Hepatocellular carcinoma, hepatitis C virus infection and miRNA involvement: Perspectives for new therapeutic approaches
Ester Badami, Rosalia Busà, Bruno Douradinha, Giovanna Russelli, Vitale Miceli, Alessia Gallo, Giovanni Zito,Pier Giulio Conaldi, Gioacchin Iannolo
Abstract Chronic hepatitis C virus (HCV) infection is the principal etiology of cirrhosis and, ultimately, hepatocellular carcinoma (HCC). At present, approximately 71 million people are chronically infected with HCV, and 10%–20% of these are expected to develop severe liver complications throughout their lifetime. Scientific evidence has clearly shown the causal association between miRNAs, HCV infection and HCC. Although it is not completely clear whether miRNA dysregulation in HCC is the cause or the consequence of its development, variations in miRNA patterns have been described in different liver diseases, including HCC.Many studies have analyzed the importance of circulating miRNAs and their effect on cell proliferation and apoptosis. In this Review, we aim to summarize current knowledge on the association between miRNA, HCV and HCC from a diagnostic point of view, and also the potential implications for therapeutic approaches.
Key Words: Hepatocellular carcinoma; miRNA; Liver; Hepatitis C virus; miRNAs; Directacting antivirals; Extracellular vesicles; Transplantation
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a well-known risk factor for hepatocellular carcinoma(HCC)[1,2]. The virus belongs to the Flaviviridae family, and is the only member of the hepacivirus genus[3-6], and is presently classified in seven main genotypes, although 11 genotypes and at least 67 confirmed subtypes are known[7-10].
HCV is a single-stranded positive-sense RNA virus consisting of an icosahedral symmetrical nucleocapsid, surrounded by a double-layer lipid envelope in which the envelope glycoproteins E1 and E2 are inserted[6]. Its genome of 9.6 kb harbors an open reading frame encoding a polyprotein precursor of about 3000 amino acids, which is processed through proteolytic cleavage by viral and host proteases into smaller molecules, including structural (core, E1 and E2), p7, and nonstructural proteins (NS2, NS3,NS4A, NS4B, NS5A and NS5B)[11].
The total global prevalence of chronic HCV infection is estimated at 71 million people by the World Health Organization (WHO)[12], and varies according to health conditions, sociodemographic characteristics, and the presence of risk factors for transmission that can alter the efficiency of the transmission routes[9,13]. HCV is transmitted primarily parenterally, through large or repeated direct percutaneous exposures to infected blood[9,13-15]. Major high-risk populations include people who inject drugs(PWIDs), men who have sex with men, and prisoners[16].
The outcome of HCV infection depends strictly on the strength and breadth of the host response during the acute phase. Around 30% of HCV-infected people spontaneously clear the virus within 6 mo of infection, although the remaining 60%–80% of people, despite developing an efficient antiviral immune response, are unable to clear the virus, resulting in persistent infection[17,18]. Activation of the immune response against HCV contributes importantly to the establishment of long-lasting inflammation and consequent liver damage. The deregulation of cytotoxic cells and the continued activation of an apoptosis pathway results in scarring and progression to cirrhosis that, in 25% of HCV infected patients, can result in HCC over a period of 20–30 years post-infection[2,19,20].
Until the last decade, interferon (IFN) and ribavirin (RBV) were the only available therapies against chronic HCV infection, although they were accompanied by significant side effects. Moreover, IFNbased therapies had only limited efficacy, as the response was genotype dependent[21-24]. The recent introduction of direct-acting antivirals (DAAs) has resulted in remarkable therapeutic improvements.These are currently the standard therapeutic choice, and a sustained virology response (SVR) > 90% is attained after 12 wk of treatment. However, some patients show relapse of HCV infection, even after DAA treatment, and achieving SVR does not completely rule out the risk of developing HCC[25]. Also,following DAA treatment and subsequent viral clearance, HCV reinfections remain a problem among individuals with high-risk behaviors, e.g., PWIDs[26,27].
Moreover, access to DAA therapy is not simple for all patients, and only the 62% of HCV-diagnosed patients have been treated with DAAs (WHO) (https://www.who.int/news-room/fact-sheets/detail/hepatitis-c).
