Active compounds in RenShenJian decoction ameliorate insulin resistance in vitro
Zhen-Hua Lan,Lan-Fang Tan,Guo-En Wang,Meng-Qiu Tang,Ruo-Hong Wang,Shu-Mei Wang*
1Guangdong Pharmaceutical University, Guangzhou 510006, China.2Key Laboratory of Digital Quality Evaluation of Chinese Materia Medica of State Administration of Traditional Chinese Medicine and Engineering & Technology Research Center for Chinese Materia Medica Quality of Guangdong Province,Guangdong Pharmaceutical University,Guangzhou 510006,China.
Abstract Background: RenShenJian decoction, a combination of Pueraria lobata (Willd.) Ohwi and Panax ginseng C.A.Mey, has been used in China since the Song Dynasty(960–1279 C.E.) to relieve symptoms of diabetes mellitus.However, the key compounds in RenShenJian that ameliorate insulin resistance remain unclear.This study identified the anti-diabetic compounds in RenShenJian by rescuing the decreased function of adenosine 5’-monophosphate-activated protein kinase (AMPK), sirtuin 3 (SIRT3), or glucose transporter isoform 4 (GLUT4). Methods: After streptozotocin-induced diabetic mice were treated with RenShenJian, fasting blood glucose levels and protein expression of SIRT3,p-AMPK, and AMPK were determined.Compounds from RenShenJian in plasma were monitored using multiple responses by liquid chromatography – mass spectrometry.Additionally, two insulin-resistant cell models were incubated with compounds identified in RenShenJian in the blood.Glucose uptake was determined using the fluorescent analog 2-(N-(7-nitrobenz-2-oxa-1,3-dia-xol-4-yl)amino)-2-deoxyglucose.Protein expression levels of p-AMPK, AMPK, SIRT3, and GLUT4 were detected by western blotting. Results:RenShenJian decreased FBG levels and upregulated SIRT3 expression and AMPK phosphorylation in diabetic mice.Thirteen RenShenJian extracts were identified in the blood, 11 of which increased the ratios of 2-(N-(7-nitrobenz-2-oxa-1,3-dia-xol-4-yl)amino)-2-deoxyglucose uptake in two insulin-resistant cell models.Nine extracts increased AMPK phosphorylation, nine increased SIRT3 expression, and six elevated GLUT4 expression in palmitate-induced HepG2 cells.Five extracts – puerarin, puerarin 6''-O-xyloside, genistein, ginsenoside Rb1, and ginsenoside Rd – simultaneously activated AMPK, SIRT3 and GLUT4. Conclusion: A series of compounds in RenShenJian that target AMPK, SIRT3, and/or GLUT4 was confirmed and indicate the chemical material basis of amelioration of insulin resistance by RenShenJian.
Keywords: SIRT3; AMPK; GLUT4; diabetes; Pueraria lobata (Willd.) Ohwi; Panax ginseng C.A.Mey
Background
Type 2 diabetes mellitus is an increasingly prevalent disease and a major global public health problem.Insulin resistance (IR), the main pathogenesis of type 2 diabetes mellitus, is a manifestation of metabolic inflexibility, such as impaired inhibition of gluconeogenesis and decreased glucose disposal function in peripheral tissues, resulting in excess glucose accumulation in the blood [1].
In the liver, metabolic imbalance, such as that caused by fatty acid metabolic disorders, leads to impaired insulin sensitivity and improves compensatory insulin secretion.Excess insulin stimulation or fatty acid-induced lipotoxicity leads to IR through a decrease in glucose uptake and glucose utilization, thereby inducing ectopic fat accumulation in the liver [2, 3].The incidence of liver-related diseases, such as nonalcoholic fatty liver disease, dyslipidemia, and metabolic syndrome, has increased.Thus, reducing fatty acids and excess insulin-induced metabolic imbalance could ameliorate IR-related liver diseases.
Palmitate, the main saturated fatty acid, induces lipotoxicity in hepatocytes and leads to IR by increasing oxidative stress [4].Palmitate-induced endoplasmic reticulum stress is related to the expression of glucose transporters, such as glucose transporter isoform 4 (GLUT4), in HepG2 cells [5].GLUT4 mediates extracellular glucose uptake into the cytoplasm and is regulated by the insulin signaling pathway in the liver [6].Sirtuin 3 (SIRT3), a nicotinamide adenine dinucleotide(+)-dependent deacetylase, regulates glucose oxidation via deacetylation of key enzymes in the Krebs cycle.Reports have indicated that impaired SIRT3 activity leads to IR through activation of oxidative stress [7].Lipotoxicity-induced IR is related to the inhibition of the energy switch adenosine 5’-monophosphate-activated protein kinase (AMPK), which disrupts the metabolic balance in peripheral tissues [8].AMPK improves GLUT4 expression and translocation and upregulates glucose uptake [9].Moreover, studies have indicated that AMPK activation rescues SIRT3 expression in skeletal muscle with decreased insulin sensitivity [10].Therefore,lipotoxicity-related hepatic IR demonstrates impaired function of the AMPK/SIRT3/GLUT4 pathway.Targeting AMPK/SIRT3/GLUT4 increases insulin sensitivity.
