Handmade tri-leaflet ePTFE conduits versus homografts for right ventricular outflow tract reconstruction
Guan-Xi Wang·Feng-Qun Mao·Kai Ma·Rui Liu·Kun-Jing Pang·Sen Zhang·Yang Yang·Ben-Qing Zhang ·Shou-Jun Li
Abstract Background This study aimed to investigate the performance of handmade tri-leaflet expanded polytetrafluoroethylene(ePTFE) conduits in the absence of a suitable homograft.Methods Patients who underwent right ventricular outflow tract reconstruction with tri-leaflet ePTFE conduits or homografts between December 2016 and August 2020 were included.The primary endpoint was the incidence of moderate or severe conduit stenosis (≥ 36 mmHg) and/or moderate or severe insufficiency.The secondary endpoint was the incidence of severe conduit stenosis (≥ 64 mmHg) and/or severe insufficiency.Results There were 102 patients in the ePTFE group and 52 patients in the homograft group.The median age was younger[34.5 (interquartile range:20.8–62.8) vs.60.0 (interquartile range:39.3–81.0) months,P =0.001] and the median weight was lower [13.5 (10.0–19.0) vs.17.8 (13.6–25.8) kg,P =0.003] in the ePTFE group.The conduit size was smaller (17.9 ± 2.2 vs.20.5 ± 3.0 mm,P <0.001) and the conduit Z score was lower (1.48 ± 1.04 vs.1.83 ± 1.05,P =0.048) in the ePTFE group.There was no significant difference in the primary endpoints (log rank,P =0.33) and secondary endpoints (log rank,P =0.35).Multivariate analysis identified lower weight at surgery [P =0.01;hazard ratio:0.75;95% confidence interval (CI)0.59–0.94] and homograft conduit use (P =0.04;hazard ratio:8.43;95% CI 1.14–62.29) to be risk factors for moderate or severe conduit insufficiency.No risk factors were found for moderate or severe conduit stenosis or conduit dysfunction on multivariate analysis.Conclusion Handmade tri-leaflet ePTFE conduits showed acceptable early and midterm outcomes in the absence of a suitable homograft,but a longer follow-up is needed.
Keywords Expanded polytetrafluoroethylene conduit·Homograft·Right ventricular outflow tract reconstruction
Introduction
Right ventricular outflow tract (RVOT) reconstruction is a common surgical procedure aimed at controlling pulmonary regurgitation and relieving RVOT obstruction,especially for congenital heart diseases.Cryopreserved valved homografts have been the conduit of choice in our institution for 30 years.It remains to be the first choice for RVOT reconstruction because of its excellent valve function and good handling properties during surgery;moreover,it does not require anticoagulation therapy [1].However,its use has been limited due to donor deficiencies and sparse availability.There is a shortage of smallsized conduits,especially for children [1,2],encouraging alternative strategies,such as size-reduced homografts[3,4],bovine jugular vein grafts [5],handmade tri-leaflet expanded polytetrafluoroethylene (ePTFE) conduits [6,7],and other materials [1].Bovine jugular vein grafts have been widely used in our institution and other institutions in China,but they are prone to calcification and failure[5].Previous studies have shown a new bovine jugular vein graft called Contegra may be an attractive alternative to pulmonary homografts,but it is not available in China[8–10].
Several case series from Japan have shown that handmade ePTFE valved conduits are an excellent alternative material for RVOT reconstruction [6,7,11].A multicentric study showed that freedom from reintervention at 5 years and 10 years were 92.3% and 76.1%,respectively[11].With the increasing demand for RVOT reconstruction,standard-stretch ePTFE graft and 0.1 mm thick PTFE membrane have been used as alternatives for RVOT reconstruction when there is no suitable homograft in our institution since December 2016.However,its comparability to homografts has not been examined.Thus,a retrospective study was conducted to compare the performance of a handmade tri-leaflet ePTFE conduit with homografts for RVOT reconstruction.
Methods
Patients
This retrospective study was conducted at the Pediatric Cardiac Center,Fuwai Hospital,Beijing,China.Patients’ demographic characteristics,echocardiographic results,operative records,and surgical outcomes were reviewed.The need for informed consent from individual patients was waived.The study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (ethical number:2017–977;approved:November 30,2019).Between December 2016 and August 2020,a total of 154 patients underwent RVOT reconstruction with either handmade tri-leaflet ePTFE conduits (n=102) or homografts (n=52) at our institution.Patients who underwent reconstruction using bovine jugular vein grafts or other materials were excluded.
Endpoints
The primary endpoint was the incidence of conduit dysfunction,which was defined as moderate or severe conduit stenosis (peak gradient ≥ 36 mmHg) and/or moderate or severe insufficiency.The secondary end point was the incidence of conduit failure,which was defined as severe conduit stenosis (peak gradient ≥ 64 mmHg) and/or severe insufficiency.
