Maternal smoking status during pregnancy and low birth weight in offspring:systematic review and meta-analysis of 55 cohort studies published from 1986 to 2020
Hong-Kun Di·Yong Gan·Kai Lu·Chao Wang·Yi Zhu·Xin Meng·Wen-Qi Xia·Min-Zhi Xu·Jing Feng ·Qing-Feng Tian·Yan He·Zhi-Qiang Nie·Jun-An Liu·Fu-Jian Song·Zu-Xun Lu
Abstract Background Maternal smoking during pregnancy may be associated with low birth weight (LBW) in offspring and global risk estimates have not been summarized previously.We aimed to systematically explore evidence regarding maternal smoking and the LBW risk in offspring globally and examine possible causes of heterogeneity across relevant studies.Methods Comprehensive search of PubMed,Ovid Embase,Ovid Medline (R),and Web of science from inception until October 2021 was carried out.A random-effects meta-analysis was used to estimate the pooled odds ratio (OR) and corresponding 95% confidence interval (CI).Restricted cubic spline analysis with three knots was used to further examine the dose–response relationship.Results Literature searches yielded 4940 articles,of which 53 met inclusion criteria (comprising 55 independent studies).Maternal smoking during pregnancy was significantly associated with the risk of LBW in offspring (OR=1.89,95%CI=1.80–1.98).Furthermore,an obvious dose–response relationship between the amount of cigarettes daily smoked in pregnancy and the risk of LBW in offspring was observed.The results of subgroup analyses indicated that the risk of maternal smoking on LBW was larger in more recently conducted studies (P =0.020) and longer period of active smoking during pregnancy (P =0.002).No evidence of publication bias was found.Conclusions In summary,maternal smoking in pregnancy was significantly associated with a higher risk of LBW in offspring on a global scale.The risk of maternal smoking on infant LBW seems to be increasing over time,and was higher with longer smoking duration throughout pregnancy and more cigarettes smoked daily.
Keywords Low birth weight·Maternal smoking·Meta-analysis·Pregnancy
Introduction
Smoking during pregnancy is known to be harmful to both mother and offspring [1,2].Maternal smoking in pregnancy remains widespread globally in spite of a decline in prevalence due to the public health interventions [3].In 2020,the global prevalence of smoking during pregnancy was 1.7%,and worldwide 250 million women smoke during pregnancy[4].The highest prevalence of smoking was observed in Europe at 8.1%,followed by Central Asia.And in 2018,6.1% of American women reportedly smoked during pregnancy,which was still far from achieving the Healthy People 2020 Objectives [5].To date,while smoking cessation programs have achieved remarkable results,such as a substantial drop in smoking rates among pregnant women in the United States over the past several decades,one in five women of reproductive age are still expected to be tobacco users by 2025 [6,7].Thus,it is still a public health priority to further reduce the harms caused by smoking during pregnancy,justifying the establishment of specific management.
Commonly defined as weight below 2500 g [8],low birth weight (LBW) is also one of the typical adverse outcomes of maternal smoking in addition to reduced fertility [9,10],increased miscarriage [11],as well as birth defects [12] and prematurity [13].LBW has been linked with increased risk of perinatal morbidity and mortality,other physical and developmental morbidities in the neonatal period,and cardiovascular disease in adulthood [13].Though no longer be one of the top five risk factors compared to 1990,LBW remains as the fifth leading risk factor for females according to the Global Burden of Disease Study 2017 [14].
The associations between maternal smoking during pregnancy and the risk of LBW in offspring were reported in population-based observational studies and a previous metaanalysis of studies that involved only the Americans [15,16].However,evidence-based data on a global basis is still lacking on how miscellaneous patterns of maternal smoking during different trimesters as well as daily amount of cigarettes smoked impacted on LBW risk in the offspring.Thus,we performed a meta-analysis of cohort studies across four continents to examine the association of maternal smoking during pregnancy and the risk of fetal LBW,and to explore the dose–response relationship to the extent possible.
Methods
Search strategy
This meta-analysis was performed according to the checklist of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [17].We performed a comprehensive systematic literature search of the PubMed,Ovid Embase,Ovid Medline (R) and Web of science from their inception to October 2021 for potential eligible publications providing evidence on maternal smoking in pregnancy and LBW of infants,with no language restrictions.The search strategy used Medical Subject Headings (MeSH) and a combination of keywords (Supplementary eTable 1).The reference lists of identified articles and relevant review articles were also searched for additional studies.
Selection criteria
We included prospective or retrospective cohort studies providing evidence on maternal smoking during pregnancy (not before) and LBW in infants.We excluded studies in which mothers used smokeless tobacco products,studies that only included women with multiple pregnancies since they carried a higher risk for complications,with immunodeficiency disease,studies conducted with illegal drug users and studies involving prenatal smoking information that did not report incidence of LBW.Additionally,estimates of relative risk(RR) or odds ratio (OR) with 95% confidence intervals (CIs)should be offered in every included study,or sufficient data were there to calculate them.