RenShenJian (RSJ) decoction was first recorded in volume 58 ofGeneral Records of Holy Universal Relief, which was compiled in 1117 C.E.by the imperial court in the period of Song emperor Huizong[11].RSJ is composed ofPanax ginsengandPueraria lobatain a 1:2 group formula and treats “Xiaoke”, known as diabetes.In ancient China, ginseng andPuerariawere used to treat diabetes mellitus [12].Modern medical research has shown that both can effectively improve IR [13–15].The use of RSJ decoction has been proven in recent years to regulate metabolic disorder in diabetic rats and reduce blood glucose levels [16, 17].
Previous studies have indicated that ginseng extract reduces serum glucose levels by upregulating AMPK and GLUT4 in diabetic mice[18].Ginseng extract also improves SIRT3 expression in aging rats[19].P.lobataextract also augments AMPK activity and GLUT4 expression [20, 21].Thus, we hypothesized that RSJ ameliorates hepatic IR via the AMPK/SIRT3/GLUT4 pathway.More than 200 and 120 components, such as ginsenoside Rg1 (Rg1), ginsenoside Re (Re),ginsenoside Rb1 (Rb1), ginsenoside Rc (Rc), ginsenoside Rd (Rd), and puerarin (Pue), have been found inP.ginsengandP.lobate,respectively; however, the chemical components of RSJ that ameliorate hepatic IR, at least via the AMPK/SIRT3/GLUT4 pathway,remain unclear [22, 23].In this study, the active compounds of RSJ extracts that ameliorate IR were identified in the blood, and the underlying mechanism involved was deduced.
Materials and methods
Preparation of RSJ extract
P.lobataandP.ginsengwere authenticated by Mr.Jizhu Liu from the College of Traditional Chinese Medicine, Guangdong Pharmaceutical University, and voucher specimens were deposited at the Department of Traditional Chinese Medicine Analysis, Guangdong Pharmaceutical University, with accession numbers GDPUTCM-PL2018-R01 (P.lobata) and GDPUTCM-PG2018-101 (P.ginseng).
P.lobata(240 g) andP.ginseng(120 g) were immersed in distilled water (1:10, w/v) for 0.5 h and then boiled for 1.5 h.After filtration,the filter residues were boiled in distilled water (1:8, w/v) for 1 h.After filtration, the filtrates were combined at a concentration of 1.5 g/mL.Protein and soluble cellulose in the extracts were removed using 70% ethanol, and the supernatant was evaporated to dryness.The dry products were dissolved in water at a concentration of 1.5 g/mL.For quantification of the main chemicals in the RSJ extract,samples were detected using a Thermo Scientific Q Exactive Focus Orbitrap liquid chromatography – mass spectrometry (LC-MS) system(Thermo Fisher Scientific, Waltham, MA, USA).
Animal experiments and treatments
Male specific pathogen-free Sprague-Dawley rats were orally administered 18.8 g/kg (the maximum tolerated dose for rats in pre-experiments) of RSJ extract twice daily for 5 days.Before the last treatment, blood samples were collected from the inner canthus,which served as a blank.At the last treatment, the rats were administered RSJ extract 1 h before being sacrificed.Blood samples were collected from the inner canthus of the eyes and used as RSJ-treated blood samples.After centrifugation (1,685 ×g, 3 min),the serum samples were collected.Each serum sample (300 µL) was added with 1.2 mL methanol for protein precipitation, followed by centrifugation at 9,718 ×gfor 20 min.The supernatant was transferred to a new tube and evaporated to dryness under nitrogen at 40 °C.After reconstitution with 60% methanol, the supernatants were filtered through 0.22-μm filters and used for LC-MS analysis.
Male specific pathogen-free C57BL/6J mice (18–22 g) were fed a high-fat diet for 8 weeks.The remaining mice were fed a normal diet.high-fat diet fed mice were administered intraperitoneal injections of streptozotocin(STZ, 40 mg/kg) once a day for 4 consecutive days.The normal diet and high-fat diet groups were injected with the same dose of citric acid-sodium buffer.STZ-induced mice with fasting blood glucose levels >11.1 mmol/L were randomly assigned to the STZ group.Two doses (3 and 5.43 g/kg) of RSJ were administered to the STZ-induced mice for an additional 6 weeks.On the last day, all mice were sacrificed.Plasma and liver tissue samples were also collected.Fasting blood glucose was determined using a glucose kit and the glucose oxidase method (Nanjing Jiancheng Bioengineering Institute,Nanjing, China).Based on the results of the preliminary experiment for dilution concentration, the plasma sample was diluted 10 times with distilled water to be tested.Then, 2.5 μL of sample was added to 250 μL of working solution and incubated at 37 °C for 10 min after gentle shaking, and the absorbance value was determined at a wavelength of 505 nm.Blank wells were replaced with 2.5 μL of distilled water, and standard wells were replaced with 2.5 μL of standard.The calculation formula is as follows:
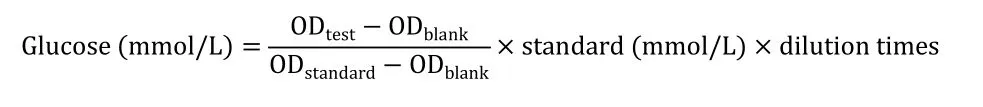
Sprague-Dawley rats and C57BL/6J mice were provided by the Laboratory Animal Center of Guangzhou University of Chinese Medicine and Guangdong Medical Laboratory Animal Center(Guangdong, China), respectively.All animal experimental procedures were approved by the Animal Ethics Committee of Guangdong Pharmaceutical University (approval number: SYXK (Yue)2017-0125).
