Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation
Florian Mourey, Amélie Decherf, Jean-François Jeanne, Mathieu Clément-Ziza, Marie-Lise Grisoni, François Machuron, Sophie Legrain-Raspaud, Arnaud Bourreille, Pierre Desreumaux
Abstract BACKGROUND Probiotics are a promising solution for managing irritable bowel syndrome (IBS).Saccharomyces cerevisiae (S. cerevisiae) I-3856 has already demonstrated beneficial effects in IBS subjects, particularly in IBS with predominant constipation (IBS-C).AIM To confirm the efficacy of S. cerevisiae I-3856 in the management of gastrointestinal symptoms in IBS-C.METHODS A randomized, double-blind, placebo-controlled clinical study was performed in a total of 456 subjects. After a run-in period, subjects were randomly assigned to the group receiving S. cerevisiae I-3856 (8 × 109 CFU daily) or the placebo for 8 wk, and they performed daily self-evaluations of gastrointestinal symptoms. The primary objective was to assess the effect of the probiotic on abdominal pain. The secondary objectives were the evaluation of other gastrointestinal symptoms,bowel movement frequency and consistency, and quality of life (QOL).RESULTS A significantly higher proportion of abdominal pain responders was reported in the Probiotic group (45.1% vs 33.9%, P = 0.017). A nonsignificant difference in the area under the curve for abdominal pain over the second month of supplementation was observed in subjects receiving probiotic vs placebo [P = 0.073, 95%CI: -0.59 (-1.23; 0.05)]. No statistically significant differences were reported in the evolution of bowel movement frequency and stool consistency between the groups. After 8 wk of supplementation, the overall QOL score was significantly higher in the Probiotic group than in the Placebo group [P = 0.047, 95%CI: 3.86 (0.52; 7.20)]. Furthermore,exploratory analyses showed statistically significant and clinically relevant improvements in QOL scores in abdominal pain responders vs nonresponders.CONCLUSION The results of this clinical study confirmed the abdominal pain alleviation properties of S. cerevisiae I-3856 in IBS-C. Abdominal pain relief was associated with improved QOL. ClinicalTrials.gov identifier: NCT03150212.
Key Words: Probiotic; Yeast; Saccharomyces cerevisiae; Irritable bowel syndrome; Abdominal pain; Quality of life
INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder of gut-brain interaction[1] that affects approximately one in ten people globally[2]. This chronic condition is characterized by recurrent abdominal pain related with altered bowel habits. In addition to the patient’s suffering, it represents a significant burden from both economic and social perspectives. It has indeed been associated with important psychosocial consequences as well as considerable costs to health care systems and society[3,4]. Limitations and impairment of social and emotional daily functioning of IBS patients is demonstrated by the consistently reported profound reductions in quality of life (QOL) compared with the general population[5,6]. QOL impairments in IBS are of similar magnitude to those in more severe diseases, such as inflammatory bowel diseases or depression[5,7,8]. The high prevalence of IBS, as well as its significant morbidity,result in substantial health care utilization and loss of work productivity that represent considerable direct and indirect costs to society[4,9,10].
IBS is a complex and heterogenous condition. Although abdominal pain is the cardinal feature, IBS patients can be classified into different subtypes depending on their predominant stool pattern: IBS with predominant constipation (IBS-C), IBS with predominant diarrhoea (IBS-D), IBS with mixed bowel habits (IBS-M) and IBS unclassified. The pathogenesis of IBS has been described as multifactorial and involves a combination of factors related to motility, visceral hypersensitivity, mucosal immune dysregulation, alterations of gut microbiota, and central and enteric nervous system dysregulation[1].However, interventions remain symptom-driven, and available pharmacological treatment options are of limited efficacy[11]. Complementary and alternative solutions to achieving satisfactory control of gastrointestinal symptoms and improve QOL in IBS patients are therefore of great interest. The gut microbiota has been proposed as central in IBS pathophysiology and has therefore drawn considerable interest[12,13]. Among microbiota-directed interventions, probiotics have been recognized as a potential solution for acting on the multifactorial causes and clinical symptoms of IBS[14,15]. A meta-analysis of fifty-three randomized controlled trials (RCTs) involving a total of 5545 patients was recently performed. It demonstrated overall beneficial effects of probiotics on global IBS symptoms and abdominal pain with favourable safety profiles, although conclusions were drawn for probiotics in general[16]. Due to the important heterogeneity of both the tested strains and study designs, identifying which probiotics are relevant for IBS management remains a challenge[17]. However, a recently conducted meta-analysis provided further evidence to determine which probiotic strains are effective in IBS symptom management. Among the probiotics analysed, Saccharomyces cerevisiae (S. cerevisiae)CNCM I-3856 emerged as a recognized solution[18].
