Effect of GGBS on performance deterioration of non-dispersible underwater concrete in saline soil
Fang Liu ,BaoMin Wang ,GuoRong Tao ,Tao Luo ,XiaoSa Yuan
1.Shaanxi Key Laboratory of Safety and Durability of Concrete Structures,Xijing University,Xi'an,Shaanxi 710123,China
2.State Key Laboratory of Simulation and Regulation of Water Cycle in River Basins,China Institute of Water Resources and Hydropower Research,Beijing 100038,China
3.School of Civil Engineering,Dalian University of Technology,Dalian,Liaoning 116023,China
ABSTRACT In saline soil areas,there are a large number of ions in soil or water environments,such as Cl−and SO42−,which havestrong corrosive interactions with buildings.To study the deterioration of non-dispersible underwater concrete in sulfate,chloride,and mixed salt environments,the compressive strength and deterioration resistance coefficient of the studied con‐crete mixed with different amounts of ground granulated blast-furnace slag (GGBS) were analyzed in this paper.At the same time,the micro morphology and corrosion products distribution of the studied concrete were observed by means of SEM,plus XRD diffraction,TG-DTG and FT-IR analyses to explore the influence of corrosive solutions on the hydration products of concrete.We also analyzed the mechanism of improving the deterioration resistance of the studied concrete by adding GGBS in a saline soil environment.The results show that the compressive strength of the studied concrete in a chloride environment was close to that in a fresh water environment,which means that chloride has no adverse effect on compressive strength.The deterioration of the studied concrete was most serious in a sulfate environment,followed by mixed salt environment,and the lowest in a chloride environment.In addition,by adding GGBS,the compressive strength and deterioration resistance of the studied concrete could be effectively improved.
Keywords:saline soil;non-dispersible underwater concrete;granulated blast furnace slag;deterioration resistance;mechanism analysis
1 Introduction
In China,there are numerous saline soil areas,whose soil or water environment contains a large number of ions with strong corrosivity to buildings,such as Cl−and SO42−.Due to the characteristics of multiple points such as wide areas and large spans,transmission line engineering will pass through these areas during construction.As a result,a portion of the concrete cast-in-place pile body in these areas is usu‐ally poured underwater.
During underwater casting concrete,water erosion can cause the segregation of concrete aggregates and the loss of cement slurry,and reduce the strength of concrete.In addition,in saline soil environments,a certain concentration of corrosion ions can dissolve into the water,some of which can directly invade the concrete,which will greatly impact the strength and durability of concrete.
Non-dispersible underwater concrete refers to the self-compacting concrete with no loss of cement slur‐ry and no segregation of aggregates formed by adding flocculant to the ordinary concrete.It is necessary for non-dispersible underwater concrete to possess self-compacting performance,because it cannot be vi‐brated during construction.Therefore,there are high‐er requirements for fluidity of non-dispersible under‐water concrete.The amount and type of flocculant will greatly affect the fluidity of non-dispersible un‐derwater concrete.Taking the case of UWB-Ⅱfloccu‐lant,with an increase of its content,the fluidity of concrete can be reduced rapidly;once the content of the flocculant exceeds 3%,the concrete is unable to be used in actual construction (Zhanget al.,2017).In contrast,the increase of fly ash content not only im‐proves the fluidity of non-dispersible underwater con‐crete in a certain range(Baeket al.,2004),but also re‐duces its time-consuming loss (Niu and Ma,2011).Also,the addition of granulated blast furnace slag powder can improve fluidity,but the improvement de‐gree is not as good as that of fly ash (Zhanget al.,2016).
The mechanical properties of non-dispersible un‐derwater concrete are also affected by the type and amount of flocculant.The study of Khayat (1996)confirmed that compared with polypropylene floccu‐lant,polysaccharide flocculant can promote concrete to reach more ideal compressive strength,and the in‐fluence of fly ash (Horszczaruk and Brzozowski,2017) as well as silica fume (Liuet al.,2018) on the strength of non-dispersible underwater concrete is al‐so obvious.
Research of durability of non-dispersible underwa‐ter concrete shows that water penetration resistance of the concrete is weaker than that of land formed con‐crete due in part to moisture entering into the concrete during underwater pouring (Wang and Chen,2006).Appropriate flocculent,flocculent dosage,the reduc‐tion of water-cement ratio and the addition of mineral admixtures are beneficial for improving anti-chloride permeability performance of underwater non-dispers‐ible concrete (Jianget al.,2001;Zhaoet al.,2017).Furthermore,concrete will expand and crack when it reacts with sulfate to form more and more ettringite crystals.These crystals will gradually reduce the strength of non-dispersible underwater concrete in seawater (Zhaoet al.,2015),lower than concrete strength in fresh water environments.
Nevertheless,when studying the durability of nondispersible underwater concrete,some scholars not only set a shorter test age (Zhaoet al.,2015;Zhaoet al.,2017),but also did not consider the problem of corrosive ions in the pouring and curing stage when non-dispersible underwater concrete was applied in a corrosive environment (Wang and Chen,2006).Therefore,in this paper,various kinds of non-dispers‐ible underwater concrete poured and cured in differ‐ent corrosive solutions were selected and their deterio‐ration resistance was tested at different ages within 360 days.