LC-MS analysis
LC separations were performed on a Dionex Ultimate UPLC 3000 system (Thermo Fisher Scientific, Waltham, MA, USA) with an ACQUITY UPLC® BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters Corp., Milford, MA, USA).The analysis was performed within 44 min at a flow rate of 0.3 mL/min.Samples were eluted using a gradient of 0.1% (v/v) formic acid (solvent A) and acetonitrile (solvent B) as follows: 0–5 min, 5%–19% (B); 5–14 min, 19%–22% (B); 14–35 min,22%–40%(B); 35–38 min,40%–90%(B); 38–40 min,90%(B); 40–41 min, 90%–19% (B); and 41–44 min, 19%–5% (B).The mass spectrometer was operated in negative electrospray ionization full MS-dd-MS/MS acquisition mode with the following parameter settings: sheath flow rate, 45; assistant flow rate, 10 AU; spray voltage, 3 KV; assistant temperature, 200 °C; and capillary temperature, 320 °C.Full scan data were recorded for ions withm/z100–1,500 (Supplementary Figure S1 and Supplementary Tables S1 and S2).The quantification of the RSJ compounds was confirmed by comparison with the MS data (mzCloud: https://www.mzcloud.org/,mzVault, ChemSpider: http://www.chemspider.com/) and retention times of Pue, Rg1, Re, and Rb1 standards.
Cell culture and treatments
Human hepatocellular carcinoma cell line HepG2 cells (purchased from the cell bank of the Chinese Academy of Sciences) was cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in the presence of 5% CO2at 37 °C.HepG2 cells were incubated in serum-free low-glucose Dulbecco’s modified Eagle medium for 12 h, then stimulated with 100 nM insulin (Macklin, Shanghai,China) or 0.2 mM palmitate (PA, Sigma, St.Louis, MO, USA) for 24 h to establish insulin-resistant hepatocyte models [24, 25].Appropriate concentrations of Pue, puerarin 6‘-O-xyloside (Pxy),3'-methoxypuerarin (3'-MP), 4'-methoxypuerarin (4'-MP), Rg1,daidzein (Dai), genistein (Gen), Rb1, ginsenoside Rb2 (Rb2), Rc, and Rd (Chroma-Biotechnology, Chengdu, China) were added to the two insulin-resistant hepatocyte models for 24 h.The purity of all compounds was higher than 98%.
2-(N-(7-nitrobenz-2-oxa-1,3-dia-xol-4-yl)amino)-2-deoxyglucose(2-NBDG) uptake assay
Glucose uptake was determined using the glucose fluorescent analog 2-NBDG (Invitrogen, Barcelona, Spain).Briefly, HepG2 cells were incubated with 50 µM 2-NBDG for 30 min.Subsequently, cells were washed twice with phosphate-buffered saline.Fluorescence intensity was immediately measured at an excitation wavelength of 485 nm and emission wavelength of 535 nm [26].The 2-NBDG content was quantified based on fluorescence intensity.Ratios of 2-NBDG uptake were calculated by normalization to the insulin or PA group.
Western blot analysis
Liver tissues and cells were homogenized using radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology,Shanghai, China).After centrifugation at 12,000 rpm for 15 min, the supernatants were collected.After quantitation, the protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinyldifluoridine membranes(Merck KGaA, Germany).Subsequently, the membranes were blocked with 5% nonfat milk for 2 h, followed by incubation overnight at 4 °C with primary antibodies, including AMPK (1:1000, Cell Signaling Technology, Boston, MA, USA), phospho-AMPK (1:1000, Cell Signaling Technology), SIRT3 (1:1000, Cell Signaling Technology),GLUT4 (1:500, Proteintech, Chicago, IL, USA), and glyceraldehyde-3-phosphate dehydrogenase (1:500, Proteintech).Membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:6000, Proteintech) for 2 h at room temperature.The blotted membrane was detected by enhanced chemiluminescence using ECL Western blotting detection substrate(Thermo Scientific, Waltham, MA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation and were analyzed using one-way ANOVA in SPSS v.19.0 (IBM Corp., Armonk, NY, USA).P<0.05 was set as a threshold of statistical significance.