Two authors independently screened titles and abstracts,and then reviewed full text to select eligible articles.Any difference was settled through consultation with a third author.
Data extraction and quality assessment
From included studies,we extracted the following information:first author,study design (prospective or retrospective study),geographical region,populations,data collection,publication year,confounding factors adjusted for in statistical models and estimated risk with a measure of uncertainty.
The Newcastle–Ottawa scale (NOS) was used to evaluate the methodological quality of included studies,awarding a star rating for each cohort studies.A total of 9 points assigned in three categories of “study population selection”(1 point for each of four items),comparability (2 points for one item),and exposure and outcome measurement (1 point for each of three items) were there in this scale.The quality of each paper is assessed on a high,moderate,and low scale of 0–3,4–6,and 7–9 points,respectively.
Data extraction and quality assessment were conducted independently by two researchers without blinding as to journal,author,or research group.Disagreement was settled through discussion with a third author.
Statistical analysis
OR with 95% CI was used as the effect measure.RR was close to the OR when the incidence rate of outcome of interest (i.e.,LBW in offspring in this paper) in the study population was less than 10%,so the reported RR was approximated as OR in our analysis [16,18].If studies reported data for independent subgroups,an effect combined by a fixedeffect model across subgroups was computed.Additionally,the studies which reported three or more groups of quantitative categories of maternal smoking during pregnancy,were included in the dose–response meta-analysis.The amount of cigarettes daily smoked,case distribution and person-year distribution,ORs and 95% CIs were extracted based on the way expounded by Greenland and Longnecker [19].Dose of smoking was the median cigarettes smoked in each category,or the mid-point of the upper and lower limits for each category if the lowest category or the highest category was half open.The mid-point was set at half of the upper limit value in the left side half open interval,while 1.5 times the lower limit value in the right hand half open interval.A restricted cubic spline regression model with three knots at percentiles 10%,50%,and 90% of the distribution was applied to assess a potential non-linear dose–response relationship between the number of cigarettes daily smoked and the risk of LBW in offspring.Through examining the null hypothesis that the coefficient of the second spline transformation was equal to zero,thePvalue for non-linearity was computed.
Heterogeneity across the included studies was assessed by Cochran Q test (Pvalue <0.10 indicated statistically significant) as well asI-square test (I2 statistic <25% manifested low,25–50% moderate,and >50% high heterogeneity).A fixed-effects model was applied if no significant heterogeneity existed,that is,whenPvalue >0.10 orI2 <50%;otherwise,the outcome data was pooled using a randomeffects model.To delve into the underlying sources of heterogeneity,we conducted subgroup analyses of the following variables:location,study design,sample size,adjusted potential confounders,the World Bank’s income categories,gestational period,daily number of cigarettes smoked,publication year and data collection period.Meta-regression analysis was used to detect the differences in results among subgroups.Sensitivity analysis was performed to explore the stability of these pooled estimates.The Begg’s and Egger’s test and funnel plots were applied to assess the publication bias.The significance level was set atP<0.05,except that the statistical significance of heterogeneity was set atP<0.10.All the reportedPvalues were bilateral,and used in all analyses except for Q statistics.The statistical analyses were performed in Stata12.0.
Results
Numbers of studies at each stage of the selection procedure are shown in Fig.1.We retrieved 4940 studies by searching electronic databases.Of these,we excluded 2206 duplicates,and 2621 irrelevant articles by scanning titles and abstracts.We excluded 58 articles by examining the full-text of 113 potentially relevant studies,based on our inclusion and exclusion criteria.Thus,a total of 55 eligible studies (23 prospective cohort studies and 32 retrospective cohort studies) were included in our analysis,including four independent studies that were reported,respectively,in two published articles.