Surgical technique
Suturing technique for hand-sewn valved conduit
A handmade tri-leaflet conduit was prepared at another table by a skilled surgeon at the time of surgery.The target diameter of the conduit was selected primarily based on weight and age,with the goal of meeting the expected need for pulmonary flow when the patient grew up.First,the length and height of the leaflets were calculated.The length of the leaflet was equal to one-third of the conduit circumference,and the height of the leaflet was equal to nine-tenths of the leaflet length.One piece of ePTFE membrane (0.1 mm thickness) was fashioned as three semilunar valves,according to the calculated results.Second,an ePTFE conduit with a standard-stretch wall was trimmed to the required length and turned inside out.Three straight lines were marked at every third of the circumference.Next,the three leaflets were sutured to the inside wall using 6–0 polypropylene sutures.Finally,the conduit was turned inside out again to enable the three ePTFE membranes to function as tricuspid valve cusps.Saline injection test was used to evaluate the pulmonary valve function before implantation.Video 1 shows the main construction process.After weaning from mechanical ventilation,anticoagulation therapy with aspirin was routinely administered at a dose of 3–5 mg/kg for at least 6 months.
Homografts
Nearly 100 heart transplantations have been performed every year at Fuwai Hospital in recent years.All the homografts were harvested from the donor hearts or explanted hearts during heart transplantation,and all the procedures had signed informed consent forms and were approved by the ethics committee.Cryopreserved pulmonary homografts(n=47) were the first choice for RVOT reconstruction,but aortic homografts (n=5) were also used when there was no suitable pulmonary homograft.Among them,size-reduced or “bicuspidalized” homografts (n=6) were also used when there was no small-sized conduit available.
All operations were performed through median sternotomy with cardiopulmonary bypass.The distal end was first anastomosed using 6–0 polypropylene sutures,followed by the proximal end.The anastomotic site was reinforced with an autologous pericardial band to prevent possible bleeding.The length of the conduit was designed and trimmed to avoid potential sternal compression.
Data collection and definition
Patient demographics and clinical data were obtained from the local database.Echocardiography was used to evaluate conduit performance,including conduit stenosis and conduit insufficiency over time,and a physician reviewed all previous echocardiograms and performed independent measurements.The grade of the conduit stenosis was measured using the maximum velocities and the pressure gradient across the conduit [12].The grades were as follows:mild stenosis,peak velocity <3 m/s and peak gradient <36 mmHg;moderate stenosis,peak velocity 3–4 m/s and peak gradient 36–64 mmHg;severe stenosis,peak velocity >4 m/s and peak gradient >64 mmHg[12].Conduit insufficiency was graded as grade 0 (absent),grade 1 (trivial),grade 2 (mild),grade 3 (moderate),and grade 4 (severe) according to the features of the jet flow as measured by pulsed Doppler echocardiography [11,13].Early mortality and late mortality were also evaluated,as well as the incidence of postoperative complications,length of intensive care unit (ICU) stay,and postoperative recovery time.Early mortality was defined as both 30-day mortality and death at any time after the operation but before discharge.Late mortality was defined as death after 30 days or after discharge if the length of hospital stay was more than 30 days.
Statistical analysis
Continuous variables are presented as means and standard deviations if the data were normally distributed,or as means and interquartile ranges if the data were abnormally distributed.Differences in continuous variables were assessed using Student’sttest or by non-parametric testing for skewed outcomes.Categorical variables are expressed as frequencies and percentages,and were compared using theχ2 statistics or Fisher’s exact test.The Kaplan–Meier method was used to analyze the patients’ survival rates and freedom from endpoints over time.All statistical tests were two-tailed and aPvalue of less than 0.05 was considered statistically significant.All data were managed and analyzed using SPSS 25.0 for Windows.All figures were made using GraphPad Prism 8.0.
Results
The demographics are presented in Table 1.A total of 154 patients were included in the study:102 patients in the ePTFE group and 52 patients in the homograft group (5 aortic homografts and 47 pulmonary homografts).Compared to the homograft group,the median age at surgery was younger in the ePTFE group [34.5 (interquartile range:20.8–62.8) vs.60.0 (39.3–81.0) months,P=0.001];the median weight at surgery was lower in the ePTFE group as well [13.5 (10.0–19.0) vs.17.8 (13.6–25.8) kg,P=0.003].A total of 50 patients underwent reoperation for previous conduit failure:33 patients (32.4%) in the ePTFE group and 17 (32.7%) patients in the homograft group (P=0.966).