Results
RSJ reduced plasma glucose and increased hepatic SIRT3 expression and AMPK phosphorylation in STZ-induced diabetic rats
To investigate the positive effects of RSJ on diabetes, STZ-induced diabetic rats demonstrating high plasma glucose levels were administered two doses of RSJ.The two doses significantly decreased plasma glucose levels compared with those in the STZ group (P<0.01, Figure 1A) and had effects comparable with those of 200 mg/kg metformin.As shown in Figure 1B, SIRT3 expression and AMPK phosphorylation in the liver were reduced in the STZ-induced mice.AMPK phosphorylation and SIRT3 expression increased in diabetic mice administered 5.34 g/kg RSJ (P< 0.01 andP< 0.05,respectively).
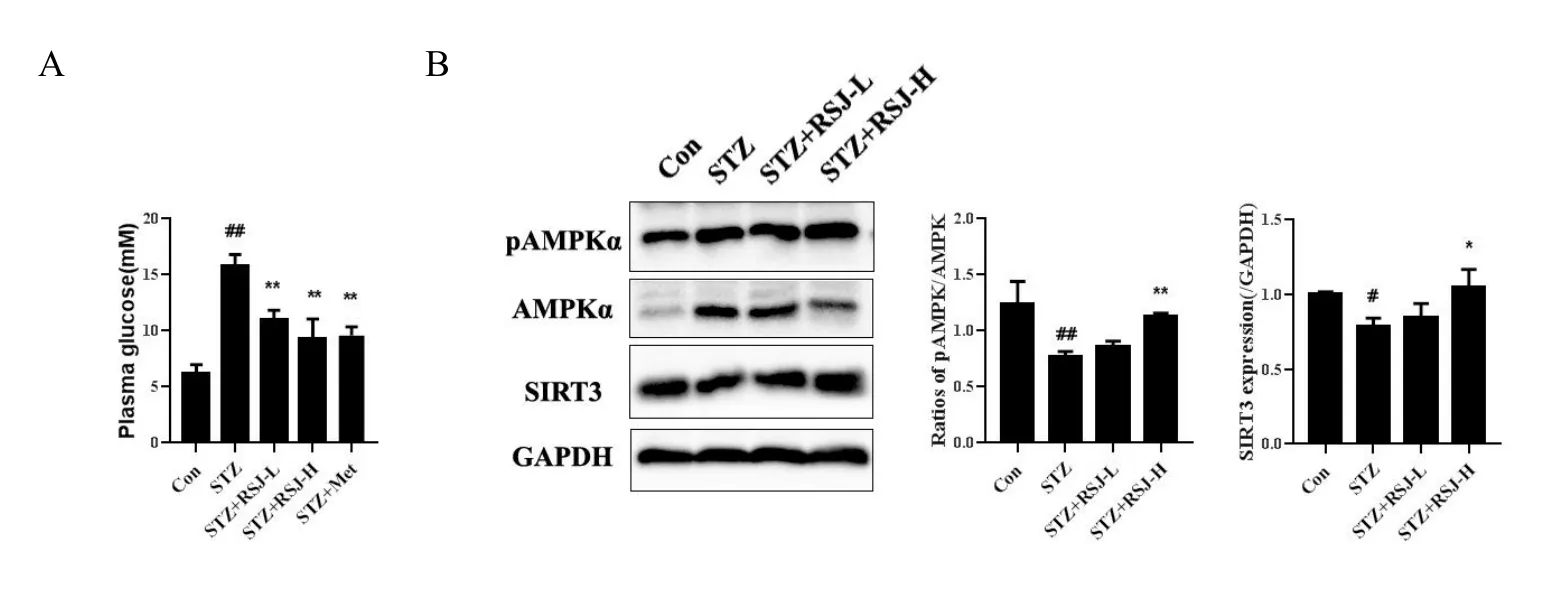
Figure 1 RenShenJian reduced fasting blood glucose levels and increased hepatic SIRT3 expression and AMPK phosphorylation in STZ-induced diabetic mice.STZ-induced diabetic mice were treated with two doses of RSJ daily for 6 weeks.(A) Plasma glucose levels were measured.(B) SIRT3 expression and AMPK phosphorylation were determined using western blotting.Data were collected from 6 mice in each group.Significant differences were observed relative to the control group at#P <0.05 and##P <0.01 and to the STZ-treated group at*P <0.05,and **P <0.01.Con, control group; STZ, streptozotocin-treated group; STZ + RSJ-L, STZ-induced mice administered 3 g/kg RSJ; STZ + RSJ-H,STZ-induced mice administered 5.43 g/kg RSJ; STZ + Met, STZ-induced mice administered 200 mg/kg metformin.RSJ, RenShenJian; AMPK,adenosine 5’-monophosphate-activated protein kinase; SIRT3, sirtuin 3; STZ, streptozotocin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Thirteen major compounds in RSJ in the blood were identified
For further investigation, we detected the compounds in RSJ in the blood of rats treated with RSJ using LC-MS.As shown in Figure 2 and Table 1, 13 compounds Pue, Dai, 3'-MP, 4'-MP, Pxy, mirificin,formononetin, Gen, Rg1, Rb1, Rb2, Rc, and Rd were identified based on structural confirmation, retention time, and them/zvalues of the molecular ions.