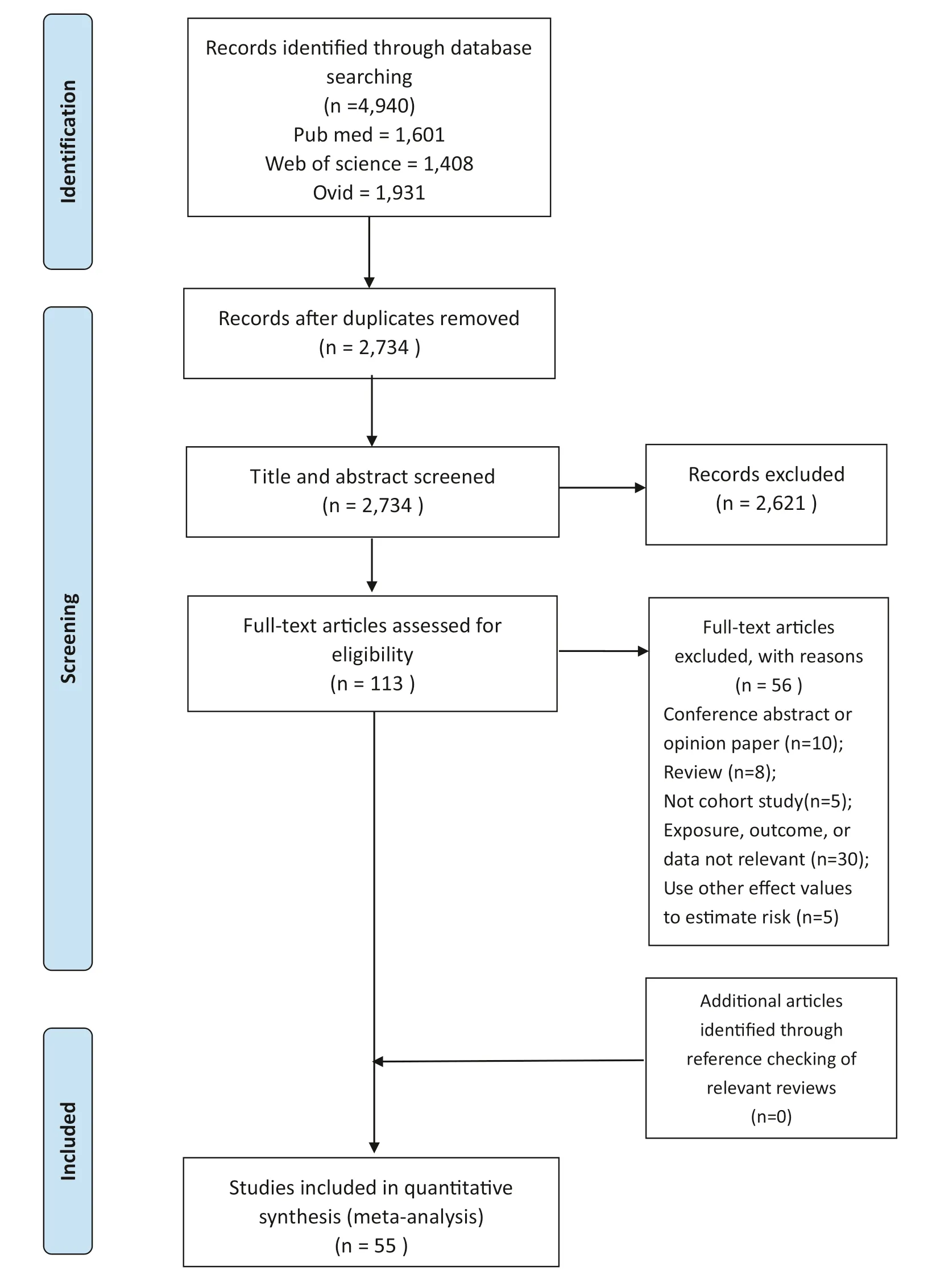
Fig.1 Flow diagram of the study selection process
Characteristics and quality of the included studies
The main characteristics of the included 55 studies,involving sample sizes ranging from 499 to 1.38 million pregnant women,are summarized in Supplementary eTable 2.These studies were published from 1980 to 2020s.The studies were carried out in 21 countries on 4 continents,among which 13 studies (23.6%) were in North America [9,10,15,20–30],9(16.4%) in South America [2,8,31–34],17 (30.9%) in Europe [1,12,13,35–47 ],12 (21.8%) in Asia [48–59],and 4 (7.3%) in Oceania [60–63].Data sources used to ascertain the maternal exposure to tobacco use during pregnancy in these studies included self-report,standard questionnaires,hospital records,and perinatal databases.Data sources for outcome ascertainment included medical records,birth registries or certificates,and perinatal or administrative databases.Among these 52 cohort studies,18 studies [1,2,9,20,23,25,26,28,29,33–35,37,40,42,58,59] reported the unadjusted outcomes and the rest 34 studies were adjusted by various confounders,including sex of newborn,maternal age,family income,maternal schooling,marital status and number of antenatal visits.According to the results of NOS quality assessment,48 (92%) studies had a high quality (7–9 points) and only 4 (8%) studies [2,12,33,41] were of moderate quality (4–6 points).
In addition,based on the information about maternal smoking reported in these articles,smoking status during pregnancy were classified into four categories:(1) smoking prior to conception only or smoking until pregnancy confirmed;(2) smoking only during the first trimester or quit during the first trimester;(3) smoking up to the second trimester but not third trimester or quit in the second trimester;and (4) smoking continued after the second trimester or throughout pregnancy.A total of 4 articles [20,23,26,54] reported information about smoking only prior to conception,7 [13,20,35,51,52,54,55] about smoking only during the 1st trimester,1[20] about smoking up to the 2nd trimester,and 7 [13,23,26,35,51,52,55] about smoking during the whole period of pregnancy.Similarly,according to the amount of cigarettes smoked daily,dose of smoking was classified approximately into three groups:(1) light smoking:≦10 cigarettes/day;(2) moderate smoking:11-19 cigarettes/day;and (3) heavy smoking:≧20 cigarettes/day.A total of 7 [33,44,45,51,55,60,64] articles reported information on the smoking dose subgroups of mothers during pregnancy,among which 6 [33,44,45,51,55,64],7 [33,44,45,51,55,64,65] and 5 [33,44,45,51,65] reported information of light,moderate and heavy smoking,respectively.