S. cerevisiae CNCM I-3856 provides statistically significant and clinically relevant benefits for individuals with IBS, as demonstrated in three RCTs[19-21] performed in a total of 679 subjects and an individual patient data (IPD) meta-analysis[22]. This meta-analysis confirmed previous observations linking S. cerevisiae CNCM I-3856 supplementation to improvement in gastrointestinal symptoms in all IBS subpopulations. Nevertheless, the strongest effects were reported in IBS with predominant constipation. Considering these results, we designed a clinical study to confirm the efficacy of S.cerevisiae CNCM I-3856 on gastrointestinal symptom management in an IBS population with predominant constipation.
MATERIALS AND METHODS
A large-scale, multicentric, placebo-controlled, randomized, double-blind, clinical study was conducted between March 2017 and May 2019. The study was approved by the Ethics Committee Ouest VI of Brest(France) and performed according to International Conference on Harmonization Good Clinical Practice and the ethical principles of the Declaration of Helsinki. Signed written informed consent for the study was obtained from all subjects before protocol-specific procedures were carried out.
The clinical trial was registered in a clinical trials registration system at ClinicalTrials.gov with the identifier NCT03150212.
Study population
Subjects with IBS were recruited by ten French general practitioners across France and in four clinical investigation centres, including Biofortis Mérieux Nutrisciences as the principal investigational centre.Females and males aged 18-75 years were eligible to participate if they met the Rome IV criteria[23] for IBS-C with a baseline score for abdominal pain ≥ 2 and < 6 assessed on a 7-point Likert scale scored from 0 (null) to 7 (severe pain) and had an IBS history between 1 and 10 years. All volunteers agreed to maintain their lifestyle behaviours during their participation in the study. Participants were excluded if they had chronic gastrointestinal conditions other than IBS, including inflammatory bowel diseases,lactose intolerance, and celiac disease. Individuals with a metabolic disorder affecting intestinal transit function or nutrient absorption, such as diabetes or unbalanced thyroid dysfunction, were excluded.Volunteers undergoing symptomatic drug treatments acting on intestinal sensitivity or motility, such as opioids and narcotic analgesics, were not eligible. Antibiotic or antifungal therapies in progress or prescribed 4 or 2 wk before randomization, respectively, were also considered an exclusion criterion.The use of probiotics, prebiotics, symbiotics or any dietary supplement that, according to the investigator, could affect study outcomes in the four weeks preceding randomization was prohibited.However, laxatives, antibloating agents, antispasmodics, anxiolytics, antidepressants, analgesics and nonsteroidal anti-inflammatory drugs were authorized if consumed for longer than three months and maintained at a stable dosage for the entire study duration.
Study design
This study consisted of a 2-wk run-in period followed by an 8-wk treatment period. Subjects visited the clinical investigation centre or general practitioner four times: V0 (screening visit), V1 (randomization visit, 2 wk after V0), V2 (follow-up visit, 4 wk after V1) and V3 (end-of-study visit, 8 wk after V1)(Figure 1). Screening visits (V0) were planned to assess whether inclusion and exclusion criteria were met and to explain study instructions. Additionally, recommendations were given to volunteers to avoid changes in lifestyle behaviours (including dietary and physical activity habits), and volunteers were asked to perform a daily evaluation of the intensity of IBS symptoms and bowel movements. At the randomization visit (V1), investigators confirmed the eligibility (including the Rome IV diagnostic criteria for IBS-C) of the volunteers by integrating the daily evaluation of abdominal pain and bowel movement consistency. Eligible subjects were randomized to receive daily S. cerevisiae CNCM I-3856(Probiotic group) or placebo (Placebo group) for 8 wk. The product allocation list was prepared using SAS®software (version 9.3, SAS Institute Inc., Cary, NJ, United States) before the study started and stored confidentially by a person not related to the clinical phase or data management. The product allocation list was generated using a dynamic randomization algorithm designed to minimize imbalance between the two supplemented groups within each investigational site. Blinding of allocation was maintained until the completed study database was locked.