In this paper,the fluidity and anti-dispersion per‐formance of non-dispersible underwater concrete were analyzed firstly,and then the deterioration resis‐tance of the concrete in a saline soil environment was studied.We also studied the influence of different cor‐rosion environments (sulfate,chloride,mixed salt)and the content of granulated blast furnace slag on the compressive strength and deterioration resistance co‐efficient of the concrete.Through SEM,TG-DTG thermal,XRD and FT-IR analyses,the influence of these three environments on hydration products of the concrete was clarified,and the mechanism of granulat‐ed blast furnace slag powder improving the deteriora‐tion resistance of non-dispersible underwater concrete in a saline soil environment is explained.
2 Experimental
2.1 Raw material
P·O 42.5R cement used in this test was produced by Dalian Onoda Cement Co.,Ltd.,China.Detailed technical indicators and chemical composition of the cement are provided in Tables 1 and 2,respectively.

Table 1 Properties of cement

Table 2 Chemical composition of cement
The fine aggregate used in this experiment was natural river sand with a fineness modulus of 2.8,and the coarse aggregate was continuous graded limestone with particle size of 5−20 mm and apparent density of 2,740 kg/m3.The type of flocculent belongs to UWB-Ⅱproduced by Research Institute of China Petroleum Engineering,which is solid powder with polysaccha‐ride as its main component.The mineral admixture was ground granulated blast-furnace slag (GGBS,S105 grade) made by Shandong Luxin New Building Materials Co.,Ltd.,China,and its properties as well as chemical composition are provided in Tables 3 and 4 separately.Tap water for mixing concrete in this ex‐periment was provided by laboratory.The Dalian Bon‐uo Biochemical Reagent Factory provided the water reducing agent polycarboxylic superplaticizer.The type and concentration of soaking solution selected in this paper are provided in Table 5.

Table 3 Properties of GGBS

Table 4 Chemical composition of GGBS
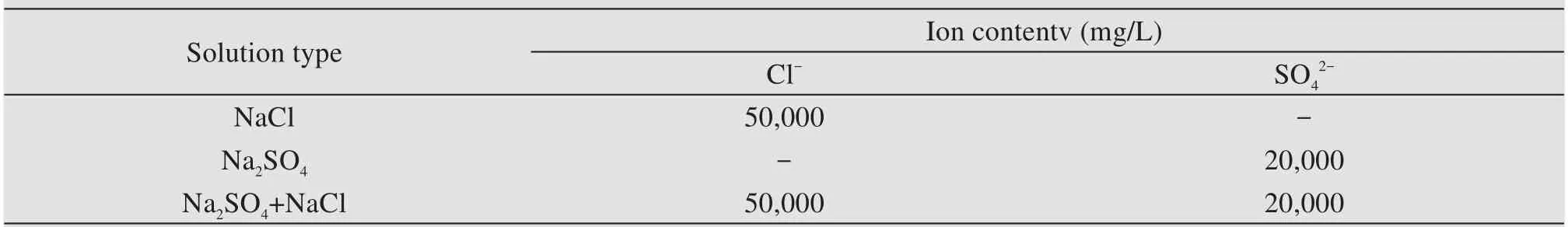
Table 5 Corrosive ion content in salt solution
2.2 Mix proportion and preparation of non-dispersible underwater concrete
The mix proportions of non-dispersible underwa‐ter concrete adopted in this test are provided in Table 6,where the number'O'indicates that granulated blast furnace slag powder is not added.'SL1','SL2' and'SL3' represent the percentage content of granulated blast furnace slag powder of 20%,40% and 60%,re‐spectively.The addition amount of reducer and floccu‐lent in Table 6 is a percentage of the mass of the bind‐ing material.
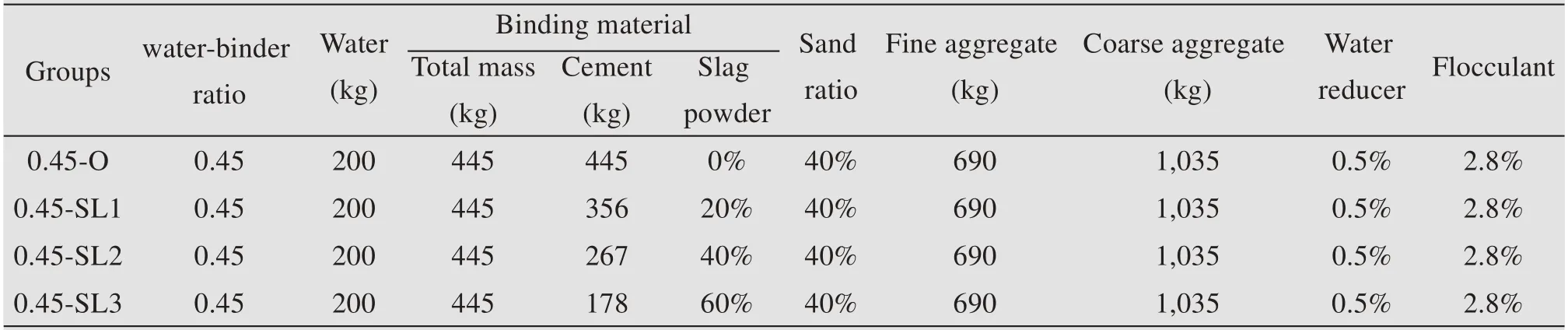
Table 6 Mix proportion design of non-dispersible underwater concrete
In accordance with DL/T 5117-2000,the cube specimens (100mm×100mm×100mm) were poured and molded in fresh water,sulfate,chloride salt and mixed salt environments.Specific operations during underwater molding proceeds as follows:
(1) The molds were placed in a 450 mm high wa‐ter tank,and water (sulfate,chloride or mixed salt so‐lutions) was added to 150 mm of the upper limit of the test mold.