Although the pathogenesis of HCV infection has not been fully elucidated, interactions between structural and nonstructural viral proteins and host cell components, such as miRNAs, have been reported by many groups. It has been shown that host miRNAs are involved in many steps of the biological cycle of HCV, such as infection and replication. Likewise, HCV infection regulates the expression of many cellular miRNAs involved, for example in liver fibrosis, hepatocarcinogenesis and HCC progression[28-31]. Due to such mutual interactions, miRNAs can be used for risk assessment and prognosis of HCV-related HCC, and could be considered for diagnostic approaches and new therapeutic strategies.
HCC
HCC is the main type of liver cancer, and the most life-threatening cancer worldwide. HCC onset consists of several processes involving multiple risk factors, but most often it presents in people with chronic liver diseases and cirrhosis[32].
The most common etiological factors that lead to liver cirrhosis, therefore predisposing to HCC transformation, are chronic infection with hepatitis B virus (HBV), HCV, or hepatitis D virus, alcoholic liver disease, and nonalcoholic steatohepatitis/nonalcoholic fatty liver disease (NAFLD). The less common causes are hereditary hemochromatosis, 1 antitrypsin deficiency, autoimmune hepatitis,porphyria, disorders of steroid hormones, Wilson’s disease, and dietary aflatoxins[33-36].
Approximately 50% of all liver cancers are related to chronic viral hepatitis, which can develop into cirrhosis and HCC[37]. In particular, HCV causes chronic infections in 70%–80% of cases, while HBV leads to chronicity in only 10% of infected people[38]. Among HCV chronically infected patients, about 20% develop liver cirrhosis within 20–30 years and, once cirrhosis is established, the rate of HCC development increases by 1%–4% per year. Moreover, chronic HCV infection is associated with a 20–30-fold increased risk of developing into HCC compared to healthy individuals[39]. HCV-related HCC is mediated both by virus-related factors and host-induced immunological responses. In addition, HCV damage is a gradual and continual process, characterized by recurrent infection that induces the immune system to attack liver cells, provoking repeated damage to the genomic material that can lead to mistakes during proofreading repair[38]. Recent studies suggest that HCV core protein can promote initial development of HCC, acting on cell signaling pathways. Indeed, HCV core protein may directly inhibit the tumor suppressor genes and the cell cycle checkpoints, inducing the activation of signaling pathways that upregulate growth and cell division[40]. The specific tumor suppressor genes inhibited by HCV core protein include retinoblastoma protein and p53 tumor suppressor, which, if synergistically lost, lead to a higher degree of carcinogenesis[41]. Repeated cell cycles are associated with the accumulation of mutations that may transform hepatocytes into cancer cells. Among the genes most mutated are telomerase reverse transcriptase, tumor protein 53, and β-catenin. These mutations not only threaten telomere maintenance, but also lead to increased oxidative stress on hepatocytes, inducing chronic inflammation secondary to HCV, thus promoting HCC progression[42].
Over the last few years, in an attempt to identify new therapies and more accurate biomarkers for early diagnosis and treatment of HCC, the pivotal role played by miRNAs in the development and progression of cancer has emerged[43].
Recently, progress has been made in the study of miRNAs in HCC, with the discovery that some of them are upregulated or downregulated in HCC. Aberrant expression of miRNAs has been linked to HCC proliferation, apoptosis and invasion, but also metastasis formation, epithelial–mesenchymal transition, angiogenesis, drug resistance, and autophagy. In addition, some miRNAs can also be potential diagnostic and prediction markers for HCC[44].
HCV THERAPY
The principal goal of anti-HCV therapy is to eradicate HCV infection, thus resolving the liver disease. In the past, the standard of care for eradication of chronic HCV treatment was IFN-based therapy; most commonly in combination with RBV. However, this combination therapy had an outcome that depended on the viral genotype, was poorly tolerated, and also associated with severe side effects such as depression, flu-like symptoms, fatigue, diarrhea, and hematological toxicity[45]. These unwanted adverse effects were the main reason for patients abandoning therapy. With the introduction of the first NS5B polymerase inhibitor, sofosbuvir, in 2014, IFN-free treatment became available[46]. Hence, DAAs were introduced in clinical practice to treat HCV+patients, obtaining SVR rates above 90%[47,48], where SVR corresponds to a cure of the HCV infection, as late relapse occurs in less than 0.2% of cases beyond 6 mo of follow-up[49]. While patients with cirrhosis are often not treatable with IFN-based treatment,DAAs, including NS5B and NS5A inhibitors, are effective for patients with any stage of liver fibrosis,including those with advanced liver disease and decompensated cirrhosis, which predispose to developing HCC at a higher degree. This has allowed a significant increase in the number of treatable patients, with excellent safety profiles[50].