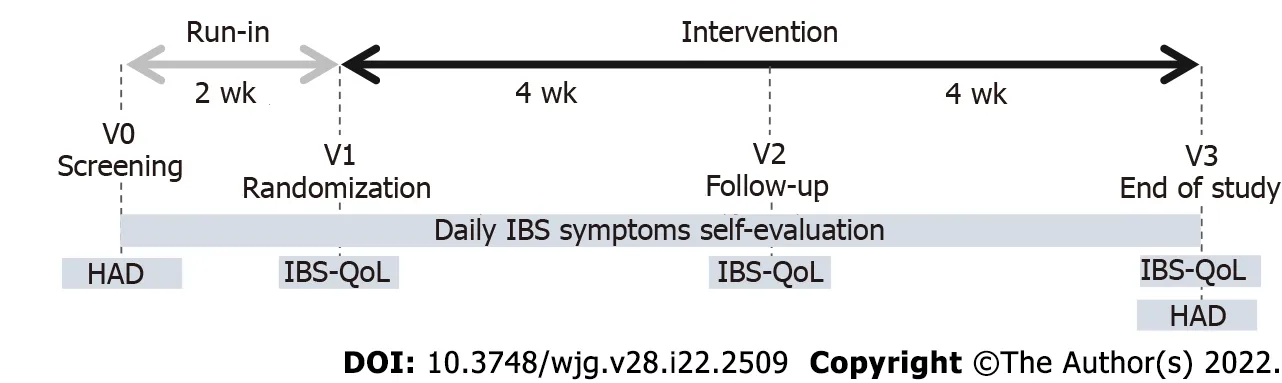
Figure 1 Study design.
Data collected
During the baseline and intervention periods, volunteers were asked to perform a daily evaluation of the intensity of IBS symptoms (abdominal pain, bloating, and flatulence/borborygmi) on a 7-point Likert scale rated from 0 (null) to 7 (severe). Bowel movement consistency assessed on the Bristol stool scale (BSS) was recorded daily. Volunteers completed an IBS-specific quality of life questionnaire (IBSQOL) at randomization (V1), follow-up (V2) and the end of the intervention (V3). The IBS-QOL is a 34-item instrument that yields an overall QOL score as well as scores for eight domains (dysphoria,interference with activity, body image, health worry, food avoidance, social reaction, sexual,relationships). Final scores were normalized to a 100-point scale, with 0 indicating the worst QOL and 100 indicating the best QOL. Anxiety and depression symptoms were also assessed using the hospital anxiety and depression questionnaire at screening (V0) and end-of-study visits (V3).
Study products and compliance evaluation
The study evaluated the probiotic yeast S. cerevisiae CNCM I-3856. This strain is a proprietary, wellcharacterized strain of Lesaffre registered in the French National Collection of Cultures of Microorganisms. The S. cerevisiae species was characterized using phenotypic (API®ID32C, Biomerieux SAS)and genotypic referenced methods (genetic amplification and sequencing of 26S DNA)[24,25].Moreover, strain I-3856 can be identified via a polymerase chain reaction interdelta typing technique[26]and complete genome sequencing. The dose tested was of 8 × 109CFU provided daily in two capsules of 500 mg each. The probiotic dietary supplement was provided by Gnosis by Lesaffre (a business unit of Lesaffre Group, France) and comprised S. cerevisiae CNCM I-3856 entirely and exclusively. The comparative product was a placebo comprising inactive ingredients, i.e., maize starch and magnesium stearate. All capsules were made from hydroxypropyl methylcellulose of the same size, weight, colour and taste regardless of the group and prepared according to good manufacturing practices. Every subject consumed two capsules of 500 mg per day in the morning, just before breakfast with a glass of water. Compliance was determined through the assessment of returned packaging and interviews of the subjects at each visit.