Association between maternal smoking during pregnancy and low birth weight
The results across the included studies were statistically heterogeneous (I2 =81.0%,P<0.001).Figure 2 shows a statistically significant association between maternal smoking in pregnancy and the risk of LBW (OR 1.89,95% CI 1.80–1.98).
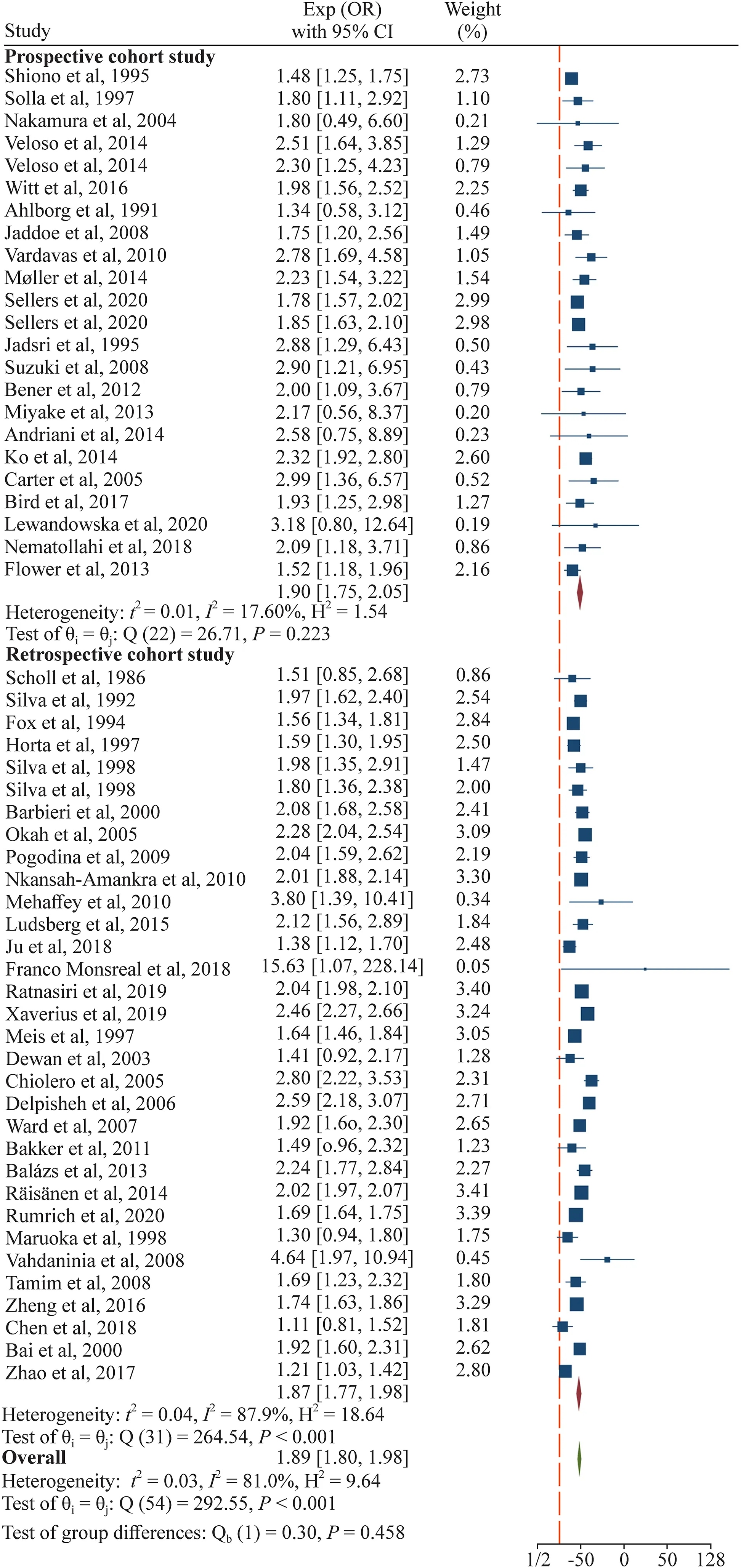
Fig.2 Effect of maternal smoking during pregnancy compared with no smoking on risk of low birth weight (LBW) of the offsprings
Dose–response analysis
Dose–response meta-analysis of seven studies [33,44,45,51,55,64,65] showed that there was evidence of a nonlinear association between the amount of cigarettes smoked per day and LBW risk in offspring.Compared with those whose mothers did not smoke in pregnancy,OR of LBW in infants estimated directly from the cubic spline model was 1.46 (95% CI 1.42–1.49) for 3 cigarettes per day,1.65(95% CI 1.60–1.70) for 5 cigarettes per day,1.84 (95% CI 1.78–1.90) for 8 cigarettes per day,1.92 (95% CI 1.85–2.00)for 15 cigarettes per day,and 2.90 (95% CI 2.62–3.22) for 25 cigarettes per day (Fig.3).