Clinically, SVR leads to stabilization and improvement of the liver function, and is thus associated with a lower risk of hepatic dysfunction, a reduced need for liver transplantation, and lower overall mortality. It has been demonstrated that DAA therapy attains much higher SVR rates than IFN-based treatment by reducing the inflammatory cargo in the liver, fibrosis, and neoplastic formation[51].
With more successful SVR obtained using DAAs in the clinic, there were higher expectations in terms of a significant reduction in the incidence of HCC. However, such expectations were not always met. In fact, despite the documented positive impact on HCV infection clearance, the effect of DAA treatment on the development and/or recurrence of HCC in patients treated for liver cirrhosis remains hard to define[52].
In a meta-analysis, Frazzoni et al[53] compared different trials based either on the use of DAAs or IFN[53]. The aim of this study was to define the long-term incidence and occurrence of HCC after the achievement of SVR using either IFN-free or IFN-based therapies. This investigation showed the higher safety profile of DAA therapy, accompanied by a lower risk of HCC recurrence[53]. A controversial subject is the effect of DAA-based SVR on the recurrence of HCC following curative treatment of early HCC. It has also been found that about 30% of patients with a history of curative treatment for HCC undergoing DAA therapy for HCV infection develop HCC recurrence, raising many surgical and clinical questions[54].
Some studies initially suggested that DAA treatment might increase the recurrence rate for HCC,with consequent doubts on the use of this therapy for patients with previous HCC[55]. However, these studies were not confirmed by further trials, and new guidelines were provided recommending the use of DAAs for patients with a history of HCC[56]. At present, patients with HCV-related cirrhosis who have undergone resection or ablation for HCC should not be dissuaded from receiving DAA therapy to prevent the progression of the liver disease.
The putative correlation between DAA treatment and de novo HCC occurrence is still a matter of debate. Some studies have shown how HCV+patients diagnosed as negative for HCC develop aggressive and fatal forms of liver cancer after DAA treatment[57-61]. These studies highlighted the importance of the surveillance of patients before and during DAA treatment, pointing to a possible correlation between the stage of fibrosis and the pre-existence of decompensated cirrhosis, with increased susceptibility to the development of de novo HCC[58,61,62].To conclude, patients failing to respond to antiviral treatment are at high risk and often need to be monitored during therapy[63]. In light of these observations, it appears clear how the use of biomarkers such as HCV/HCC/liver-related miRNA are important for early HCC diagnosis before and during DAA treatment.
HCV INFECTION AND miRNAs
As discussed above, miRNAs are active players in tumor initiation and progression for their involvement in the regulation of expression for proteins implicated in the pathophysiological mechanisms of cancer development, including cell growth, apoptosis, and metastasis[64-66]. In the liver,it has been shown that liver-enriched transcription factors such as HNF1A, HNF3A and HNF3B regulate miR-122 expression, which can function as a tumor suppressor factor by inhibiting angiogenesis, HCC growth/invasion, and high levels of HCC apoptosis and cell cycle arrest[67,68]. This miRNA represents 70% of the total miRNAs in hepatocytes[69,70], and has been therefore nicknamed hepatic miRNA. It has been found that HCV RNA possesses two tandem miR-122 complementary sites at the 5’ end of the viral genome[71], responsible for viral RNA accumulation[72]. The miR-122 binding with the HCV RNA has been described at multiple levels: miR-122 affects folding of the viral internal ribosomal entry site(IRES)[73], required for viral translation, and modulates viral replication and polyprotein translation[74]. In addition, it has been found that the stability of HCV RNA is mediated by RNA-induced silencing-like complex (RISC-like), suggesting the coordination of Ago2 and miR-122 in stabilizing and protecting the viral genome from 5′ exonuclease activity[75]. Furthermore, HCV RNA acts as a sponge for miR-122, leading to de-repression of host mRNAs normally targeted by miR-122, thus providing a fertile environment for the long-term oncogenic potential of HCV[76]. The serum levels of miR-122 are clearly higher in HCV-infected patients with HCC[77-79]. During the course of HCV infection, miR-122 is coupled with miR-34a[80] (Figure 1). While several studies have reported a downregulation of miR-34a in neoplastic transformation in various tissues[81], miR-34a was found increased in HCC[82].Moreover, we recently reported an increase of miR-34a in HCC cells after HCV infection, the effect of which can be modulated by p53 induction[31]. It has recently been proposed that flaviviruses can upregulate expression of miRNAs that inhibit viral replication in target cells[31], demonstrating that miR34 overexpression induces the IFN-mediated response in dengue virus, West Nile virus and Japanese encephalitis virus infection[83]. In HCC cells, miR-34a induces cell cycle arrest and apoptosis.Concomitantly, other groups have also demonstrated that miR-34 inhibits fibrosis in stellate cells by regulating the TGF-β1/Smad3 pathway[84].