Study endpoints
The primary endpoint was the area under the curve, i.e., the sum of weekly means of daily scores of abdominal pain calculated with the trapezoidal method (interpolation of scores between weeks) from week 5 to week 8 [area under the curve (AUC) (W5-W8)], and it was determined based on previously reported data on the strain[22]. Prespecified secondary endpoints were the total AUC (W0-W8) of IBS symptoms (abdominal pain, bloating, and flatulence/borborygmi) and AUC (W5-W8) of bloating and flatulence/borborygmi scores. Mean weekly scores of gastrointestinal symptoms (abdominal pain,bloating and flatulence/borborygmi) were analysed at each week of the intervention. The evolution of abdominal pain was also assessed through the analysis of the percentage of responders. Abdominal pain response was set according to recommended guidelines[27,28]. Responders were defined as subjects who experienced a reduction of at least 30% of the mean daily abdominal pain score reported at baseline (week 0) from week 5 to week 8. The global QOL score and the eight derived subscores corresponding to the different domains of QOL were assessed at V1 and V2 and at the end of the intervention.Mean stool consistency (BSS scores) and the mean daily number of bowel movements were assessed at each week of the intervention.
Safety endpoints
The safety of the intervention was assessed considering the occurrence of adverse events and the followup of vital signs.
Statistical analyses
Sample size:Based on previous studies performed on IBS-C subjects, considering a 5% type-one error rate and 80% power, 205 subjects per arm would allow the detection of a 1 arbitrary unit (a.u.) difference between groups in AUC (W5-W8) of abdominal pain scores (12% difference) assuming a standard deviation of 3.6 a.u./d.wk. Enrolling a total of 456 subjects would allow for a 10% drop-out.
Efficacy analyses:Efficacy analyses were performed for the intention-to-treat (ITT) population(including all randomized subjects in the study) and for the per-protocol population (ITT population who completed the study and presented no major protocol deviations). The primary endpoint, i.e., the AUC (W5-W8) for abdominal pain, and the other AUCs of IBS symptoms were analysed using an analysis of covariance (ANCOVA) model. Repeated measurements such as the mean daily number of bowel movements, the mean stool consistency and IBS-QOL questionnaire were analysed using ANOVA models. The percentage of abdominal pain responders was analysed by a Chi2test. IBS-QOL scores were additionally examined in exploratory analyses that focused on abdominal pain responders.These analyses were conducted through ANCOVA models for repeated measurements with the baseline (V1) as the cofactor. For all statistical tests, the 5% level of significance was used to justify a claim of a statistically significant effect and standardized mean difference (SMD) was calculated.Hochberg adjustments were used to correct for multiple testing.
Safety analyses:The safety population included all subjects who had consumed at least one dose of the products. For each term of severity and causality, the proportion of subjects showing at least an adverse event was compared between groups using a Chi2or Fisher’s exact test. The number of events was compared between groups using a Poisson regression model.
Software:Statistical analyses were performed using SAS®software version 9.4 (SAS Institute Inc., Cary,NC, United States). Exploratory analyses were performed using R software version 4.0.3.
RESULTS
Study flowchart and baseline characteristics of the population
A total of 456 IBS subjects (392 females, 64 males) with predominant constipation matching the Rome IV criteria were included in this study and randomly assigned to the Probiotic group (n = 230) or the Placebo group (n = 226). The flowchart of subjects throughout the study is presented in Figure 2. The baseline characteristics of the subjects are presented in Table 1. The quantitative variables are described through the mean ± SD, and qualitative variables are described through the number of subjects and percentages (%).
Abdominal pain-AUC
The abdominal pain scores were similar at baseline in the Probiotic (3.97 ± 1.04 a.u.) and the Placebo(3.98 ± 1.07 a.u.) groups. Significant reductions in abdominal pain scores throughout the 8 wk of supplementation were observed in the Placebo group and in the Probiotic group, with reductions of 24.1% and 28.7%, respectively, from baseline at the end of the intervention (P < 0.001 in both groups). The abdominal pain over the 8-wk intervention period decreased similarly to previously reported results. A nonsignificant difference was observed in the AUC for abdominal pain over the second month of supplementation in subjects receiving probiotic vs placebo [-6.3%, P = 0.073, 95%CI: -0.59 (-1.23; 0.05)],between-group difference from ANCOVA model) (Figure 3). Similarly, no difference was reported in the AUC over the 8 wk of intervention.
