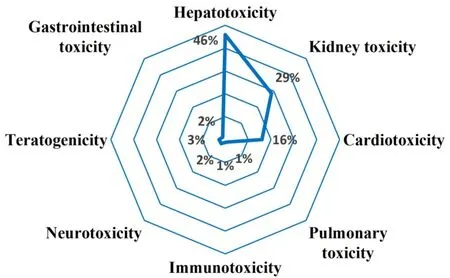
Figure 2 Statistical distribution of annual publications on target organ toxicity induced by TM.TM, traditional medicine.
Liver was regarded as the primary target organ in TM toxicity
As an important tissue for drug metabolism, the liver is the primary target organ for TM toxicity.Alkaloids, terpenoids, and anthraquinones induce hepatotoxicity by inducing abnormal expression of drug metaboling enzyme genes, dysregulation of hepatocyte apoptosis, lipid peroxidation damage, mitochondrial damage, and inflammatory responses [18].
Mitochondrial damage-induced apoptosis is one of the causes of liver injury.For example, chrysophanol-8-O-glucoside in TM, such asRheum PalmatumL.,Fallopia Multiflora(Thunb.) Haraldson, andReynoutria JaponicaHoutt.,inhibits the function of the mitochondrial complex, leads to abnormal oxidative phosphorylation, decreases matrix metalloproteinase, increases reactive oxygen species, and ultimately causes mitochondrial damage and hepatic cellular apoptosis [19].Evodiamine, the volatile fraction of aconite, and pyrrolizidine alkaloids found in the root and aerial parts of the Japanese camellia induced oxidative stress in liver mitochondria,which led to mitochondrial vacuolization and decreased mitochondrial membrane potential, adenosine triphosphate depletion,and cytochrome C release[9, 15, 20].
Damage to lipid peroxidation is also involved in liver injury.For example,Sophorae Tonkinensis Radix et Rhizoma,Dioscorea BulbiferaL.,and the aqueous extract ofSemen Cassiaedisrupted lipid metabolism,increased lipid peroxidation reaction products, and decreased antioxidant activity in the body, causing pathological changes such as edema and acidophilic degeneration [21–23].
The inflammatory response is also one of the main causes of liver injury.For example, it has been shown thatSophorae Tonkinensis Radix et Rhizomainduces mitochondrial apoptosis in hepatocytes by increasing levels of tumor necrosis factor-α (TNF-α) and enhancing the expression of cell adhesion molecule-1 [21].The polyglycosides ofTripterygii Radix et Rhizomainhibit natural killer cells, activate hepatic Kupffer cells, and induce TNF-α [24].
Kidney was considered the second target organ in TM toxicity
The kidney functions as the excretion center for drugs and toxic substances to be removed from the body, and is considered the second ranking target organ of TM toxicity.Nephrotoxicity involves various pathways, including oxidative stress, apoptosis, inflammatory response, mitochondrial damage, fibrosis, Na+-K+-ATPase, and transport proteins [25].For example, nephrotoxicity induced bySemen Strychniis related to abnormal amino acid metabolism,disorders of glycolysis and lipid metabolism, and oxidative stress [7].Triptolide significantly elevated cellular reactive oxygen species levels and induce nephrotoxicity [24].Tripterygiumglycosides activate macrophages to synthesize and secrete TNF-α; affect Fas expression in renal tubular epithelial cells; upregulate the expression of Fas, p53,and nuclear factor kappa-B; and downregulate B-cell lymphoma-2 to induce kidney cellular apoptosis [24].Additionally, evodiamine induces cellular autophagy-related nephrotoxicity [20].
Cardiac toxicity
In addition to hepato-and nephrotoxicity, cardiotoxicity is also an important toxicity target for TM.For example, diterpene alkaloids are toxic compounds inAconitum CarmichaeliDebx induced cardiotoxicity through Ca2+overload, lipid peroxidation, apoptosis, and increased vagal excitability [26].Aconitine, a diester-type alkaloid inAconitum,acts on the muscarinic receptor M2 to slow the heart rate, decrease blood pressure, and cause arrhythmia.Aconitine also acts directly on cardiomyocytes to affect Na+and K+ion transport, Ca2+ion channels,and ion homeostasis, causing cardiovascular damage [4].In addition,Chloranthus HenryiHemsl.may damage cardiac muscle cells or act on central vital cells, destroy the heart muscle or brain stem, and thus lead to the development of heart failure or central respiratory failure[27].
Other target organs of TM toxicity
It has been found thatGardenia JasminoidesEllis extract disrupted endogenous substances such as vitamin B6, phenylalanine, and arachidonic acid metabolism, induced an inflammatory response,caused severe endoplasmic reticulum stress, and apoptosis, thereby resulting in gastrointestinal toxicity [28].Seed extract ofSenna AlexandrinaMill.(Leguminosae) significantly reduces the oxidase activity of interneurons and smooth muscle cells in the large intestine,resulting in excessive disturbances in the intestine and decreased colonic motility [29].
Regarding neurotoxicity, rhynchophylline upregulated NR1 protein expression in the nuclei of nerve cells, downregulated NR2B protein expression in dendritic trunks, dendritic spines, and axons, and led to a decrease in the number of NR1/NR2B receptor family members[30].The water-eluting part ofSiegesbeckia OrientalisL.and datura caused abnormal energy metabolism and oxidative stress injury in rat lungs, thereby inducing pulmonary toxicity [31].Tripterygii Radix et Rhizomapolyglycosides induce teratogenicity by regulating the hypothalamic-pituitary-gonadal axis, testosterone secretion,mitochondrial damage, germ cell apoptosis in male rats, and oxidative stress in female rats [24].