There were also significant differences in the distribution of primary diagnosis,mainly affected by the available conduit size and conduit type (P<0.001).The main diagnoses in the ePTFE group were pulmonary atresia/ventricular septal defect (54.9%),complex double outlet right ventricle(15.7%),transposition of the great arteries/ventricular septal defect/pulmonary stenosis (8.8%),truncus arteriosus (2.9%),corrected transposition of the great arteries/pulmonary stenosis (8.8%),complex tetralogy of Fallot (4.9%),double outlet left ventricle (1.0%),and severe aortic valve stenosis or regurgitation who underwent the Ross procedure (2.9%).As Table 1 shows,there was a significantly higher proportion of patients with truncus arteriosus (13.5%) and who underwent the Ross procedure (28.8%) in the homograft group,while the proportion of corrected transposition of the great arteries/pulmonary stenosis (0%) was significantly lower,compared to the ePTFE group.
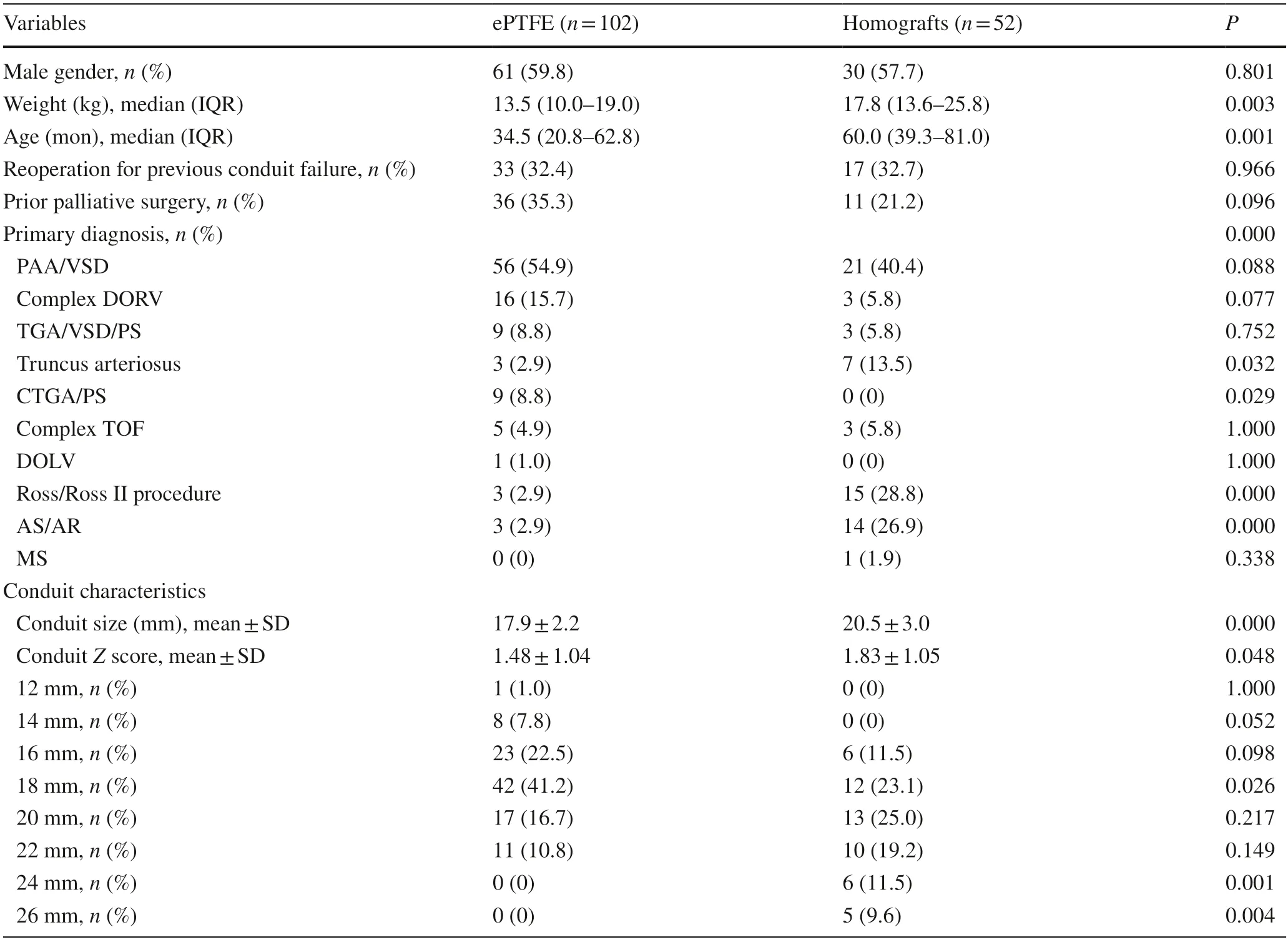
Table 1 Patients’ characteristics
The choice of conduit size depended mainly on the weight and age at surgery,but also on the available conduit size.The distribution of conduit size and weight at surgery is shown in Fig.1.Overall,patients with a higher weight at surgery were more likely to have a larger conduit.Heart transplantations were mostly performed for adults in our hospital,resulting in a shortage of small-diameter homografts.The ePTFE conduit accounted for 84.2% of all the small-sized conduits (≤ 16 mm),including a 12 mm conduit in 1 patient,14 mm conduits in 8 patients,and 16 mm conduits in 23 patients.The smallest diameter in the homograft group was 16 mm,but it was only used in six patients.Larger conduits(≥ 24 mm) were mostly homografts.Thus,the conduit size was smaller in the ePTFE group compared to the homograft group,measuring 17.9 ± 2.2 mm versus 20.5 ± 3.0 mm(P<0.001);the conduitZscore was likewise lower in the ePTFE group,with a mean of 1.48 ± 1.04 versus 1.83 ± 1.05(P=0.048) in the homograft group.