Expression of miR-21 increases in patients with HCV-related HCC[85], while miR-223 expression is increased in patients with advanced fibrosis compared to moderate/minimal fibrosis[86]. In contrast,during HCV-related chronic hepatitis, miR-21 and miR-223 display a pro-fibrotic/tumorigenic effect[85](Figure 1).
Depending on the role played in viral infection, some miRNAs have been classified as pro- or antiviral. For example, miR-135a has been described as proviral miRNA because of its ability to enhance HCV RNA replication in hepatocytes. In addition, miR-135a inhibits the expression of host restriction factors, such as CXCL2, MyD88 and IRPK2, which are involved in antiviral immunity[87]. Likewise,miR-146a-5p enhances HCV infection by playing an immunoregulatory role through the downregulation of the inflammatory signaling, and by turning off the immune response in hepatocytes. Moreover,miR-146a-5p promotes the late steps of the HCV replication cycle, likely by modulating HCV assembly[88]. Another example is represented by miR-21-5p, which triggers HCC growth and metastasis by modulating a PTEN-dependent pathway[89].
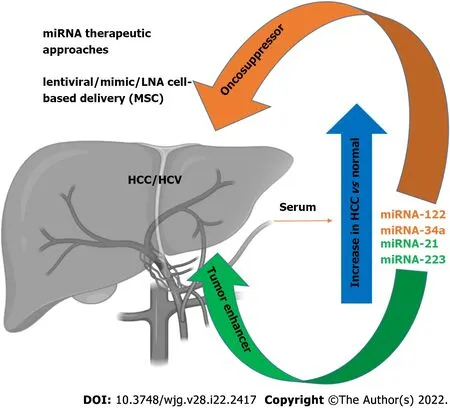
Figure 1 Schematic representation of some major miRNAs upregulated in serum after hepatocellular carcinoma occurrence. Noteworthy,some of them (mir-122 and mi-R34a) display a tumor suppressor effect and represent not only a diagnostic target, but have been proposed in novel therapeutic approaches (Figure was created using BioRender.com). HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; LNA: Locked nucleic acid; MSC: Mesenchymal stem cell.
Conversely, let-7 family miRNAs have demonstrated strong anti-HCV activity, thus being classified as antiviral miRNA[90]. It has been found that let-7b potentially reduces HCV replication through the targeting of IGF2BP1 required for HCV replication[90], and increases the cell apoptosis rate[91]. Similar to other antiviral miRNAs, mir-199a* is a HCV RNA binder in IRES, targeting the HCV 5′-UTR[64,92].
HCV-RELATED HCC miRNA-BASED APPROACHES
The mechanisms that drive liver injury during HCV infection have not been fully elucidated. Several studies have attempted to understand the interactions between viral proteins and the cellular host machinery[93]. An important effort has been made to address and understand the complex interconnections between HCV infection and miRNAs, aimed at finding new therapeutic and diagnostic tools.