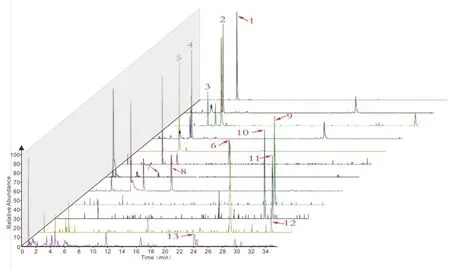
Figure 2 Thirteen major compounds in RSJ in the blood after rats treated with RSJ were identified.Blood samples were collected 1 h after RSJ administration.Blood samples were disposed of and analyzed using LC-MS/MS in negative mode.Data acquisition and total ion chromatograms were processed using Thermo Xcalibur 3.0.The X-axis represents retention time, and the Y-axis represents relative abundance.According to the product spectra and fragmentation patterns of the analytes,the numbers from the figure above represent the following compounds in RSJ: 1, Pue; 2, Pxy; 3, 3'-MP; 4, mirificin; 5, 4'-MP; 6, Rg1; 7, Dai; 8, Gen; 9, Rb1; 10, Rb2; 11, Rc; 12, Rd; 13, formononetin.RSJ, RenShenJian;Rg1, ginsenoside Rg1; Re, ginsenoside Re; Rb1, ginsenoside Rb1; Rc, ginsenoside Rc; Rd, ginsenoside Rd; Pue, puerarin; LC-MS, liquid chromatography – mass spectrometry; Pxy, puerarin 6‘-O-xyloside; 3'-MP, 3'-methoxypuerarin; 4'-MP, 4'-methoxypuerarin; Dai, daidzein; Gen,genistein; Rb2, ginsenoside Rb2.
The primary MS data of peak 2 indicated thatm/z547.15948 was[M-H]-, and the molecular formula predicted by Thermo Xcalibur 3.0 software was C26H28O13.Secondary MS analysis was performed usingm/z547.15948 as the parent ion, and the secondary ion fragments obtained were 547.15948 [M-H]-→295[M-H-xyl-C4H8O4]-→267[M-H-xyl-C4H8O4-CO]-.Based on data from a previous report, peak 2 was characterized as Pxy.Similarly, for peak 3, the molecular formula was determined to be C22H22O10[27].The secondary ion fragments obtained were 445.11368 [M-H]-→ 325 [M-H-C4H8O4]-→ 282[M-H-163]-.For peak 5, the molecular formula was C22H22O9, and the secondary ion fragments obtained were 429.08228 [M-H]-→309[M-H-C4H8O4]-→ 266 [M-H-163]-.For peak 10, the molecular formula was C53H90O22, and the secondary ion fragments obtained were 1123.59082 [M+HCOO]-→ 783 [M-H-132-glc]-→ 621[M-H-132-2glc]-.For peak 13, the molecular formula was C16H12O4,and the secondary ion fragments obtained were 267.06607 [M-H]-→252[M-H-CH3]-→223 [M-H-CO2]-.
According to the characteristic fragmentations of compounds described in references, peaks 3, 5, 10, and 13 were characterized as 3'-MP, 4'-MP, Rb2, and formononetin [23, 27].Peaks 4 and 2 were two isomers, and peak 4 was determined according to the retention time to be mirificin.Peaks 1, 6, 7, 8, 9, 11, and 12 were identified using MS data (supplementary results) and retention time.
Eleven compounds in RSJ in the blood increased 2-NBDG uptake in insulin-resistant HepG2 cells
To investigate whether the 13 main plasma compounds in RSJ ameliorate IR, the glucose uptake activities of the compounds were determined using insulin- or palmitate-induced insulin-resistant HepG2 cells.These HepG2 cells demonstrated decreased ratios of 2-NBDG uptake compared with that in the control group.Eleven compounds Pue, 3'-MP, Pxy, Dai, 4'-MP, Gen, Rg1, Rb1, Rb2, Rc, and Rd significantly increased 2-NBDG uptake in insulin-induced insulin-resistant HepG2 cells (Figure 3A) and palmitate-induced insulin-resistant HepG2 cells (Figure 3B).The results indicated that these components contributed to the positive effects of RSJ on relieving hepatic IR.