Current advances
Various models were used to evaluate the safety of TM
Safety evaluation is now performed at the cellular, organ, and individual levels.Rodents are used as common models for analyzing the safety of TM because they have the advantages of rapid reproduction, relatively low cost, small size, ease of husbandry, and share many physiological and anatomical similarities with humans.For example, mice were used to evaluate the acute oral maximum tolerated dose values of stevioside M, which has been determined to be non-toxic grade 2 [32].A mouse model was also used to analyze the molecular toxic mechanism of triptolide, which is involved in oxidative stress and inflammatory response and further aggravates liver injury [33].
Meanwhile, the zebrafish model is gradually being recognized as a reliable, rapid, moderate throughput, and cost-effective model for assessing embryotoxicity and elucidating the toxic mechanisms of TM.However, it has limitations due to its anatomical structure and small blood volume, which makes it difficult to detect blood pressure and collect blood.Although zebrafish are highly similar to humans at the genetic level, there are still some differences between them.Therefore,zebrafish can be used for safety evaluation,together with other animal models [34].For example, acute toxicity studies using a zebrafish model revealed thatSemen Strychnihad reversible toxic side effects on the heart, central nervous system, liver, and kidneys [7].Wuccinine changes heart rate and blood circulation and induces heart failure and malformation of the pericardium in zebrafish [20].In addition,Phytolacca AcinosaRoxb.induces hepatotoxicity in zebrafish by decreasing both hepatic cysteine and methionine, thus causing abnormal cysteine and methionine metabolism redox imbalance[35].
Recently, 3D cell culture systems have been easily adapted and scaled up with a high throughput.Liver sphere models have been used in hepatotoxicity evaluations and clearance predictions [36].Drosophila have also become popular in the safety evaluation of various compounds [37].However, few studies have focused on the safety evaluation of TM.Future studies should focus on the application of 3D cell culture systems and drosophila as models for toxicity evaluation of TM.
A clinical phase I trial involves the evaluation of drug toxicity in healthy people.Currently, there are more than 20 Chinese herbal medicines in phase I clinical trials.For example, icaritin displayed safety and survival benefits in patients with advanced hepatocellular carcinoma in a clinical phase I trial [38].
Metabolomics and other novel toxicology technologies
In recent years, metabolomics has been regarded as an effective strategy for toxicological evaluation of TM [39].For example,SemenStrychnicauses both liver and kidney toxicity by inducing disorders of glycolysis as well as lipid and amino acid metabolism disruption [7].Phytolacca AcinosaRoxb.polysaccharides induce hepatotoxicity based on dysregulation of metabolism in a zebrafish model [35].
Other techniques, such as toxicokinetics, bioprinting methods,molecular blotting techniques, microchip organ technology, and network toxicology methods, are gradually being employed in the toxicity evaluation of TM.For example, toxicokinetics have been used in the assessment of anthraquinone and microcystins [40, 41].3D bioprinting technology reproducibly and efficiently manufactures cellular microsphere models to achieve high-throughput drug screening and preliminary knowledge of the pharmacological toxicology of complex TM [42].Additionally, 3D bioprinting technology can be used to study the potentially toxic mechanism of TM by further revealing drug effects on organs and tissues.
Molecular blotting technology can remove toxic components in isolated TM, which is vital in reducing toxicity and increasing effectiveness [43].Organ-on-a-chip can realize drug metabolism and action processes simultaneously within a single-chip system, providing a high-throughput platform for drug development and research, which is not available in two-dimensional cell culture models and animal models, while simultaneously enhancing their functions and sensitivity to toxic stimuli [44].In addition, network toxicology is used for toxicity prediction and evaluation of new drugs to shorten the development cycle, reduce development costs, and improve the success rate of new drug development.It plays an important role in the prediction of hepatotoxic and nephrotoxic components in traditional Chinese medicine [22].
Perspective
Toxicological research progress in 2021 shows that the toxicity evaluation of TM, such as evodiamine,Gardenia JasminoidesEllis,anthraquinone, daphnetin, and dioscin, has become a topic of increasing interest.Their target organs of toxicity are primarily the liver, kidneys, and heart.The main toxic mechanisms include oxidative stress, inflammatory response, apoptosis, mitochondrial damage, and disorders of lipid and amino acid metabolism.Rodent and zebrafish models are still widely used to evaluate the toxicity of TM.In addition, numerous new technologies have been applied to the toxicity assessment of TM.At the same time, many technologies, such as metabonomics, toxicokinetics, bioprinting methods, molecular blotting techniques, microchip organ technology, and network toxicology methods, are gradually being applied.Toxicological research aims to protect people’s physical and mental health.Although TM is relatively safe and effective, it has side effects.Therefore, toxicological research plays a vital role in the protection of human health.Applying different models to analyze the toxicity mechanisms involved in different toxic target organs will promote the safe and rational utilization of TM in the future.
 Traditional Medicine Research2022年4期
Traditional Medicine Research2022年4期
- Traditional Medicine Research的其它文章
- Integration of multi-omics in investigations on the mechanisms of action of Chinese herbal medicine interventions in metabolic diseases
- Active compounds in RenShenJian decoction ameliorate insulin resistance in vitro
- Quality evaluation of Pinelliae Rhizoma using network pharmacology and multicomponent quantitative analysis
- Inhibition of rat prostate smooth muscle contractility by extracts of Costus speciosus(crepe ginger)
- Biologically active components for cosmeceutical use extracted from Chaetomorpha aerea
- Advances in traditional Chinese medicine for respiratory disease therapy in 2021