It has recently been shown that the expression of specific patterns of miRNAs in HCC patients may be used for diagnostic purposes, with a sufficient level of reliability, thus highlighting a potential role of miRNAs as biomarkers[94]. Specific circulating miRNA profiles are associated with several diseases,including HCV infection. These miRNAs have been proposed as biomarkers of HCV-related HCC pathophysiology and prognosis. Many studies have focused on setting miRNA panels to be tested in HCV-related HCC patients, and used to discriminate healthy individuals from ill patients; above all,during the early phases of disease. For example, Ali et al[95] described and validated a panel of nine liver-associated miRNAs (miR-21, miR-30c, miR-93, miR-122, miR-125b, miR-126, miR-130a, miR-193b and miR-222)[95], while Zekri et al[96] identified miR-122, miR-885-5p and miR-29b as associated with fluctuations in the levels of the diagnostic liver-specific biomarker -fetoprotein (AFP)[96]. Wahb et al[97]suggested the use of circulating miR-9-3p and endocan as novel biomarkers for risk assessment in the early diagnosis of HCV-related HCC[97]. It has been shown that circulating extracellular vesicles (EVs)containing specific patterns of noncoding RNAs are highly related to disease progression of HCVassociated HCC[98]. Recent reports have highlighted the importance of serum miR-34a and miR-122 as biomarkers of HCV-related HCC[80]. We recently showed that HCV infection induces an increase in miR-34a expression in the HCC cell line HuH7.5, and acts as a tumor suppressor[31]. We also found that HCV infection induces the release of EVs containing miR-34a, and these EVs can exert a growth inhibition effect and induce apoptosis[31]. We were able to demonstrate that miR-34 action is not limited only to the infected cells, as EV release has a paracrine effect on neighboring hepatocytes, thus underscoring the potential use of this miRNA for therapeutic purposes[31] (Figure 1). A newly generated polymer-based nanosystem redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) loaded with miR-34a inhibits HCC proliferation in vitro and in vivo,reducing the epithelial mesenchymal transition[99].
Conversely, it has been demonstrated that miR-21 can act as an oncogene by stimulating HCC growth, invasion, and migration[89,100]. Indeed, it has been shown that the inhibition of miR-21 suppresses HCC growth both in vitro and in vivo[100].
It is worth mentioning that miR-122 is known to potentiate HCV replication by inhibiting the degradation of the viral genome[70]. Several clinical trials are testing the ability of reducing HCV replication using locked nucleic acid inhibition of miR-122[101-103], perhaps evidence for the efficacy of this treatment, which is devoid of adverse effects[64]. For example, miR-122 antagonists can be used as a therapeutic approach in synergistic association with DAA therapy to obtain an enhancement of the clinical outcome. These results point out the potential therapeutic interest of miR-122, in particular, for patients who do not respond to antiviral agents[104]. Recently, the use of EVs to treat HCC has become an interesting topic, but one that requires further studies. Lou et al[105] revealed that the injection of EVs derived from miR-122-modified mesenchymal stem cells (MSCs) can significantly improve chemotherapeutic sensitivity of HCC, and increase the efficacy of sorafenib treatment[105] (Figure 1).Wei et al[106] found that there are different miRNA expression patterns between HCC cells and their EVs, suggesting a self-modulating mechanism whereby HCC-cell-derived EVs were able to shuttle miRNAs to recipient cells, promote cell growth, and migration and invasion of HCC cells[106]. EVs contain both oncogenic and tumor suppressor miRNAs, and their deregulated expression in HCC tissues can promote HCC development. Therefore, EV-mediated miRNA transfer might represent a crucial mechanism, exploitable on the one hand as a diagnostic tool by identifying circulating EVderived miRNA, and, on the other, EV-derived miRNAs could be a useful target to inhibit HCC growth.In agreement, Zhang et al[107] generated virus-like vectors containing both miRNA-21-sponge and premiRNA-122, and demonstrated that virus-like particles were able to correct HCC miRNA dysregulation,and to decrease proliferation, migration, and invasion of HCC[107].
Currently, 20 clinical trials (Table 1) are investigating both tissue-specific and circulating miRNAs as a diagnostic/prognostic tool for HCC or for monitoring HCC during a specific treatment(clinicaltrial.gov). Future clinical trials will be needed to provide appropriate analysis of the performance of miRNAs as biomarkers, and to discover new miRNA-related HCC targets for implementation of new treatments.