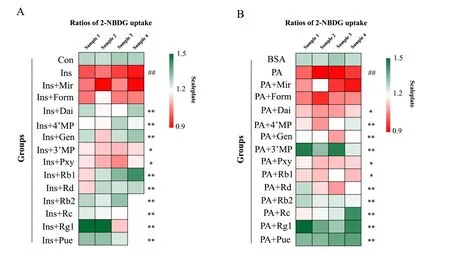
Figure 3 Thirteen compounds in RenShenJian in the blood increased 2-NBDG uptake in insulin-resistant HepG2 cells.Insulin-resistant HepG2 cells were treated with insulin or palmitate for 24 h.(A) Compounds in RSJ were administered to insulin-induced HepG2 cells for 24 h.(B)Compounds in RSJ were administered to palmitate-induced HepG2 cells for 24 h.Heat maps indicate the ratios of 2-NBDG uptake in different groups.The relative ratio of 2-NBDG uptake in each group was normalized to that in the Ins or PA group.Red indicates downregulation, whereas green indicates upregulation.Data were collected from 3 or 4 samples from each group.Significant differences were observed relative to the control or bovine serum albumin (BSA) group at##P <0.01 and to the Ins or PA group at*P <0.05 and**P <0.01.Control and BSA,control groups;Ins,insulin-induced insulin-resistant HepG2 cells; PA, palmitate-induced insulin-resistant HepG2 cells; Ins + Pue et al., insulin-induced cells treated with the different compounds; PA + Pue et al., palmitate-induced cells treated with the different compounds.2-NBDG,2-(N-(7-nitrobenz-2-oxa-1,3-dia-xol-4-yl) amino)-2- deoxyglucose; RSJ, RenShenJian; Mir, mirificin; Form, formononetin; Rg1, ginsenoside Rg1;Re, ginsenoside Re; Rb1, ginsenoside Rb1; Rc, ginsenoside Rc; Rd, ginsenoside Rd; Pue, puerarin; Pxy, puerarin 6‘-O-xyloside; 3'-MP,3'-methoxypuerarin; 4'-MP, 4'-methoxypuerarin; Dai, daidzein; Gen, genistein; Rb2, ginsenoside Rb2.
Glucose uptake activators from RSJ targeted AMPK, SIRT3, or GLUT4 in insulin-resistant HepG2 cells
Next, we investigated whether the 11 compounds activated AMPK,SIRT3, or their downstream regulator, GLUT4, in insulin-resistant cells.As shown in Figure 4A and B, Pue, 3'-MP, Pxy, 4'-MP, Gen, Rb1,Rb2, Rc, and Rd significantly increased AMPK phosphorylation in palmitate-induced insulin-resistant HepG2 cells (P<0.05,P<0.01).Nine of the eleven compounds Pue, 3'-MP, Pxy, Dai, 4'-MP, Gen, Rb1,Rd, and Rg1 increased SIRT3 expression (P<0.05,P<0.01).In addition, GLUT4 expression in insulin-resistant HepG2 cells was improved by incubation with Pue, Pxy, Gen, Rb1, Rd, and Rg1 (P<0.05,P<0.01).Five of these Pue, Pxy, Gen, Rb1, and Rd activated AMPK, SIRT3, and GLUT4 simultaneously.
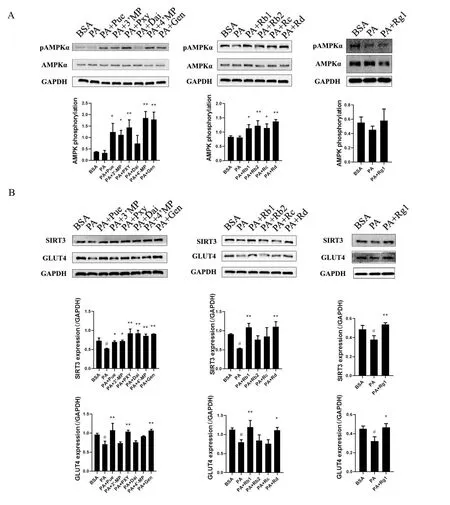
Figure 4 Glucose uptake activators from RSJ targeted AMPK, SIRT3, or GLUT4 in insulin-resistant HepG2 cells.Palmitate-induced insulin-resistant HepG2 cells were incubated with glucose uptake activators from RSJ for 24 h.(A) Phosphorylation of AMPK and (B) expression of SIRT3 and GLUT4 were determined by western blotting.Data were collected from three samples in each group.Significant differences were observed relative to the BSA group at#P <0.05 and##P <0.01 and to the PA group at *P <0.05 and **P <0.01.BSA, control group; PA,palmitate-induced insulin-resistant HepG2 cells; PA + Pue et al., palmitate-induced cells treated with the different compounds.GLUT4, glucose transporter isoform 4; AMPK, adenosine 5’-monophosphate-activated protein kinase; 2-NBDG, 2-(N-(7-nitrobenz-2-oxa-1,3-dia-xol-4-yl) amino)-2-deoxyglucose; SIRT3, sirtuin 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RSJ, RenShenJian; Mir, mirificin; Form, formononetin; Rg1,ginsenoside Rg1; Re, ginsenoside Re; Rb1, ginsenoside Rb1; Rc, ginsenoside Rc; Rd, ginsenoside Rd; Pue, puerarin; Pxy, puerarin 6‘-O-xyloside;3'-MP, 3'-methoxypuerarin; 4'-MP, 4'-methoxypuerarin; Dai, daidzein; Gen, genistein; Rb2, ginsenoside Rb2.