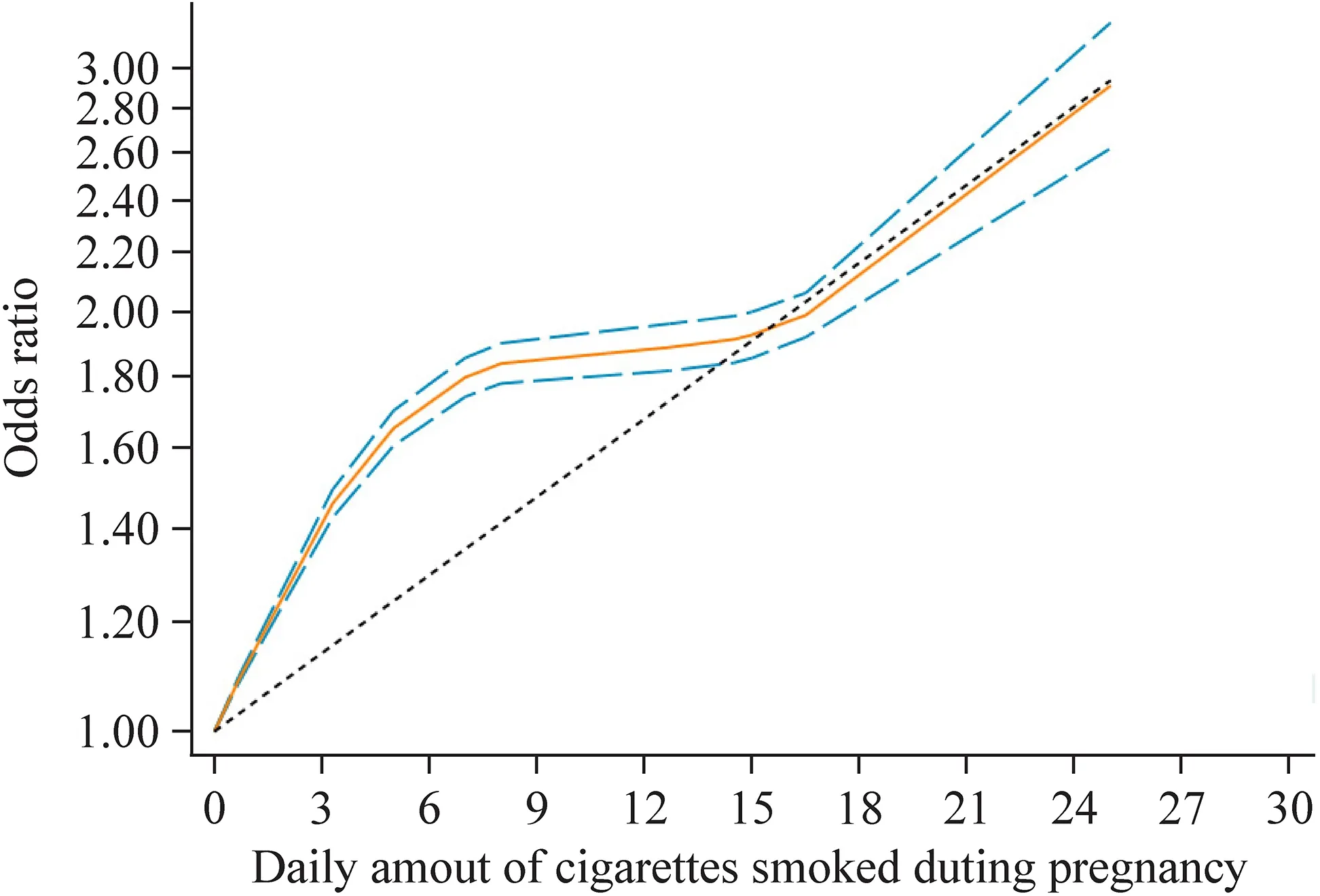
Fig.3 Dose–response relation plot between the number of cigarettes daily smoked and the risk of LBW in offspring (the solid red line and the short dash blue line represent the estimated relative risk and corresponding 95% confidence intervals of the non-linear relationship;long dash black line represents the linear relationship)
Subgroup analysis
Table 1 and Supplementary eFigures 1–9 show the results of subgroup analyses.The subgroup analyses revealed a positive association of maternal smoking during pregnancy with the risk of LBW,and most variables were identified as the relevant heterogeneity moderators.