PERSPECTIVES FOR TUMOUR DETECTION AND INTERVENTION
As described above, there is a strong correlation between miRNA expression and HCC. However, some of the regulated miRNAs are not only related to HCV infection, but also with other pathological liver conditions. In particular, alterations in some miRNAs have been also found in NAFLD[108-110]. Among the identified miRNAs, a significant upregulation of miR-122 in the serum of NAFLD patients was noted. This variation is comparable to the upregulation of the same miRNA observed in the serum of HCC/HCV patients. Another group reported a significant increase of miR-34a expression in the serum of NAFLD patients[110], in agreement with our observations in HCV HCC cells infections[31]. These results indicate that these miRNAs could be considered potential markers of pathological liver conditions induced by inflammation or transformation (Figure 1). The most direct implication of this finding is the possibility of having new, noninvasive, and reliable biomarkers for early HCC detection that could be applied in the near future. In particular, a specific circulating miRNA expression, in association with conventional tumor markers such as AFP, protein induced by vitamin K absence/antagonist II (PIVKA-II), and classical clinical parameters has been observed[111,112]. The definition of an miRNA expression signature (88 miRNAs) provides a substantial increase of accuracy in the detection of HCC (up to 99.5%), associated with a strong sensitivity (100%), while AFP evaluation as a tumor biomarker has an accuracy and a sensitivity of 76.5% and 63.8%, respectively[111]. The strength of these results refers mostly to the detection of small HCC tumors (< 3 cm). This analysis also revealed that an miRNA expression signature shows specific variations for HCC compared to other pathological liver conditions, where cirrhotic patients or chronic hepatitis B infection can be distinguished by a characteristic alteration of expression pattern[110]. These results strengthen the possibility of using miRNAs as a new approach for early tumor detection and subsequent early intervention, particularly considering that, after HCV DAA treatment, there is still the need to monitor HCC occurrence after viral eradication, as also suggested elsewhere[31,113,114].
CONCLUSION