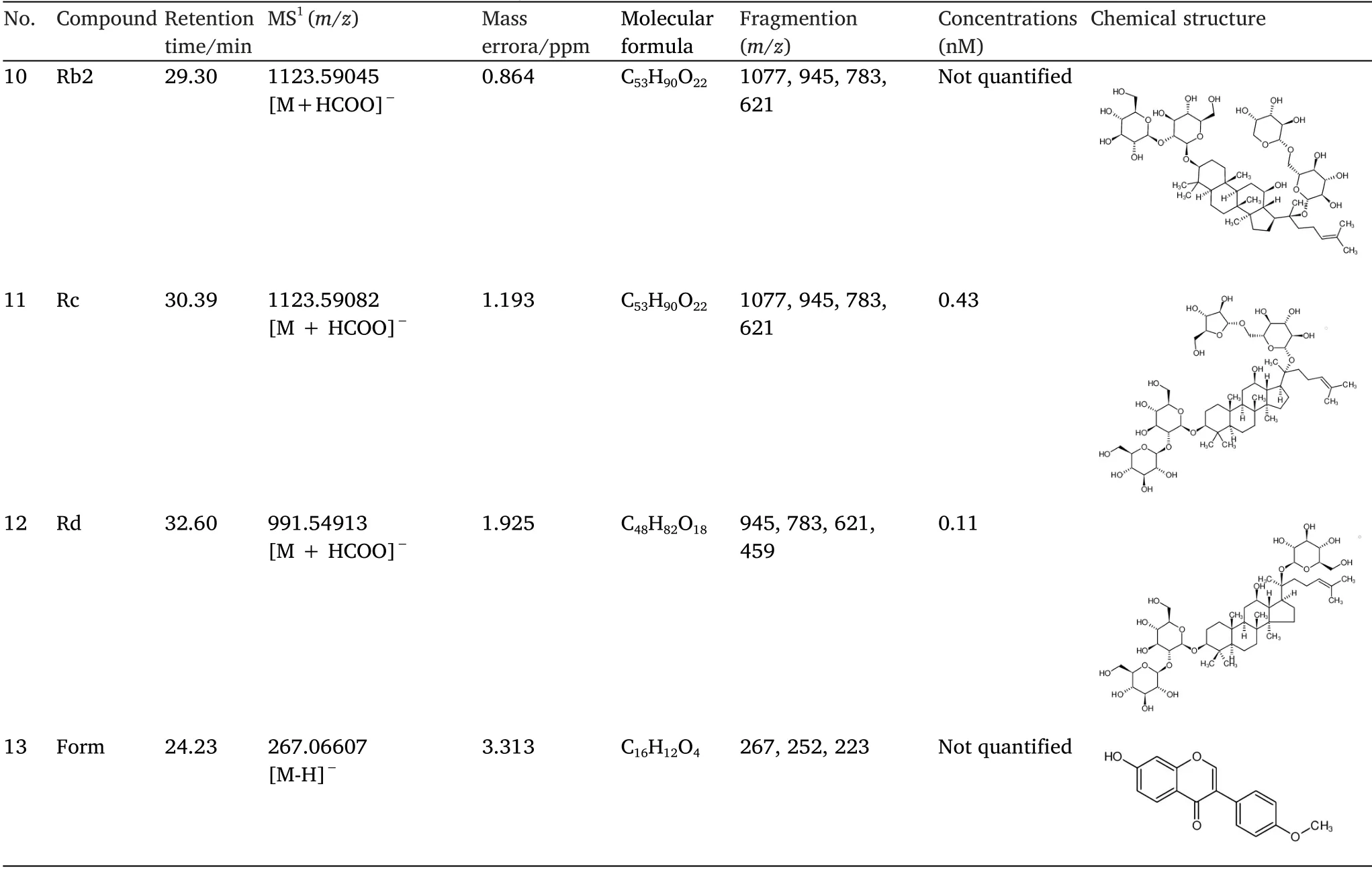
Table 1 Chemical data of the ingredients of RSJ in the blood and their concentrations(Continued)
Discussion
RSJ demonstrated anti-diabetic effects in the clinic and in STZ-induced diabetic mice, and the chemical basis and mechanism of RSJ in ameliorating hepatic IR were investigated.According to the pharmacokinetics of active compounds from ginseng andPueraria,components such as Pue, Dai, Rg1, Re, Rb1, Rc, and Rd can be absorbed into the blood with different pharmacokinetic parameters in rats and mice, but there are few differences in biotransformation pathways[27–29].Thirteen proto-constituents of RSJ Pue, 3'-MP, Pxy,Dai, 4'-MP, Gen, Rg1, Rb1, Rb2, Rc, Rd, formononetin, and mirificin were identified in the blood after RSJ administration.
Hepatic IR causes various diabetes-related complications, such as nonalcoholic fatty liver disease, dyslipidemia, and metabolic syndrome.Our study found that chemicals such as Pue, 3'-MP, Pxy,Dai, 4'-MP, Gen, Rg1, Rb1, Rb2, Rc, and Rd reversed the reduction in glucose utilization in the liver.Our data confirmed the antidiabetic basis of RSJ.