Abdominal pain-percentage of responders
A significant difference between groups was found in the percentage of responders. The percentage of responders was significantly higher in the group receiving S. cerevisiae CNCM I-3856 (45.1%, 101 subjects among 224 subjects with available data) than in the Placebo group (33.9%, 74 subjects among 218 subjects with available data), resulting in an increase of 33% in the proportion of responders in the treated group vs placebo (Figure 3, Supplementary Figure 1) (P = 0.017, Chi2test).
QOL (IBS-QOL)
A higher overall IBS-QOL score, indicative of better QOL, was reported at the end of the intervention in the Probiotic group (79.51 ± 2.34 a.u.) compared with the Placebo group (75.65 ± 2.39 a.u.) in the PP population [P = 0.047, 95%CI: 3.86 (0.52; 7.20), SMD = 0.18]. Improved QOL in the Probiotic group translated into a mean increase in the overall IBS-QOL score of 11.9% from baseline. The analysis of the eight domains of the IBS-QOL questionnaire showed statistically significant differences in favour of the Probiotic group for 3 domains, i.e., interference with activity [P = 0.05, 95%CI: 4.30 (0.54; 8.05), SMD =0.18)], body image [P = 0.040, 95%CI: 3.96 (0.17; 7.75), SMD = 0.17)], and food avoidance [P = 0.030,95%CI: 5.69 (1.10; 10.28), SMD = 0.22)] (Figure 4, Supplementary Figure 2, Supplementary Table 1).Exploratory analyses of IBS-QOL scores were conducted to evaluate the evolution of the QOL among the responders during the time of supplementation with S. cerevisiae CNCM I-3856. The QOL wassignificantly more improved in the abdominal pain responder group than in the nonresponder group;these differences in improvement following eight weeks of intervention with S. cerevisiae CNCM I-3856 were observed for the overall score and for the eight subscores of the IBS-QOL questionnaire. The improvements with the largest difference between responders and nonresponders were for the body image score [between-group difference from ANOVA model: P < 0.001, 95%CI: 11.94 (8.53; 15.35), SMD= 0.56], health worry score [P < 0.001, 95%CI: 10.91 (7.16; 14.66), SMD = 0.43], food avoidance score [P <0.001, 95%CI: 9.90 (5.14; 14.66), SMD = 0.38] and dysphoria score [P < 0.001, 95%CI: 8.86 (5.79; 11.92),SMD = 0.42] (Figure 5, Supplementary Figure 3, Supplementary Table 2).

Table 1 Baseline characteristics of the study population

Figure 2 CONSORT flow diagram.

Figure 3 Abdominal pain. A: Abdominal pain score evolution; B: Area under the curve (W5-W8) of abdominal pain; C: Percentage of abdominal pain responders in the ITT population. Significant result (aP < 0.05, Chi2 test). Similar profiles were obtained for the PP population (Supplementary Figure 1).

Figure 4 Irritable bowel syndrome-specific quality of life questionnaire scores at the end of the intervention (V3) in the PP population.Significant result (aP < 0.05, ANOVA model).
Other gastrointestinal symptoms, bowel movement frequency and consistency

Figure 5 Irritable bowel syndrome-specific quality of life questionnaire scores at the end of the intervention (V3) adjusted to baseline (V1)in ITT abdominal pain responders supplemented with Saccharomyces cerevisiae CNCM I-3856. PP population radar plot: Supplementary Figure 3.Significant result (aP < 0.05, ANCOVA for repeated measures). Between-group differences are shown in Supplementary Table 2.
At baseline, the bloating scores were similar in both groups (3.99 ± 1.52 a.u. in the Probiotic group vs 4.04 ± 1.83 a.u. in the Placebo group). Kinetic profiles of the bloating score over the 8-wk supplementation period showed a statistically significant decrease in both groups corresponding to a bloating score reduction of 28.4% (95%CI: 23.9-33.0) and 24.5% (95%CI: 19.7-29.5) in the Probiotic and Placebo groups, respectively (between W0 and W8, P < 0.001 in both groups, within-group differences from the ANCOVA model). A similar decrease in the flatulence/borborygmi score was observed in both the Placebo and Probiotic groups. The bowel movement frequency and BSS scores increased in Probiotic and Placebo groups between W0 and W8 (P < 0.01 from W1 to W8), but no statistically significant differences were found between the groups.