The introduction of DAA therapy signals a milestone in the history of HCV treatment, with achievement of SVR at high rates. However, whether the development of HCC is related to pre-existing HCV infections after DAA treatment is disputed. Not all HCV patients are eligible for DAA treatment,and HCV reinfection in high-risk populations frequently occurs. In light of this, understanding the molecular mechanisms of HCV viral infection that lead to development of liver cancer in the host isfundamental.
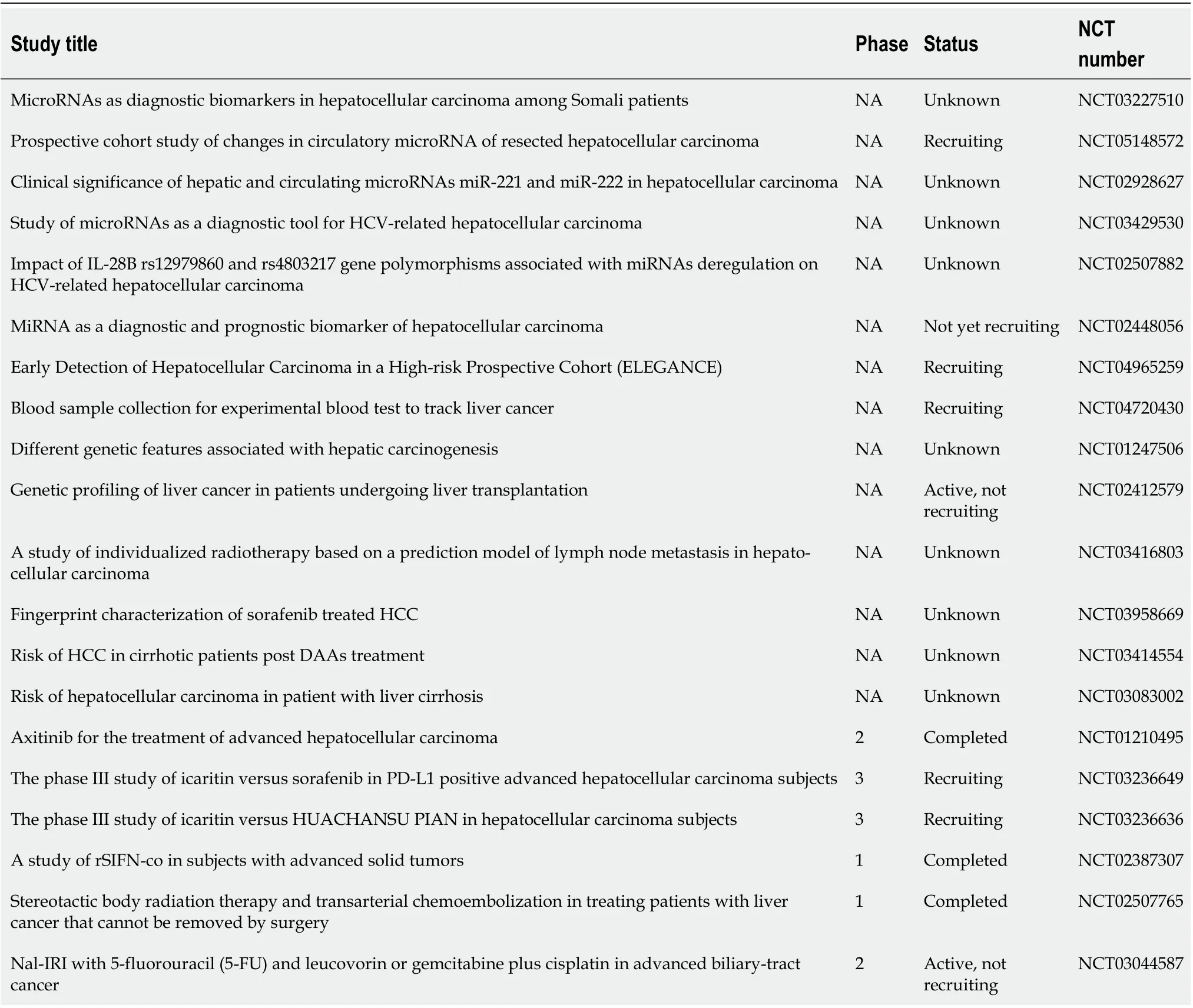
Table 1 List of registered trials that use miRNAs as diagnostic and/or prognostic tool for hepatocellular carcinoma
It has been reported that cellular miRNAs may contribute to HCV pathogenesis, either by direct or indirect interactions with the viral genome or proteins. Several miRNAs and their targets have been shown to be associated with HCV and HCC progression, and might represent a diagnostic biomarker for the early prognosis of HCC. Early HCC prognosis acquires relevance during DAA treatment to minimize the risks of HCC development in HCV+patients. Likewise, many miRNAs could be used also for therapeutic purposes. However, further studies are needed to elucidate the molecular interplay between miRNA and HCC, and the development of novel therapeutic strategies for the treatment of HCC is a major critical goal to be achieved.
FOOTNOTES
Author contributions:Badami E, Busà R, Douradinha B, Russelli G, Miceli V, Gallo A, Zito G, Conaldi PG and Iannolo G carried out the research for the manuscript and edited all drafts of the paper; Badami E, Busà R and Douradinha B equally contributed to the manuscript.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORCID number:Ester Badami 0000-0002-0154-6270; Rosalia Busà 0000-0002-7546-7209; Bruno Douradinha 0000-0002-9980-4505; Giovanna Russelli 0000-0002-1920-0892; Vitale Miceli 0000-0001-8922-9909; Alessia Gallo 0000-0001-6737-9770; Giovanni Zito 0000-0002-0482-6729; Pier Giulio Conaldi 0000-0003-1994-8005; Gioacchin Iannolo 0000-0002-7710-4735.
S-Editor:Fan JR
L-Editor:Kerr C
P-Editor:Fan JR
 World Journal of Gastroenterology2022年22期
World Journal of Gastroenterology2022年22期
- World Journal of Gastroenterology的其它文章
- Future therapies for pancreatic carcinoma: Insights into cancer precision medicine
- Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation
- Endoscopic classification and pathological features of primary intestinal lymphangiectasia
- Prognostic value of preoperative enhanced computed tomography as a quantitative imaging biomarker in pancreatic cancer
- Application of endoscopic ultrasonography for detecting esophageal lesions based on convolutional neural network
- Metabolic aspects of hepatitis C virus