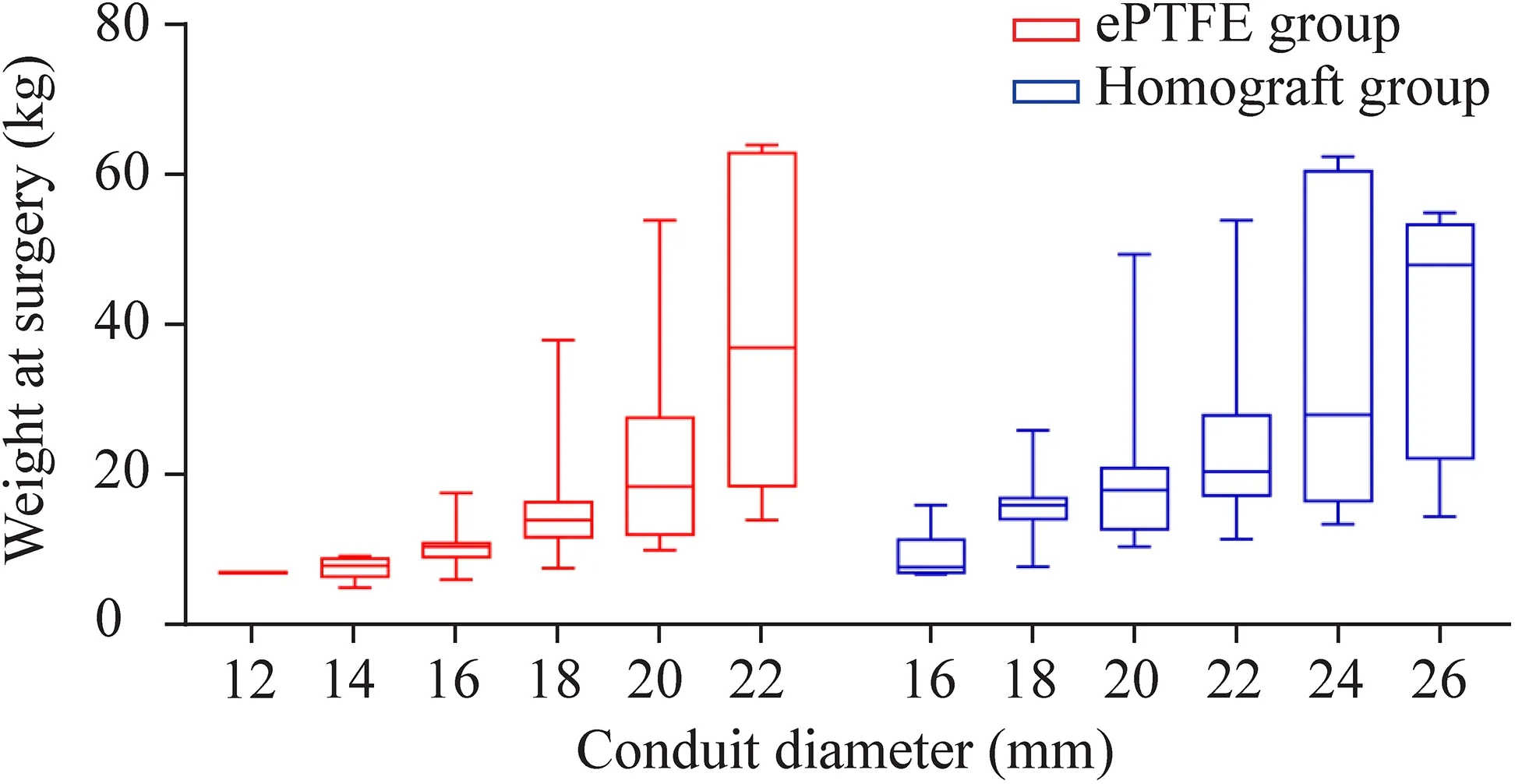
Fig.1 Distributions of body weight in each conduit diameter between the two groups.The middle horizontal line represented the median,and the upper and lower borders of the box represented the upper and lower quartiles.The upper and lower whiskers represented the maximum and minimum values of non-outliers.ePTFE expanded polytetrafluoroethylene
Early death occurred in both groups (P=1.000).There was no significant difference in postoperative recovery between the two groups,including the incidence of delayed sternal closure (2.0% vs.1.0%),extracorporeal support (2.0%vs.1.0%),peritoneal dialysis (6.9% vs.5.8%),diaphragmatic paralysis (2.9% vs.3.8%),third-degree atrioventricular block(1.0% vs.1.9%),and respiratory tract infection (22.5% vs.21.2%).The median mechanical ventilation time was 27.5(19.0–94.5) hours versus 24.5 (15.3–97.3) hours (P=0.586).The median ICU stay was 4.0 (2.0–8.0) days versus 4.5(2.3–8.0) days (P=0.797).However,the peak gradient of the conduit was higher in the ePTFE group after surgery,measuring 11.6 ± 7.0 mmHg,compared to 6.9 ± 6.3 mmHg in the homograft group (P<0.001) (Fig.2),possibly due to the smaller conduit size and lower conduitZscore.The degree of conduit regurgitation after surgery was none in 79(77.5%),trivial in 14 (13.7%),mild in 8 (7.8%),and moderate in 1 (1.0%) of the patients in the ePTFE group,while the degree of conduit regurgitation was none in 31 (59.6%),trivial in 12 (23.1%),mild in 8 (15.4%),and moderate in 1(1.9%) of the patients in the homograft group (P=0.098).
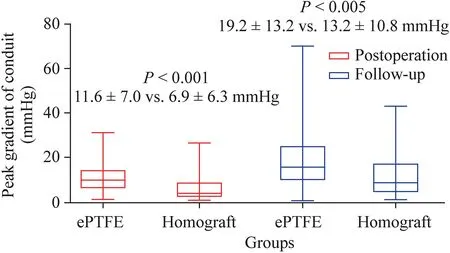
Fig.2 Distributions of peak gradient of conduit after operation and follow-up.The middle horizontal line represented the median,and the upper and lower borders of the box represented the upper and lower quartiles.The upper and lower whiskers represented the maximum and minimum values of non-outliers.ePTFE expanded polytetrafluoroethylene