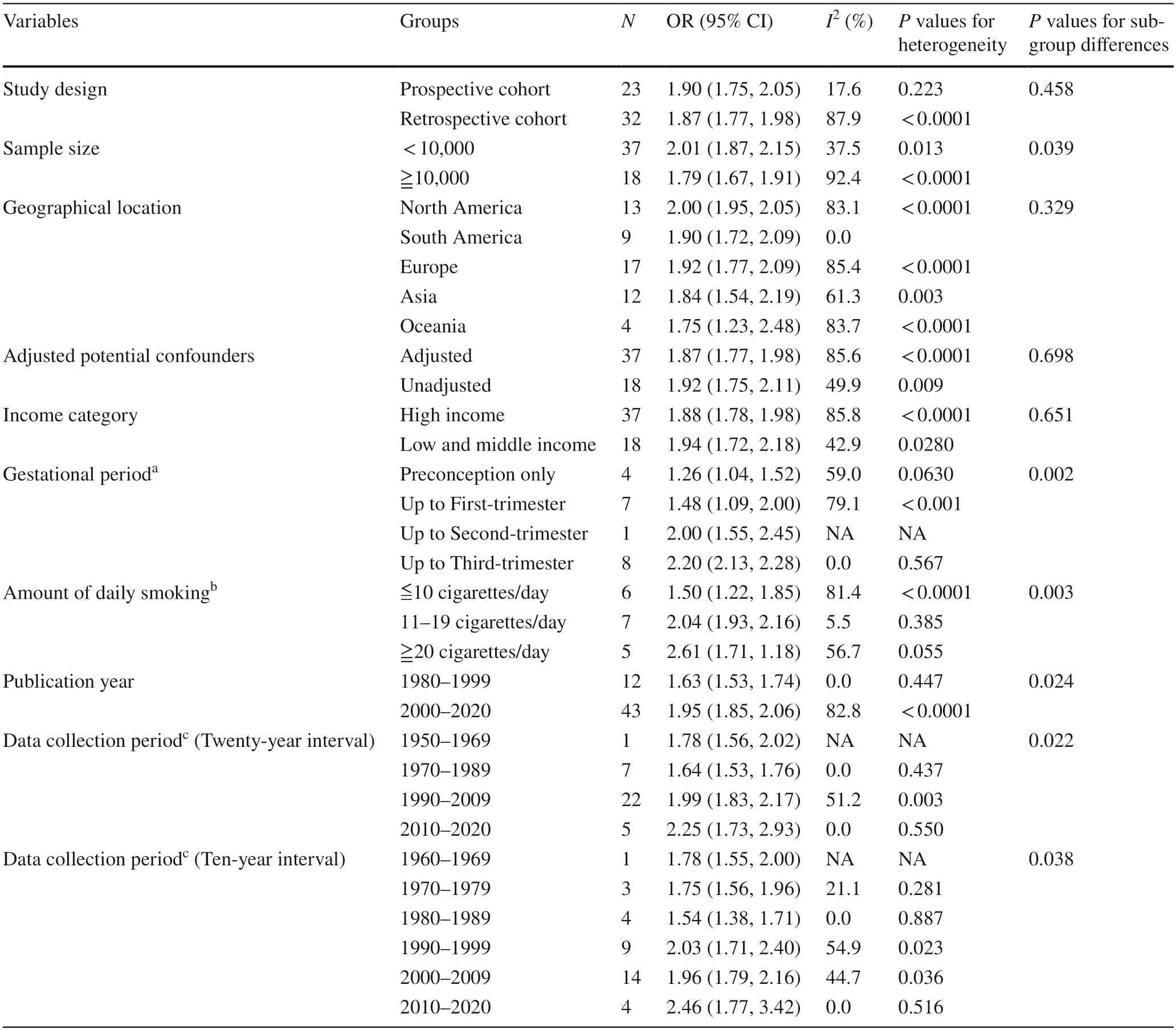
Table 1 Subgroup analyses of the effect of maternal active smoking during pregnancy on low birth weight in offspring
Compared to the overall analysis,heterogeneity was reduced in prospective cohort group (I2 =4.8%,P=0.396),sample size <10,000 group (I2 =28.7%,P=0.055),smoking up to third-trimester group (I2 =0.0%,P=0.567),11–19 cigarettes/day group (I2 =5.5%,P=0.385),1980–1999 group (I2 =0.0%,P=0.447),1970–1989 group (I2 =0.0%,P=0.437),2010–2020 group (I2 =0.0%,P=0.550),1970–1979 group (I2 =21.1%,P=0.281),1980–1989 group(I2 =0.0%,P=0.887) and 2000–2009 group (I2 =44.7%,P=0.036).
No significant interactions were existed between the effect and four stratification variables (study design,geographical location,adjusted potential confounders and the World Bank’s income categories) in relation to LBW risk(Pvalues for subgroup differences >0.05).However,there were significant subgroup effects by sample size,gestational periods,amount of daily smoking,publication year and data collection periods.
Of note,we quantified the effects of maternal smoking on LBW of offspring by diverse gestational periods.From the first to the fourth categories,the risk of LBW in infants was increased by 1.24,1.32,2.00,and 2.21 times,respectively,than those whose mothers did not smoke.Similarly,the subgroup analysis illustrated that the risk of LBW was increased by 53%,103% and 152%,respectively,in infants whose mothers were mild smokers (≦10 cigarettes/day),moderate (11–19 cigarettes/day) and heavy (≧20 cigarettes/day) smokers.
Bar and scatter chart showing pooled ORs of LBW risk by maternal smoking status in diverse gestational periods is shown in Supplementary eFigure 10.And the temporal trend by data collection period of the association of maternal smoking in pregnancy and LBW risk observed is shown in Supplementary eFigure 11.