(2) Fresh non-dispersible underwater concrete was dropped from the water surface to let it freely fall into the test mold.The pouring process remained continu‐ous until the pouring amount exceeded the surface of the test mold.The pouring time of each test mold was controlled within 30 s to 60 s.
(3)Afterwards,the molds were taken out of the so‐lution,standing for five minutes,and then two sides of it were gently tapped with a rubber hammer to ac‐celerate the drainage.The concrete was returned to the corresponding solution after its excess part was smoothed with a spatula.After two days,samples are demolded,when the concrete develops to a certain strength and is preliminarily formed.
The demolded specimens were put into the corre‐sponding environment (fresh water,sulfate,chloride and mixed salt solutions)for curing.
2.3 Test methods
The fluidity (slump and slump expansion) and an‐ti-dispersion performance (suspension content) of non-dispersible underwater concrete were tested ac‐cording to DL/T 5117-2000.
The compressive strength test ages of concrete specimens were 7 days,28 days,56 days,90 days,180 days and 360 days.A YAW-2000 microcomputer controlled electro-hydraulic servo pressure testing ma‐chine (manufactured by Changchun Xinte Co.,Ltd.,China) was selected for our test.The specific test steps were carried out in terms of relevant provisions in DL/T 5117-2000.
After the compressive strength test,crushed con‐crete fragments (5−20 mm in size and within 5 mm in thickness) were placed into a plastic bottle,then anhy‐drous ethanol was poured into the bottle to stop hydra‐tion.After 24 to 48 h,the samples were taken out and dried in an oven at 40 °C for 24 to 48 h,then scanned by SEM following the careful removal of gravel.Ac‐cording to the water binder ratio designed in Table 6,non-dispersible underwater cement paste was prepared and poured into 40mm×40mm×40mm cube specimens in fresh water,sulfate,chloride salt and mixed salt envi‐ronments.Then each specimen was placed into the cor‐responding solution for curing,and crushed when reaching the specified age.Next,after adding anhy‐drous ethanol for a period of time to stop hydration,the samples were taken out and dried in an oven with a temperature of 40 °C.Once the drying was over,they were gently ground,and screened with an 80 μm sample sieve to obtain samples for XRD,TG-DTG and FT-IR tests.The instruments and models used in this experiment were scanning electron microscope(QUANTA 450,FEI,USA),X-ray diffractometer(D8 ADVANCE,Bruker AXS,Germany),TG-DSC thermal analyzer(METTLER TOLEDO STARE,Met‐tler Toledo,Switzerland) and Fourier infrared spec‐trometer(EQUINOX 55,Bruker,Germany).
3 Results and discussions
3.1 Basic performance
3.1.1 Fluidity
The fluidity of all mix proportions was tested in this study,and the test results are provided in Table 7.The slump values in the table all meet the require‐ments of the specification for fluidity not less than 210 mm.
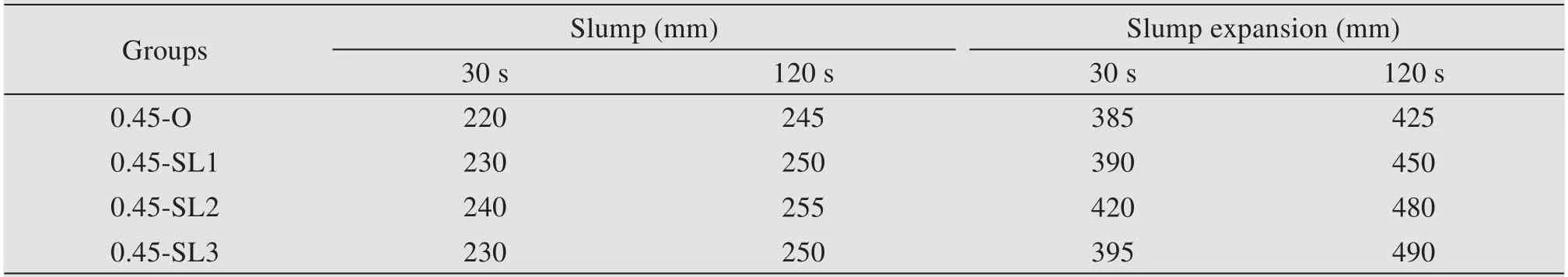
Table 7 Fluidity results of non-dispersible underwater concrete
It is clear from Table 7 that the addition of GGBS could effectively improve the slump and slump expansion of non-dispersible underwater concrete.There are two main reasons for GGBS improving the fluidity of concrete (Shiet al.,2000;Shiet al.,2004;Wei,2005).One is that the particle size of GGBS is much smaller than that of cement particles,which is favorable for dispersing the latter,increasing the slurry content and reduc‐ing the friction between aggregates.Second,be‐cause the density of GGBS is less than that of ce‐ment,the volume of cementitious material will be increased and the effect of flocculent will be weak‐ened by replacing part of the cement with an equal amount of GGBS.