Safety
The number of adverse events per subject showed no statistically significant difference between the groups [P = 0.50, 95%CI: 1.10 (0.83; 1.46)], between-group difference from Poisson regression model).Similarly, there was no statistically significant difference between groups regarding the proportion of subjects with at least one adverse event (assessed for each term of severity and for adverse events whose causality with the study product or with the research was considered “not excluded”) (Table 2).Descriptive statistics of the adverse events by body system are presented in Supplementary Table 3.Three serious adverse events occurred during the study (1 in the Probiotic group and 2 in the Placebo group). The relationship with the study product was considered “not excluded” for one serious adverse event in the placebo group (bowel obstruction).
DISCUSSION
The beneficial properties of S. cerevisiae CNCM I-3856 for gastrointestinal symptom management in the IBS population have been reported in three RCTs conducted in a total of 679 participants[19-21]. An individual patient data meta-analysis reported the strongest effects of the strain in IBS with predominant constipation[22]. To validate these observations, the effect of S. cerevisiae CNCM I-3856 was specifically investigated in this population in the present clinical study.
Effect of S. cerevisiae CNCM I-3856 on abdominal pain
The evolution of abdominal pain reported in IBS-C with moderate symptom severity in this study was consistent with previous findings. Lower abdominal pain intensity over the second month of supplementation was observed in the Probiotic group than in the Placebo group, although the difference was not significant. Abdominal pain alleviation during supplementation with S. cerevisiae CNCM I-3856 was confirmed by the higher proportion of abdominal pain responders in the Probiotic group than in the Placebo group. The definition of abdominal pain responder used in this study is aligned with current recommendations and corresponds to a reduction of abdominal pain intensity from baseline of at least 30%[27,28]. The higher rate of responders observed in this study during daily supplementation with S.cerevisiae CNCM I-3856 compared with placebo is consistent with previously reported results[19,22].Pineton de Chambrun et al[19] reported a significantly higher percentage of responders in the probiotic group than in the placebo group (63% vs 47%, P = 0.04). The IPD meta-analysis conducted on S. cerevisiae CNCM I-3856 further confirmed this finding. Participants in the probiotic group had 1.51 higher odds ofbeing a responder than participants in the placebo group (P = 0.02)[22]. The abdominal pain responder definition used in previous studies was slightly different. Responders were defined as subjects experiencing a decrease in abdominal pain intensity greater than or equal to 1 a.u. of abdominal pain score,assessed on a 7-point Likert scale, for at least 50% of the second month of supplementation. Based on the mean level of abdominal pain intensity reported at baseline in this study, a decrease of 1 a.u. would correspond to a mean 25% decrease in abdominal pain intensity. The stricter definition of the responders used in our study likely contributes to the lower percentage of abdominal pain responders reported here. However, when using a more demanding definition, the present study confirmed the beneficial effect of S. cerevisiae CNCM I-3856 on abdominal pain. This result is of great clinical interest as it meets the clinically relevance threshold previously proposed[29]. Therefore, the relevance of S.cerevisiae CNCM I-3856 in addressing abdominal pain in IBS-C is confirmed. Effective solutions to managing abdominal pain may be even more critical in this subpopulation, as recent findings have shown that individuals with IBS-C experience more bothersome, frequent and diffuse abdominal pain[30].

Table 2 Descriptive statistics of the proportion of subjects with adverse events
Effect of S. cerevisiae CNCM I-3856 on QOL
Importantly, this clinical study also showed improvements in QOL in the probiotic group. Indeed,significant differences were found at the end of the intervention period in the overall IBS-QOL score as well as in several domains. Notwithstanding, the magnitudes of these differences were relatively low.The consequences of IBS on daily function in addition to physical symptoms were further investigated in abdominal pain responders to provide a more accurate understanding of the overall impact of diet supplementation with S. cerevisiae CNCM I-3856.
Specific tools have been developed to capture emotional, social and physical functions. The IBS-QOL is currently the most validated self-reported QOL measure[31-33]. Here, we showed that the abdominal pain alleviation reported in responders was accompanied by significant and clinically relevant improvements in IBS-QOL. A strong association between abdominal pain and QOL has been previously described, and a causality relation has been suggested[34]. Moreover, it has also been shown that IBS patients attributed their impairment in daily functioning directly to IBS symptoms[35].