During the follow-up (18.4 ± 10.6 vs.15.2 ± 10.0 months),one late death occurred in the ePTFE group for an unknown reason;no late death occurred in the homograft group(Table 2).The primary endpoint consisted of moderate or severe conduit stenosis (11.9% vs.7.7%,P=0.580)and/or moderate or severe regurgitation (2.9% vs.11.5%,P=0.062).One patient in the homograft group had moderate or severe conduit stenosis and regurgitation.
Two patients in the ePTFE group and none in the homograft group developed conduit failure (P=0.550).Although asymptomatic,interventions have been recommended for these two patients;however,their parents were reluctant to accept conduit replacement or interventional therapy due to economic factors,and were instead willing to followup.Hence,to date,no interventional catheter-based procedures have been performed in these patients.There was no significant difference in the primary endpoints (P=0.33,Fig.3) and secondary endpoints (P=0.35,Fig.4).However,the peak gradient of the conduit was still higher in the ePTFE group,measuring 19.2 ± 13.2 mmHg,compared to 13.2 ± 10.8 mmHg in the homograpft group (P<0.005,Fig.2).

Fig.3 Kaplan–Meier:freedom from primary end points.Dashed lines indicate the upper and lower limit for 95% CI.ePTFE expanded polytetrafluoroethylene,HR hazard ratio,CI confidence interval
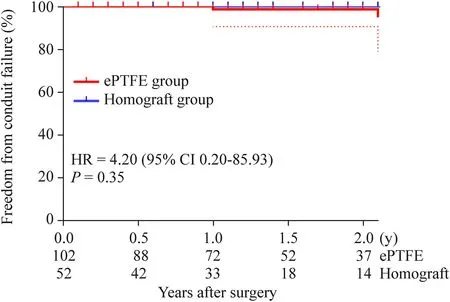
Fig.4 Kaplan–Meier:freedom from secondary end points.Dashed lines indicate the upper and lower limit for 95% CI.ePTFE expanded polytetrafluoroethylene,HR hazard ratio,CI confidence interval
Excluding the small conduits (≤ 16 mm) in the ePTFE group and the larger conduits (≥ 24 mm),there was no significant difference in age,weight,and other demographic characteristics between the two groups.It was estimated that the 1-year and 2-year freedom from the primary endpoint were 92.7% and 78.3% in the ePTFE group,and 98.3% and 82.7% in the homograft group,respectively(P=0.464).No patient developed conduit failure in either group,and the 1-year and 2-year freedom from the secondary endpoint were 100.0% and 100.0% in both groups(P=1.000).