3.1.2 Anti-dispersion property
The content of suspended substance was used to characterize the anti-dispersion performance of non-dis‐persible underwater concrete,and the test results are provided in Table 8.The content of suspended sub‐stance in all mixing ratios was less than the standard limit of 150 mg/L,meeting the specification.
As can be seen from Table 8,the content of sus‐pended substances continually increases with an addi‐tional increase of GGBS,indicating that the anti-dis‐persible property of non-dispersible underwater con‐crete is declining.When a small amount of GGBS is added,the anti-dispersible performance of non-dis‐persible underwater concrete will not be evidently weakened,while its anti-dispersible property is great‐ly reduced with too many GGBS.The reason is that the density of GGBS is less than that of cement,so the volume of cementitious material will increase and the effect of flocculent will weaken after the equiva‐lent amount of cement is replaced by GGBS.

Table 8 Results of anti-dispersion property of
3.1.3 Development law of compressive strength of non-dispersible underwater concrete in fresh water
The compressive strength results of non-dispers‐ible underwater concrete specimens cast and cured in fresh water are provided in Figure 1,where 'S' repre‐sents fresh water environment.The influence of GG‐BS content on compressive strength is shown,which testifies that the compressive strength of non-dispers‐ible underwater concrete with each GGBS content is greater than that of the control group(0.45-O-S).
3.2 Sulfate resistance and microscopic analysis of non-dispersible underwater concrete
3.2.1 Development of compressive strength and deterioration resistance coefficient of concrete in a sulfate environment
The test results of compressive strength and deteri‐oration resistance coefficient of concrete in a sulfate environment are provided in Figure 2 and Table 9,where'SY'represents sulfate environment.
The influence of GGBS addition on compressive strength of concrete in a sulfate environment is provided in Figure 2,which indicates that the compres‐sive strength of concrete mixed with GGBS is greater than that of the blank sample in the period of 7 days to 360 days.That is to say,adding GGBS in a sulfate environment can effectively enhance the compressive strength of non-dispersible underwater concrete.
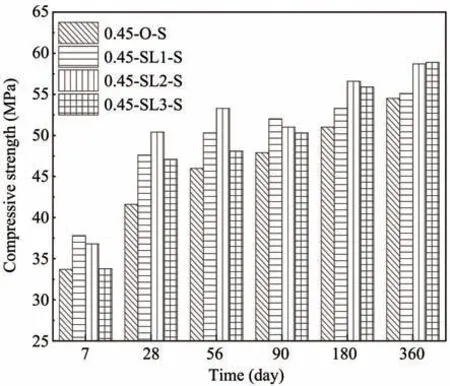
Figure 1 Influence of GGBS addition on compressive strength

Figure 2 Influence of GGBS addition on compressive strength in a sulfate environment
Table 9 shows the influence of GGBS content on deterioration resistance coefficient in a sulfate envi‐ronment.It can be seen that,the deterioration resis‐tance coefficient fluctuates greatly with no obvious regularity during the period from 7 days to 56 days.From 90 days to 360 days,the coefficients of all non-dispersible underwater concrete show a decreas‐ing trend,among which the coefficient of ordinary non-dispersible underwater concrete is obviously lower than that of concrete mixed with GGBS.More‐over,the more granulated the blast furnace slag pow‐der,the higher the deterioration resistance coeffi‐cient and the better the sulfate resistance.
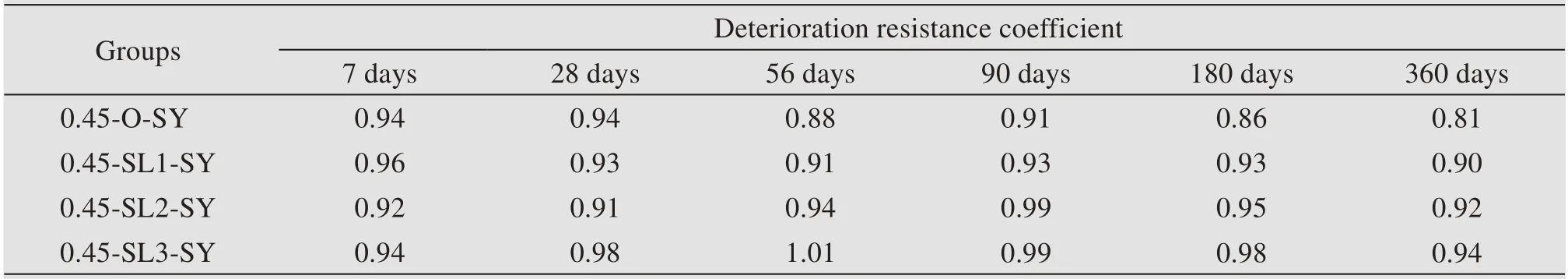
Table 9 Deterioration resistance coefficient of non-dispersible underwater concrete in a sulfate environment
3.2.2 Microscopic analysis and discussion
(1) SEM analysis
Figures 3 and 4 are SEM images of non-dispers‐ible underwater concrete in a fresh water environment and a sulfate environment at 28 days,respectively.It can be seen from Figures 3 and 4a that compared with a fresh water environment,non-dispersible underwa‐ter concrete in a sulfate environment produces a large number of needle-like ettringite crystals,most of which occurred in cracks inside the concrete,while some were attached to plate-shaped calcium hydrox‐ide.From Figure 4b,there is no obvious calcium hy‐droxide crystals and needle-like ettringite crystals in the concrete sample with 60%GGBS.