As a traditional Chinese medicine, RSJ is characterized by multiple components, targets, and pathways.Previous data have demonstrated that compound K in ginseng can improve insulin sensitivity by regulating the PI3K/Akt signaling pathway to achieve a hypoglycemic effect [30].Re alleviates IR by activating the peroxisome proliferator-activated receptors-γ pathway, upregulating peroxisome proliferator-activated receptors-γ2, promoting GLUT4 translocalization, and increasing glucose uptake [31].Pue can regulate the IkappaB kinase beta/insulin receptor substrates signaling pathway to inhibit inflammation and alleviate endothelial IR [32].The present data indicate that Pue, Pxy, Gen, Rb1, and Rd from RSJ regulate AMPK/SIRT3/GLUT4 activation in insulin-resistant hepatocytes.Some traditional Chinese medicines relieve symptoms of polydipsia, polyuria, and marasmus via activation of the AMPK/SIRT3/GLUT4 pathway [33, 34].Therefore, upregulation of AMPK/SIRT3/GLUT4 function might be a potential mechanism by which RSJ ameliorates IR.Impaired SIRT3 activity leads to IR through increased reactive oxygen species production and inhibition of β-oxidation [7].SIRT3 activators of RSJ may improve energy metabolism in hepatocytes, thereby increasing glucose metabolic flux.
Several mechanisms underlying the hypoglycemic effects of ginseng andPuerariawere also proposed in the current study, including inhibition by ginseng of cytokine-induced apoptosis of pancreatic insulin-producing β-cells.Several components of RSJ extracts in blood, such as Pue, Gen, Rg1, Rb1, and Rd, have been used to treat diabetic or nonalcoholic fatty liver disease through AMPK-related signaling pathways [35–40].Our data also showed that 3'-MP, Pxy,4'-MP, Rb2, and Rc activate AMPK phosphorylation.
In conclusion, six active compounds that alleviate IR were confirmed, which indicated the chemical material basis of RSJ increasing insulin sensitivity (Figure 5).These components exert a synergistic effect by targeting AMPK/SIRT3/GLUT4.The component group Pue, 3'-MP, Pxy, Dai, 4'-MP, Gen, Rg1, Rb1, Rb2, Rc, and Rd partly explains the mechanism of the anti-diabetic effects of RSJ.
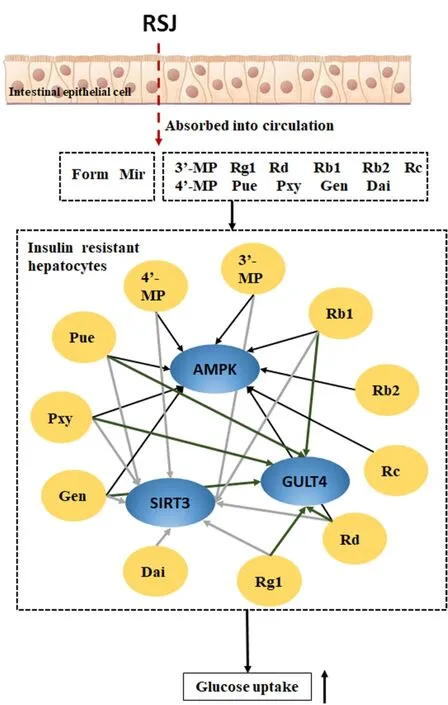
Figure 5 Eleven glucose uptake activators from RSJ confirmed the probable basis of IR amelioration by RSJ partly by upregulation of AMPK/SIRT3/GLUT4 function.GLUT4, glucose transporter isoform 4; AMPK, adenosine 5’-monophosphate-activated protein kinase; RSJ, RenShenJian; SIRT3, sirtuin 3; 3’-MP,3'-methoxypuerarin; Rg1, ginsenoside Rg1; Rd, ginsenoside Rd; Rb1,ginsenoside Rb1; Rb2, ginsenoside Rb2; Rc, ginsenoside Rc; 4'-MP,4'-methoxypuerarin; Pue, puerarin; Pxy, puerarin 6‘-O-xyloside; Gen,genistein; Dai, daidzein.
Chemical data of the RSJ compounds in the blood and their concentrations were determined by LC-MS/MS analysis.Compounds 1, 6, 7, 8, 9, 11, and 12 were identified using standard substances,Pue, Rg1, Dai, Gen, Rb1, Rc, and Rd, based on their product spectra and fragmentation patterns.Compounds 2, 3, 4, 5, 10, and 13 were identified by analyzing their secondary cleavage rules and comparing them with data from previous research [17, 22, 23].The concentrations of Pue, Dai, Gen, Rg1, Rb1, Rc, and Rd in the blood were calculated using external standards as quantitative methods.
 Traditional Medicine Research2022年4期
Traditional Medicine Research2022年4期
- Traditional Medicine Research的其它文章
- Integration of multi-omics in investigations on the mechanisms of action of Chinese herbal medicine interventions in metabolic diseases
- Quality evaluation of Pinelliae Rhizoma using network pharmacology and multicomponent quantitative analysis
- Inhibition of rat prostate smooth muscle contractility by extracts of Costus speciosus(crepe ginger)
- Biologically active components for cosmeceutical use extracted from Chaetomorpha aerea
- Advances in traditional Chinese medicine for respiratory disease therapy in 2021
- Toxicological advances of traditional medicine in 2021