The fate of small conduits (≤ 16 mm) was similar to that of large conduits (>16 mm) in the ePTFE group.It was estimated that the 1-year and 2-year freedom from the primary endpoint were 95.8% and 83.9% in the small conduit group,and 98.3% and 82.6% in the large conduit group (log rankP=0.458).Two pulmonary atresia/ventricular septal defect patients who used 16 mm ePTFE conduits developed conduit failure.One patient developed severe conduit stenosis with a peak pressure gradient of 70.6 mmHg,while the other patient developed severe conduit regurgitation.It was estimated that the 1-year and 2-year freedom from the secondary endpoint were 95.8% and 87.1% in the small conduit group,and 100.0% and 100.0% in the large conduit group,respectively (log rankP=0.061).
A Cox regression model was used to analyze the risk factors for moderate or severe conduit insufficiency.Univariate analysis of variables allowed the selection of variables(P<0.10) for inclusion in the multivariate analysis.Multivariate analysis identified lower weight at surgery [P=0.01;hazard ratio:0.75;95% confidence interval (CI) 0.59–0.94]and use of a homograft conduit (P=0.04;hazard ratio:8.43;95% CI 1.14–62.29) to be risk factors for moderate or severe conduit regurgitation (Table 3).No risk factors were found for moderate or severe conduit stenosis or conduit dysfunction on multivariate analysis.
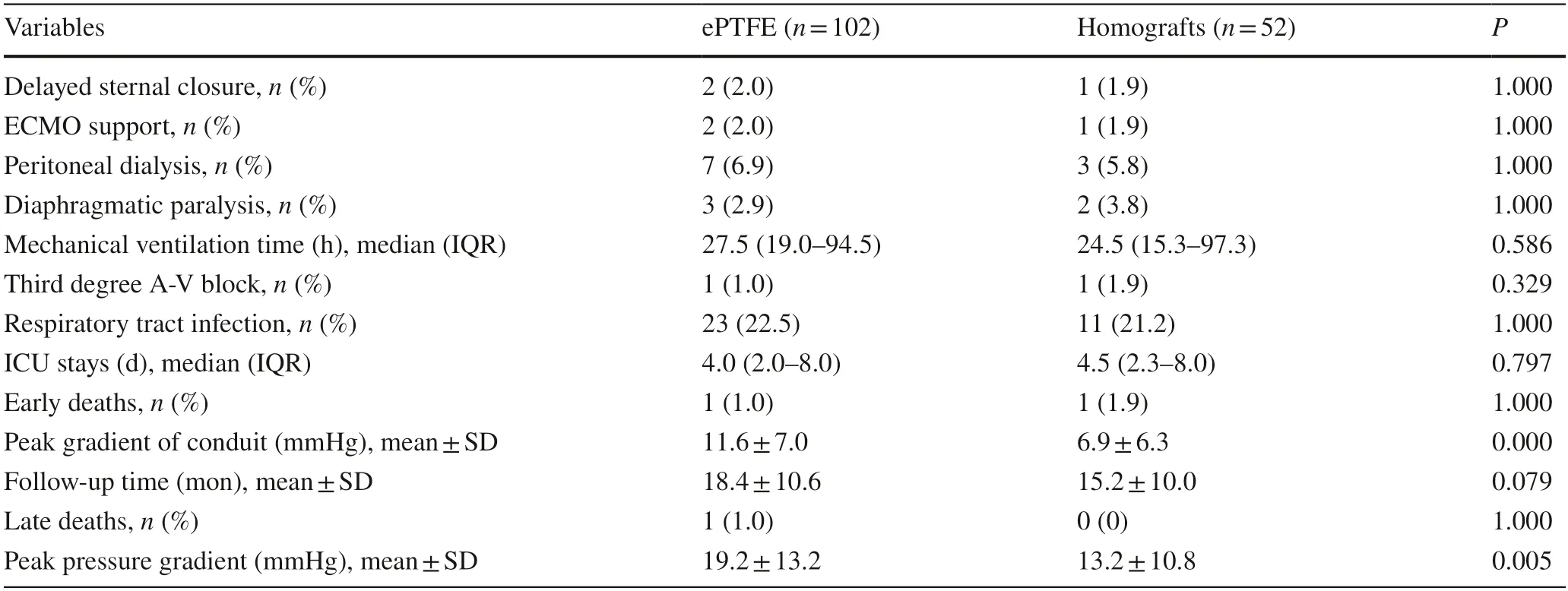
Table 2 Postoperative and follow-up results
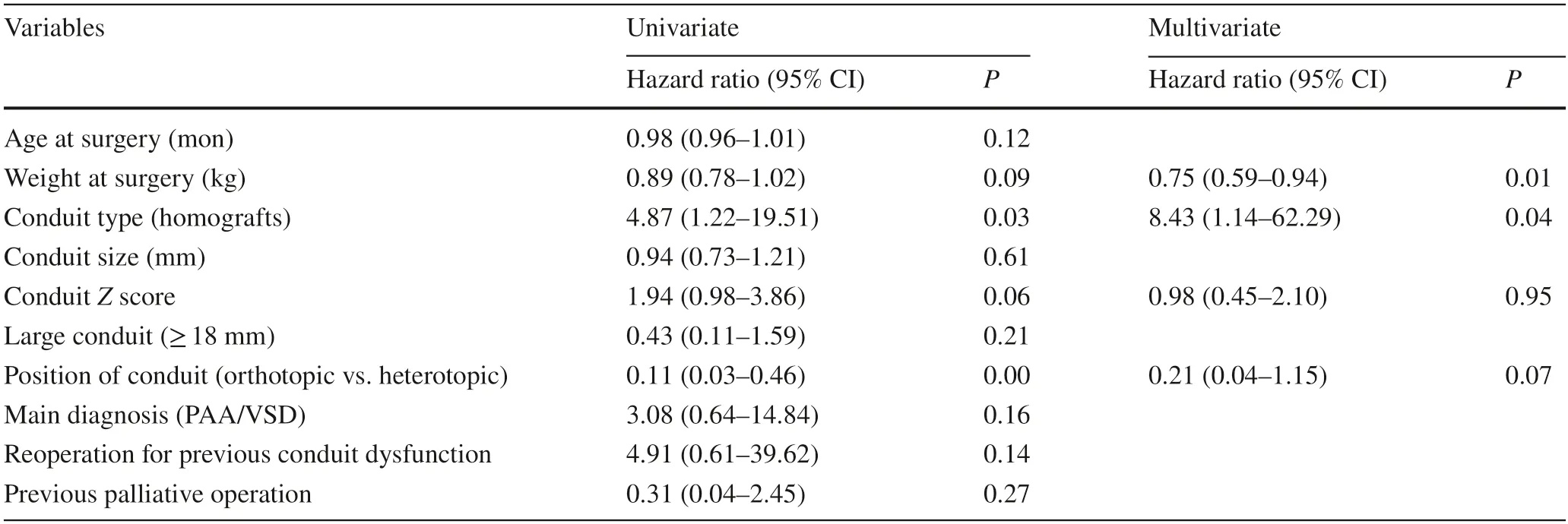
Table 3 Risk analysis for moderate or severe conduit regurgitation (Cox regression)
Discussion