Figures 5 and 6 are SEM images of non-dispers‐ible underwater concrete in a fresh water environment as well as a sulfate environment at 360 days,respec‐tively.Compared with Figures 5 and 6a,it can be seen that a large number of well-crystallized flake calcium hydroxide crystals are formed in the hydration prod‐ucts in a sulfate environment.A large quantity of nee‐dle-like ettringite crystals are attached to the calcium hydroxide crystals and the internal pores of concrete,some of which are clustered together due to the large amount.This phenomenon shows that numerous sul‐fate ions infiltrate into the non-dispersible underwater concrete,and these ions combine with hydration prod‐ucts to generate a large amount of corrosive product(ettringite).From Figure 6b,calcium hydroxide and ettringite crystals are difficult to be seen in the SEM image after 60% GGBS are added.Also,calcium hy‐droxide crystals are consumed after the secondary hy‐dration reaction with active substances in GGBS,and the cementitious material produced in the secondary hydration reaction makes the whole matrix more com‐pact,so it is difficult for sulfate to penetrate and form a large number of ettringite crystals.

Figure 3 SEM image of non-dispersible underwater concrete in a fresh water environment for 28 days

Figure 4 SEM image of non-dispensable underwater concrete in a sulfate environment for 28 days

Figure 5 SEM image of non-dispersible underwater concrete in a fresh water environment for 360 days
(2) XRD analysis
The XRD result of cement paste in a sulfate envi‐ronment is provided in Figure 7,where'0.45-O-S'in a fresh water environment is the reference group.In Figure 7,peak shapes of the three samples are rough‐ly similar,indicating that no new crystals are formed in the cement matrix under a sulfate environment.Through XRD test,ettringite,dihydrate gypsum,cal‐cium hydroxide and calcium carbonate can be found in the samples,where calcium carbonate is from car‐bonation of calcium hydroxide.
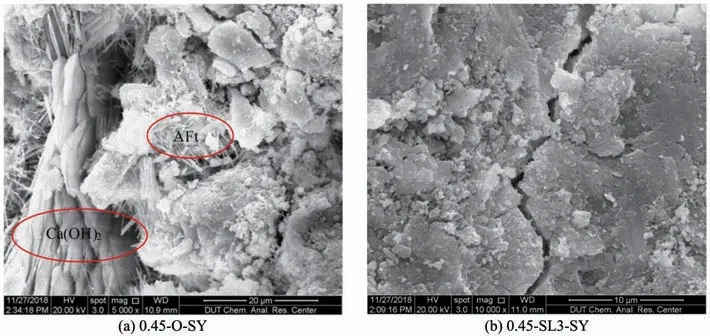
Figure 6 SEM image of non-dispensable underwater concrete in a sulfate environment for 360 days
Table 10 lists the diffraction peak intensities of cement paste specimens at diffraction angles of 9.12°,11.70°,and 18.15° at the age of 28 days and 360 days,in which 9.12° and 11.70° are the diffraction angles of ettringite and dihydrate gypsum,respectively.It can be concluded from this table that the diffraction peaks of ettringite and dihydrate gypsum in a sulfate environ‐ment are significantly higher than those in a fresh wa‐ter environment no matter the test age of 28 days or 360 days,which means that the corrosion products in a sulfate environment include those two substances.In addition,when the diffraction angles are 9.12° and 11.70°,the diffraction peak intensities of concrete with 60% GGBS in a sulfate environment are significantly lower than that of concrete without GGBS,which shows that the addition of GGBS can reduce the production of ettringite and dihydrate gypsum,and improve the sulfate resistance of concrete.Compared with 28 days,the in‐tensities of diffraction peaks at 9.12° and 11.70° have marked increases at 360 days,that is,as the concrete age increases,the corrosion becomes increasingly se‐vere and more corrosive products are generated.The peak value of Ca(OH)2in the non-dispersible underwa‐ter concrete with GGBS is the lowest,indicating that the content of Ca(OH)2is greatly reduced by adding GGBS.
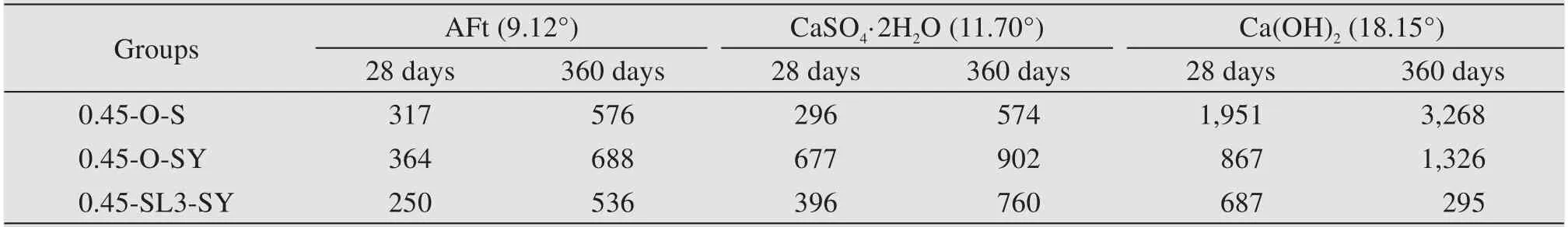
Table 10 Peak intensity of main phase of cement pastes at ages of 28 days and 360 days
Figures 8 and 9 show the TG/DTG analysis of ce‐ment paste samples cured in freshwater and sulfate en‐vironments for 180 days,respectively.It can be con‐cluded that there are four obvious weight loss peaks in the TG/DTG analysis diagram both in fresh water and sulfate environments,and the first two are in the range of 50°C to 200°C.