QOL improvements appeared to be mainly driven by specific domains of QOL: dysphoria, food avoidance, health worry and body image. Improvement in the food avoidance subscale of the IBS-QOL is of interest, as perceived restrictions in lifestyle related to gastrointestinal symptoms are commonly reported in the literature and encountered in clinical practice[33,35]. A considerable proportion of IBS patients report an association between food and gastrointestinal symptoms[36]. Food-related gastrointestinal symptoms have been associated with more severe IBS symptoms and lower QOL[37].Individuals with IBS have been reported to experience emotional dysphoria, which can also be described as feeling helpless, out of control and depressed. It may therefore not be surprising that individuals experiencing significant abdominal pain alleviation also reported improvement in the dysphoria subscale of the IBS-QOL. In addition, significant improvement was seen in the body image subscale of the IBS-QOL. This result also appears consistent with the literature, as individuals with IBSC have been described as more likely to internalize their distress and to feel self-conscious about their body image[38].
Effect of S. cerevisiae CNCM I-3856 on gastrointestinal symptoms
In contrast with previous findings, no significant between-group differences were observed in the AUC for gastrointestinal symptoms. Although the clinical studies conducted on S. cerevisiae CNCM I-3856 in IBS present consistent designs, a significant change here is the use of Rome IV criteria to select the participants. A worldwide comparison of IBS prevalence by Rome IV and Rome III diagnostic criteria demonstrated that individuals diagnosed by Rome IV criteria exhibit higher IBS severity. Consistently, a higher level of abdominal pain was reported at baseline in this study than in previous clinical trials conducted on S. cerevisiae CNCM I-3856. Among factors associated with response to placebo in IBS-C,higher baseline symptom severity was reported as an important predictor of placebo response[40].Differences in the studied population could therefore have contributed to the lower between-group size effect reported in this study.
Previous studies have reported statistically significant effects of this probiotic on bowel transit in IBSC, IBS-D and IBS-M, assessed by daily reporting of bowel movement frequency and consistency[22,21].In contrast, we did not find between-group differences in bowel movement frequency or consistency in this study. Spiller et al[20] highlighted the possible role of accelerated bowel transit as central in the relief of abdominal pain and bloating in IBS-C. Instead, the absence of a significant effect on bowel transit in this study as well as the reported improvements in abdominal pain in all IBS subtypes suggest that other mechanisms of action may be involved and participate in the clinical benefits.
Regarding putative mechanisms of action of S. cerevisiae CNCM I-3856
Several putative mechanisms of action of S. cerevisiae CNCM I-3856 can be hypothesized. First, the antiinflammatory effects of S. cerevisiae CNCM I-3856 have been reported in several preclinical models[41-45]. The immune-modulating properties of the strain may be relevant in IBS, as chronic low-grade intestinal mucosal inflammation has been implicated in IBS pathophysiology. Second, interactions with the gut microbiota might influence the multiple factors involved in IBS pathophysiology[12,46]. The results from trials conducted in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME®)suggest that S. cerevisiae CNCM I-3856 can modulate gut microbiota activity towards an increase in short-chain fatty acids (SCFAs)[47]. This result may be of particular importance, as a recently published longitudinal multiomics study showed significantly lower levels of SCFAs in the stool samples of IBS-C patients than in those of healthy controls[48]. In addition, several lines of evidence suggest that increased levels of SCFAs, such as butyrate, may be beneficial in IBS management[49-51]. Nevertheless,these possible mechanisms of action remain speculative and would need to be addressed separately.Differences in microbiota composition and function between abdominal pain responders and nonresponders may provide a mechanistic basis for the beneficial effects of S. cerevisiae CNCM I-3856 in IBS management and warrant further exploration.
CONCLUSION
In conclusion, the results of this large-scale clinical study are consistent with previous findings and confirm the safety and efficacy of S. cerevisiae CNCM I-3856 for abdominal pain management in IBS-C.In addition, abdominal pain alleviation was associated with significant improvements in IBS-related QOL. Dietary supplementation with S. cerevisiae CNCM I-3856 therefore appears to be an interesting complementary or alternative solution for IBS management with positive implications for the day-today life of individuals with IBS-C.