An increasing number of studies have demonstrated the excellent outcomes of ePTFE conduits and introduced new methods of making ePTFE valved conduit [6,7,11,14–16].Many studies have compared the ePTFE conduit with the bovine jugular vein [2,5] and showed lower incidences of perioperative complications,graft failure,and early phase mortality [5].Professor Mercer and his colleagues compared a bicuspid valved ePTFE conduit with a homograft in 2018,showing satisfactory results for RVOT reconstruction with the advantages of availability,low cost,and lack of potential sensitization in patients aged less than 2 years[17].However,few studies have compared the performance of handmade tri-leaflet ePTFE conduits with homografts.The results of our study suggest that handmade tri-leaflet conduits produce acceptable early and midterm outcomes,in the absence of a suitable homograft.
The method of conduit preparation using a standardstretch PTFE graft and 0.1-mm thick PTFE membrane was easy to learn and could be completed in our institution.In contrast to standard-stretch ePTFE conduits,many centers have recommended the design of ePTFE conduits with bulging sinuses in Japan [2,6,11,14,18].Yamagishi et al.developed bulging sinuses on the conduits and patches based on the vortex flow along the sinus of Valsalva,which demonstrated the advantages of a lower pressure gradient through the valves,a larger effective opening area,a faster response in valve movement,and lower energy loss and less regurgitant flow at the valve [19,20].However,the necessity of a bulging sinus remains controversial.The valved ePTFE conduit,with or without bulging sinuses,showed excellent early to midterm outcomes for RVOT reconstruction [21].Some centers have adopted the standard-stretch PTFE graft in Japan [17,21].In addition,standard-stretchePTFE conduits with bicuspid valves showed satisfactory results compared with homografts in patients aged less than 2 years [17].The existence of a bulging sinus may add to difficulties in preparation.Longer follow-up results are needed to confirm the benefits of the bulging sinus.