There is some controversy in the analysis of cement hydration products using TG/DTG in the range below 200 °C,and scholars have different views on the de‐composition temperatures of ettringite and dihydrate gypsum.Durdziński (2016) considered the decomposi‐tion temperature of ettringite to be 50−150°C and dihy‐drate gypsum to be 150−180°C,while some researches(Zhou and Glasser,2001;Shimada and Young,2004;Lothenbach and Wieland,2006) considered the de‐composition temperature of ettringite to be 50−110°C while Elbeyliet al.(2003) considered 128−188 °C as the weight loss interval for dihydrate gypsum with a valley value of 164 °C.There are also literature (Step‐kowskaet al.,2004;Yeet al.,2007) showing that the range of 100−130 °C is dominated by the evaporation of adsorbed water,and in the range of 160−185 °C,there is partial evaporation of bound water.Based on the aforementioned studies,we believe in this test that the decomposition temperature range of ettringite is 50°C to 140°C,dihydrate gypsum is 140°C to 200°C,and the evaporation of water in C-S-H gel occurs throughout the whole temperature range of 50−200 °C.The third weight loss peak ranges from 420°C to 480°C,and its peak valley appears at 450 °C,which is caused by the decomposition of Ca(OH)2.In the temperature range of 700 °C to 800 °C,the fourth weight loss peak appears due to the decomposition of CaCO3.
The CaO content of samples 0.45-O-S,0.45-O-SY and 0.45-SL3-SY is calculated to be 15.1%,14.16%and 8.99%,i.e.,the Ca(OH)2content in the first two samples is much more than the third one.This indi‐cates that the blending of granulated blast furnace slag powder can consume a large amount of Ca(OH)2.It can also be seen from Figures 8 and 9 that positions of the weight loss peaks do not change throughout the test,indicating that no new hydration products are produced after the incorporation of granulated blast furnace slag powder.
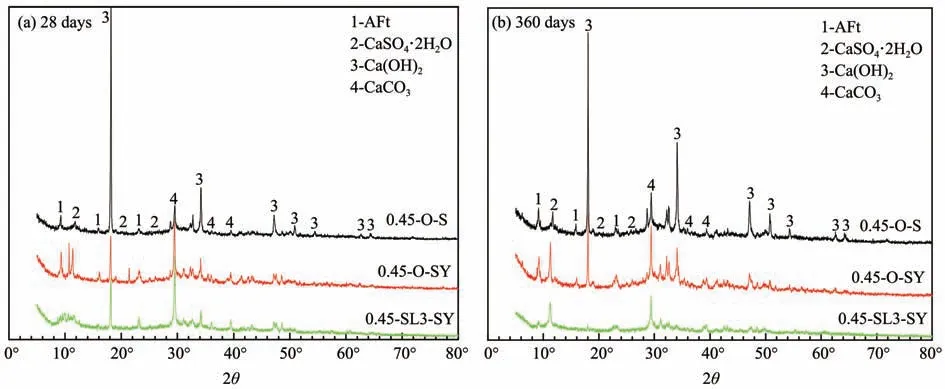
Figure 7 XRD patterns of cement pastes in a sulfate environment
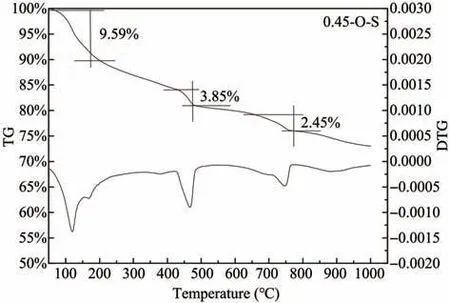
Figure 8 TG/DTG analysis of cement paste samples cured in freshwater for 180 days
(4) FT-IR analysis
The FT-IR results of cement paste at 28 days and 360 days in a sulfate environment are provided in Fig‐ure 10,in which the sample '0.45-O-S' is the reference group.The absorption peak at 3,644 per cm is caused by the vibration of O-H in Ca(OH)2(Silvaet al.,2002),and the absorption peak produced by symmetry stretching vi‐bration of SO4(v1)located at 1,103 per cm is an obvious characteristic peak of ettringite(Trezza and Lavat,2001).
By comparing samples 0.45-O-SY and 0.45-SL3-SY in Figure 10,it can be found that the absorption peak of Ca(OH)2at 3,644 per cm disappears after adding 60% GGBS,which indicates that the addition of slag powder could consume a lot of Ca(OH)2produced by cement hydration.At 1,103 per cm,sample 0.45-O-SY has an obvious absorption peak,while samples 0.45-O-S and 0.45-SL3-SY have no obvious absorption peak.The results show that the corrosive product ettringite is generated from sample 0.45-O-SY in a sulfate envi‐ronment,and it can be inferred that the sulfate resis‐tance of concrete can be effectively improved by add‐ing 60%GGBS.