ARTICLE HIGHLIGHTS
Research background
The gut microbiota has been proposed as central in irritable bowel syndrome (IBS) pathophysiology,and microbiota-directed intervention has therefore drawn considerable interest. Among them, Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856 is a probiotic yeast that has emerged as a recognized solution for managing IBS.
Research motivation
S. cerevisiae I-3856 has demonstrated beneficial effects in IBS subjects, particularly in IBS with predominant constipation.
Research objectives
To confirm the efficacy of S. cerevisiae CNCM I-3856 on gastrointestinal symptom management in an IBS population with predominant constipation.
Research methods
A total of 456 subjects were enrolled in a randomized, double-blind, placebo-controlled trial. After a run-in period to confirm IBS diagnosis, subjects were randomly assigned to the group receiving the probiotic or the placebo for 8 wk and performed daily self-evaluations of gastrointestinal symptoms.The primary objective was to assess the effect of the probiotic on abdominal pain. The secondary objectives were the evaluation of other gastrointestinal symptoms, bowel movement frequency and consistency, and quality of life (QOL).
Research results
Abdominal pain alleviation during supplementation with S. cerevisiae CNCM I-3856 was confirmed by the higher proportion of abdominal pain responders in the probiotic group than in the placebo group.Importantly, this clinical study also showed improvements in QOL in the probiotic group.
Research conclusions
The results of this large-scale clinical study are consistent with previous findings and confirm the safety and efficacy of S. cerevisiae CNCM I-3856 for abdominal pain management in IBS population. In addition, abdominal pain alleviation was associated with significant improvements in IBS-related QOL.
Research perspectives
Dietary supplementation with S. cerevisiae CNCM I-3856 appears to be an interesting complementary or alternative solution for IBS management with positive implications for the day-to-day life of individuals with IBS with predominant constipation. Differences in microbiota composition and function between abdominal pain responders and nonresponders may provide a mechanistic basis for the beneficial effects of S. cerevisiae CNCM I-3856 in IBS management and warrant further exploration.
ACKNOWLEDGEMENTS
The authors want to acknowledge Biofortis Merieux Nutrisciences and all the investigators for conducting the study.
FOOTNOTES
Author contributions:Decherf A designed the study; Desreumaux P and Bourreille A contributed to the design of the study; Clément-Ziza M, Grisoni ML and Machuron F supervised the statistical analysis operations; Mourey F drafted the publication manuscript; Legrain-Raspaud S gave final approval of the version to be published; All authors participated in interpretation of the results, critically reviewed the manuscript and approved the final manuscript for submission.
Institutional review board statement:The study was reviewed and approved by the Ethics Committee Ouest VI of Brest, France.
Clinical trial registration statement:This study is registered at ClinicalTrials.gov. The registration identification number is NCT03150212.
Informed consent statement:All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement:Mourey F, Decherf A, Jeanne JF, Clément-Ziza M, Machuron F and Legrain-Raspaud S are employees of Lesaffre. Grisoni ML was an employee of Lesaffre during her main contribution to the study.Bourreille A and Desreumaux P received financial support for research from Lesaffre.
Data sharing statement:No additional data are available.
CONSORT 2010 statement:The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:France
ORCID number:Florian Mourey 0000-0002-0826-9632; Amélie Decherf 0000-0001-7721-8073; Jean-François Jeanne 0000-0002-5074-4679; Mathieu Clément-Ziza 0000-0003-2763-249X; Marie-Lise Grisoni 0000-0002-8240-6834; François Machuron 0000-0002-5709-762X; Sophie Legrain-Raspaud 0000-0001-7747-5766; Arnaud Bourreille 0000-0003-4903-3535; Pierre Desreumaux 0000-0002-6127-5281.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Gastroenterology2022年22期
World Journal of Gastroenterology2022年22期
- World Journal of Gastroenterology的其它文章
- Future therapies for pancreatic carcinoma: Insights into cancer precision medicine
- Endoscopic classification and pathological features of primary intestinal lymphangiectasia
- Prognostic value of preoperative enhanced computed tomography as a quantitative imaging biomarker in pancreatic cancer
- Application of endoscopic ultrasonography for detecting esophageal lesions based on convolutional neural network
- Metabolic aspects of hepatitis C virus
- Hepatocellular carcinoma, hepatitis C virus infection and miRNA involvement: Perspectives for new therapeutic approaches