The inevitability of conduit replacement in infants and young children was in part related to somatic outgrowth of the conduit [22 ],and patients who accepted a smaller conduit had a higher likelihood of reoperation [6,11,18].However,controversies remain regarding the recommended conduitZscore.A retrospective study of small ePTFE conduits(≤ 16 mm) was conducted at 63 Japanese hospitals between 2003 and 2014,which showed that a conduitZscore of approximately 1.4 was optimal for RVOT reconstruction in younger patients [23].A subsequent study showed that the median interval time from implantation to explantation in 10,12,and 14 mm ePTFE conduits were 3.5,4.3,and 4.0 years,respectively [6].They recommended that an ePTFE conduit over 18 mm could be implanted in patients who were approximately 4 years old and/or 12.8 kg in body weight [6].The smallest ePTFE conduit was 12 mm in our institution,and we agreed to use larger conduits at implantation when feasible.For newborns who require very small conduits,ePTFE conduits and homografts demonstrated similar durability as defined by reoperation and reintervention rates in neonatal truncus arteriosus repair [24].
Two patients in the ePTFE group experienced conduit failure but were asymptomatic and did not undergo any reintervention;hence,pathologic results were not obtained.There was no definitive evidence to determine whether or not torn leaflets were present in patients with regurgitation,or if accelerated calcification occurred in patients with stenosis.Miyazaki et al.removed ten conduits for exchange,and their experience demonstrated calcification of the leaflet attachment region,including the interstices of the leaflet,conduit,and suture,which may lead to compromised mobility of the leaflet [11].Histopathological analysis of 15 leaflets from 5 failed conduits suggested that proteinaceous infiltration into the leaflet was responsible for future calcification of the leaflet and subsequent stenotic conduit failure [25].
The ePTFE conduit is a promising material for RVOT reconstruction,and the results encourage us to expand the use of ePTFE conduit for all patients,because of a shortage of homografts of all sizes due to donor deficiencies and limited availability.Our limited experience identified that lower weight at surgery and the use of homograft conduits were risk factors for moderate or severe conduit regurgitation.When there are no suitable homografts for younger children,ePTFE conduits may be more protective for conduit insufficiency.There remains to be a shortage of ideal conduits for RVOT reconstruction;more research is needed to identify the causes of conduit failure and to improve the durability of the ePTFE conduit in the future.
Limitations
This study utilized a retrospective design,and the sample size was small and limited to a single institution.There were significant differences in the preoperative baseline characteristics between the two groups,such as age,weight,primary diagnosis,and characteristics of the conduits.In addition,the median follow-up time was less than 2 years,and a longer follow-up is needed.More research is needed to verify the potential causes of conduit failure in our study.
In conclusion,handmade tri-leaflet ePTFE conduits showed acceptable early and midterm outcomes in the absence of a suitable homograft,but a longer follow-up is needed.
Supplementary InformationThe online version contains supplementary material available at https://doi.org/10.1007/s12519-021-00498-x.
AcknowledgementsWe gratefully acknowledge all the doctors and nurses who were involved in this study.We presented an oral presentation of the paper at the 101th Annual Meeting of The American Association for Thoracic Surgery.This manuscript was done at March 25,2021.
Author contributionsWGX and MFQ contributed equally to this manuscript.WGX designed the study,collected and analyzed the data,and drafted the manuscript.MFQ collected and analyzed the data and drafted the manuscript.MK designed the study,and contributed to the critical revision of the manuscript for important intellectual content.PKJ and ZBQ designed the study.LR,ZS,and YY contributed to the critical revision of the manuscript for important intellectual content.LSJ designed the study and obtained funding,and approved the final version of the manuscript.All authors have read and approved the final manuscript.
FundingThis work was supported by National Key R &D Program of China (No.2017YFC1308100) and Beijing Municipal Science and Technology Commission (No.Z201100005520001).
Data availabilityThe datasets generated and/or analyzed during the current study were available from the corresponding author on reasonable request.
Declarations
Ethical approvalThe study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the Ethics Committee at Fuwai Hospital.The specific ethical number was 201-977 and it was approved on November 30,2019.
Conflict of interestNo financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2022年3期
World Journal of Pediatrics2022年3期
- World Journal of Pediatrics的其它文章
- Long-term physical,mental and social health effects of COVID-19 in the pediatric population:a scoping review
- Prediction modelling in the early detection of neonatal sepsis
- Maternal smoking status during pregnancy and low birth weight in offspring:systematic review and meta-analysis of 55 cohort studies published from 1986 to 2020
- Associations of early-life factors and indoor environmental exposure with asthma among children:a case–control study in Chongqing,China
- Urinary cystatin C:pediatric reference intervals and comparative assessment as a biomarker of renal injury among children in the regions with high burden of CKDu in Sri Lanka
- Occurrence of early epilepsy in children with traumatic brain injury:a retrospective study