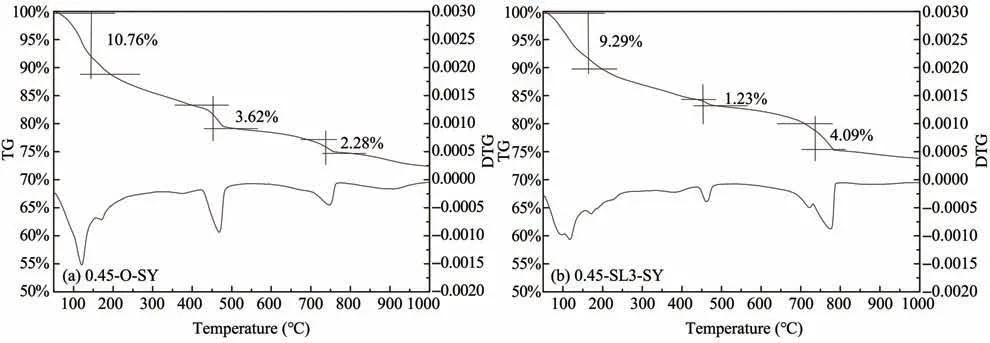
Figure 9 TG/DTG analysis of cement paste samples cured in a sulfate environment for 180 days
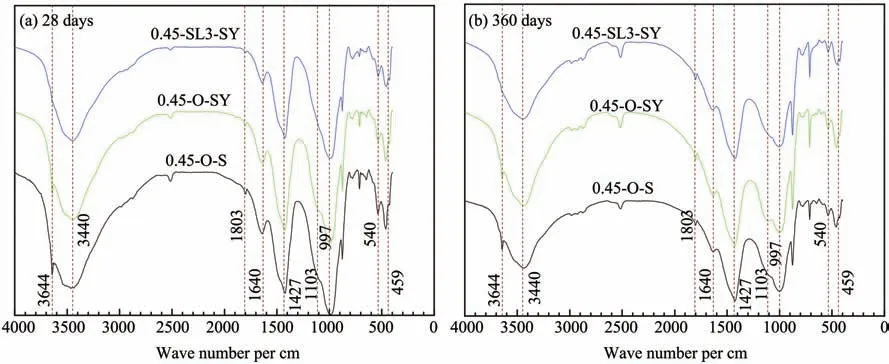
Figure 10 FT-IR analysis of cement paste samples cured in a sulfate environment
3.3 Chloride corrosion resistance and microscopic analysis of non-dispersible underwater concrete
3.3.1 Development of compressive strength and deterioration resistance coefficient of concrete in a chloride environment
And when she had had enough, she said, as the wise woman had told her, Little goat, bleat, Little table, away, and immediately the table and all that was on it disappeared again
Test results of compressive strength and deteriora‐tion resistance coefficient of concrete in a chloride en‐vironment are provided in Figure 11 and Table 11,where'LY'represents chloride environment.
The influence of the content of GGBS on compres‐sive strength of non-dispersible underwater concrete in a chloride environment is provided in Figure 11.It is clear from Figure 11 that the compressive strength of ordinary non-dispersible underwater concrete in a chlo‐ride environment increases with an increase of age.After adding GGBS,the compressive strength is also in an increasing state from 7 days to 360 days,which shows that the compressive strength of non-dispers‐ible underwater concrete can be effectively improved by adding GGBS in a chloride environment.

Figure 11 Influence of GGBS addition on the compressive strength in a chloride environment
The influence of GGBS addition on the deteriora‐tion resistance coefficient of non-dispersible underwa‐ter concrete in a chloride environment is provided in Table 11.Overall,deterioration resistance coefficients of the four groups are very similar,roughly between 0.95 to 1.In a chloride environment,the expansion of Cl−erosion products is small,and before the Cl−ero‐sion products accumulate in the specimen,fill the pores and cause extrusion,the pores within the con‐crete are gradually filled to increase the density of the concrete and may cause an enhancement in strength,resulting in some values greater than 1.0.
3.3.2 Microscopic analysis and discussion
(1) SEM analysis
Figures 12 and 13 are SEM images of non-dispers‐ible underwater concrete in a chloride environment for 28 days and 360 days,respectively.By comparing Figures 3 and 12a,Figures 5 and 13a,it can be found that concrete in a chloride environment has similar mi‐cro morphology to that in a fresh water environment without obvious corrosion products.In addition,com‐paring Figures 12a and 12b,Figures 13a and 13b,it can be seen that the compactness of the concrete ma‐trix is improved after adding granulated blast furnace slag powder.
(2) XRD analysis
It is clear from Figure 14 and Table 12 that sam‐ples 0.45-O-LY and 0.45-SL3-LY have distinct dif‐fraction peaks at 11.12° and the peak intensity of the latter is greater than that of the former,which implies that new substance Friedel's salt is produced due to the hydration of cement in a chloride environment.Also,the quantity could be increased by mixing of GGBS,because the aluminum phase in GGBS could improve the binding ability of cement paste to chlo‐ride ions(Hewlett,2003).The diffraction peak intensi‐ty of Friedel's salt at 360 days is much greater than that at 28 days,which indicates that more Friedel's salt will be produced in cement paste with the in‐crease of soaking time.