Publication bias and sensitivity analysis
The funnel plot (Supplementary eFigure 12) shows no significant asymmetry (the Begg's testP=0.298 and theEgger's testP=0.745).In the sensitivity analysis,the estimates of the overall effect remained stable after omitting each study in turn,ranging from 1.88 (95% CI 1.78,1.98) to 1.91 (95% CI 1.80,2.01).
Discussion
Consistent with earlier evidence,the present meta-analysis suggested that maternal smoking during pregnancy was an avoidable risk factor for LBW in infants.Based on 55 studies including more than 21 million participants from 4 continents,this meta-analysis demonstrated that infants whose mothers smoked during pregnancy were 89% more possible to develop LBW,compared with those of nonsmoking women.Moreover,the association remained significant in all subgroups analyzed.We also observed substantial heterogeneity,which may be explained in part by years of studies being conducted,gestation periods,sample size,and the amount of cigarettes smoked.
Medical biological facts indicated that maternal smoking during pregnancy had a causal effect on fetal growth in utero and weight after birth [1,66].The underlying mechanisms of the impact of cigarette smoke on fetal growth and development have been well clarified–hundreds of toxic substances out of more than 7000 chemicals may cross the placental barrier,limit placental development and restrict fetal growth[15,67].Among them,nicotine and carbon monoxide in tobacco smoke were fetal neurotoxins,which could cross the placenta into the fetal circulation,reduce the fetal blood circulation and subsequently impaire fetal oxygen delivery and micronutrients [35,68].Moreover,tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons may cause genetic toxicity,carcinogenic and teratogenic effects on the fetus [35,69].
Our results were in line with the previous review [16] and complemented more interesting findings in several important aspects.First of all,18 recently published prospective cohort studies and 22 retrospective cohort studies from 4 continents have increased the sample size and number of cases in our study,which has apparently reinforced the statistical power to discover potential association of maternal smoking during pregnancy with the risk of LBW in offspring around the world.
More importantly,this dose–response analysis revealed a non-linear association between amount of cigarettes daily smoked in pregnancy and LBW in offspring.We found that,the detrimental effect of maternal smoking on LBW in infants increased most at the lowest levels of exposure,and appeared to level off at moderate-to-heavy exposure levels,and then increased rapidly again at the high levels.A previous study [70] reporting a dose–response relationship between the amount of cigarettes smoked per day and birth weight supported our finding.Additionally,a previous study has shown that the risk of adverse pregnancy outcomes declined with reduced daily smoking amount during pregnancy,but the risk remains for mothers who smoked only 1–9 cigarettes per day and maintain that frequency throughout pregnancy [71].Therefore,for women who smoked moderately or heavily during pregnancy,it may be still not enough to simply reduce the amount of cigarettes a day without quitting.
Furthermore,the large number of relevant studies enabled us to explore causes of heterogeneity by conducting meaningful subgroup analyses.The previous review [16]suggested that sample size could not explain the observed heterogeneity,whereas our subgroup analysis showed that the pooled OR was relatively low in studies with sample sizes greater than 10,000,which may also be due to higher statistical power.
In addition,based on a subgroup analysis of gestation periods,we found that smoking cessation at any time points during pregnancy reduced the risk of LBW in infants,compared with mothers who continued to smoke.These results reinforced the current public health policy and clinical guidelines,emphasizing smoking cessation as early as possible in pregnancy is of utmost importance.Although the results of the study showed that quitting smoking before or during the first trimester were associated with LBW at borderline significance,based on which several recent studies[1,35,39] have made new findings.They have researched the effects of maternal smoking in pregnancy,classified as quit in the 1st trimester and kept on smoking later,on premature delivery,infant weight,head circumference,body size and proportion at birth.Their results indicated that while quitting smoking in the 1st trimester of pregnancy can guarantee a low risk of LBW,it was not associated with a lower risk of abnormal head circumference and body length.Similarly,this meta-analysis illustrated that the risk of LBW increased sequentially among infants whose mothers were mild smokers (≤10 cigarettes/day),moderate (11–19 cigarettes/day) and heavy smokers (≥20 cigarettes/day) during pregnancy,which still underlined the importance of reducing and quitting smoking.
Another interesting finding was the change in the estimated effect of maternal smoking in pregnancy on the LBW risk in offspring over time.Based on the results of 55 studies,there was a statistically significant trend that the estimated risk of maternal smoking for infant LBW was reported higher in more recently conducted and/or published studies.
In most countries,especially in the West,the rate of LBW declined steadily before 1980s [72].However,there seemed to be a noticeable increase of LBW cases on the whole while the proportion of mothers who smoked and the PAR% of maternal smoking during pregnancy from 1980 to 1990 declined [32,72].An increase in the proportion of preterm very-low-birth-weight (VLBW) infants may account for the upward trends in LBW,which was attributed to changes in perceptions of viability.Moreover,a consequent increase in assisted reproduction and a rise in the registration of live births may heavily play a role especially in North America,Europe,and Australia [73].Other factors could be social and behavioral problems,economic recession and economic adjustment program,medical interventions such as elective caesarean section and induced labor or welfare reform during the 1980s/early 1990s in America which resulted in a slight drop in the amount of pregnant women receiving prenatal care in the first 3 months,etc.[74,75].For most Asia countries,due to both signally lowered maternal smoking rate in pregnancy and birth weight of the fetus [8],little difference has been observed in maternal smoking risk over time.
Since 1990s,the LBW rate and maternal smoking rate decreased in several countries,owing to improved living conditions,prenatal and childbirth care,as well as smoking cessation services and anti-smoking campaigns [14,49,75].However,our meta-analys not only confirmed the increased risk of LBW infants in mothers who were used to smoking in pregnancy,but also revealed that the effects of maternal smoking during pregnancy on LBW were larger in more recently conducted studies.This may be related to the trend of global air quality.Based on risk assessments of 84 behavioral,environmental and occupational factors in 195 countries and territories from 1990 to 2017,household air pollution has shown a significant decline with socioeconomic development,which may be one of the reasons for this phenomenon [14].Improvements in air quality over the past 40 years may have reduced the baseline inhaled pollution among nonsmokers so that the risk-effect values in the most recent studies were relatively high.Although most possible causes of this phenomenon are unclear,it is indicated that the prevention of harmful behaviors such as smoking in pregnancy should be a top priority.
Strengths and limitations
The meta-analysis has several strengths.Firstly,we conducted the most comprehensive literature search to date,included a large number of relevant articles,all of which were birth cohort studies from four continents and most studies were of high-quality;Secondly,our findings were stable,as the subgroup analyses and sensitivity analyses indicated,and had no evidence of publication bias;Thirdly,larger sample sizes increased the statistical power to provide robust and precise estimates,and enabled us to conduct meaningful subgroup analyses to investigate the sources of heterogeneity;Fourthly,the present study is the first to involve European,Asian and Oceanian studies,which makes the findings more representative and generalizable,filling the information gap about this large segment of the world's population;Finally,using all classes of data to judge linear and non-linear relationships,we performed dose–response analysis,which contributed to quantify associations and verify the shape of them.
Our study has several limitations.Firstly,there were significant heterogeneities across studies.Some heterogeneities could be attributable to year of study being conducted or published,gestation period and daily smoking amount.However,aided by sensitivity analyses and subgroup analyses,our results were robust.Secondly,in the subgroup analysis stratified by data collection period,many studies were not grouped accurately,with majority of the studies included were from the Americas and a relatively small number of studies from other three continents.Thirdly,accuracy of exposure information cannot be guaranteed due to smoking status of pregnant women was self-reported and prone to under-reporting.Fourthly,socioeconomic status plays a role in the effect of smoking during pregnancy on LBW,but we were unable to stratify this variable into subgroups because only five original studies adjusted for it.Finally,we focused only on LBW and did not consider other prenatal and postnatal outcomes such as preterm birth,infant mortality,and head circumference.
In conclusion,convincing evidence was found regarding the association of maternal smoking during pregnancy and the risk of LBW in offspring on a global scale;and the association was stronger with longer smoking duration throughout pregnancy and larger amount of cigarettes daily smoked.Our findings highlight the importance of quitting completely as early as possible in the world's population before and during pregnancy.
Supplementary InformationThe online version contains supplementary material available at https://doi.org/10.1007/s12519-021-00501-5.
AcknowledgementsWe thank all the authors of the studies included in our meta-analysis.
Author contributionsHKD and YG contributed equally to this work.HKD,YG,and ZXL conceived the study.HKD and YG searched the databases and checked them according to the eligible criteria and exclusion criteria.KL,CW and YZ helped develop search strategies.HKD and KL did the data extraction and quality assessment.HKD and YG analyzed the data.YZ,XM,JAL and FJS gave advice on metaanalysis methodology.H.K.D and Y.G wrote the draft of the paper.HKD,YG,YZ,WQX,XM,MZX,JF,JAL,FJS and ZXL contributed to reviewing or revising the paper.All authors read and approved the final manuscript.ZXL is the guarantor of this work and,as such,had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FundingThis study was funded by the by the National Social Science Foundation of China (Grant No.18ZDA085).
Data availability statementStudies used for meta-analysis are listed in Supplementary eTable 2.
Declarations
Ethical approvalNot needed.
Conflict of interestWe declare that we have no conflicts of interest.
 World Journal of Pediatrics2022年3期
World Journal of Pediatrics2022年3期
- World Journal of Pediatrics的其它文章
- Long-term physical,mental and social health effects of COVID-19 in the pediatric population:a scoping review
- Prediction modelling in the early detection of neonatal sepsis
- Associations of early-life factors and indoor environmental exposure with asthma among children:a case–control study in Chongqing,China
- Urinary cystatin C:pediatric reference intervals and comparative assessment as a biomarker of renal injury among children in the regions with high burden of CKDu in Sri Lanka
- Handmade tri-leaflet ePTFE conduits versus homografts for right ventricular outflow tract reconstruction
- Occurrence of early epilepsy in children with traumatic brain injury:a retrospective study