Figure 12 SEM images of non-dispersible underwater concrete in a chloride environment for 28 days
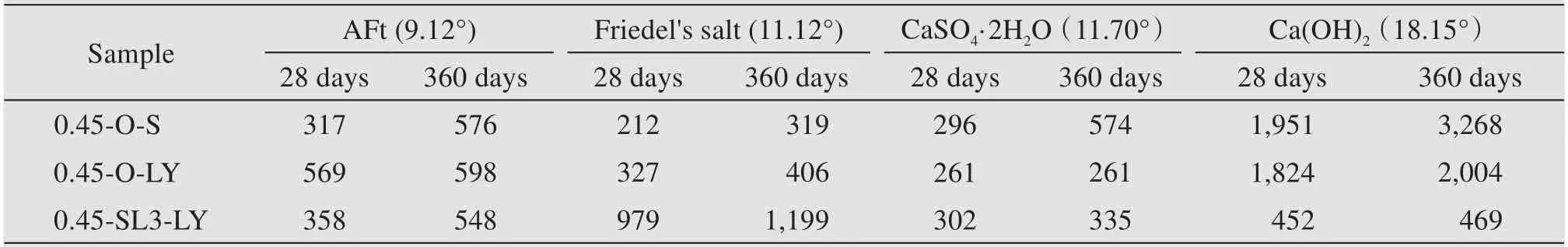
Table 12 Peak intensity of main phase of cement pastes at ages of 28 days and 360 days

Figure 13 SEM images of non-dispersible underwater concrete in a chloride environment for 360 days

Figure 14 XRD patterns of cement pastes in a chloride environment

Table 11 Deterioration resistance coefficient of non-dispersible underwater concrete in a chloride environment
(3) TG/DTG analysis
Figure 15 shows TG/DTG analysis of cement paste samples after curing in a chloride environment for 180 days.Comparing Figures 8 and 9 with Figure 15,it can be concluded that there is an obvious absorp‐tion peak in the range of 250 °C to 350 °C with peak valley near 320 °C,except for the other four in the range of 50°C to 200°C,420°C to 480°C and 700°C to 800 °C,indicating that new hydration products are produced in a chloride environment.Referring to rele‐vant literature (Csizmadiaet al.,2001;Goñi and Guer‐rero,2003;Saikiaet al.,2006),it can be inferred that the endothermic peak at 320 °C in Figure 15 is caused by the dehydration and decomposition of Friedel's salt.The weight loss rate of Friedel's salt in Figures 15a and 15b is 2.44% and 3.56%,respectively,indicating that the amount of Friedel's salt will increase significantly after adding 60% GGBS.This is because GGBS con‐tains a large amount of aluminum phase (Lu,2015),which is conducive to the adsorption of a large num‐ber of chloride ions,thus generating Friedel's salt.Moreover,there is a competitive relationship between hydroxyl ions and chloride ions,therefore,as the con‐centration of the former decreases,the binding ability of the latter with cement-based materials enhances(Wang,2013).The addition of GGBS can produce secondary hydration reaction with hydration product Ca(OH)2,which greatly reduces the concentration of hydroxyl ions in cement matrix.
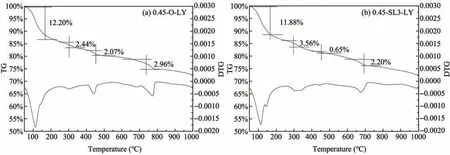
Figure 15 TG/DTG analysis of cement paste samples in a chloride environment for 180 days
(4) FT-IR analysis
Figure 16 shows FT-IR results of cement paste samples in a chloride environment at 28d and 360d,and 0.45-O-S is the reference group.In Figure 16,the spectrum of 0.45-O-S is similar to that of 0.45-O-LY,and the absorption peak of Ca(OH)2at 3,644 per cm of 0.45-SL3-LY disappears owing to the secondary hydration reaction between GGBS and Ca(OH)2pro‐duced by cement hydration.
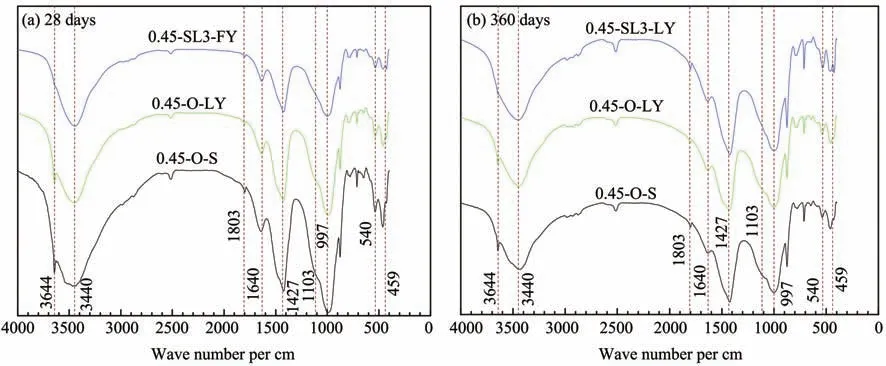
Figure 16 FT-IR analysis of cement paste samples in a chloride environment
3.4 Deterioration resistance and microscopic analysis of non-dispersible underwater concrete in a mixed salt environment
3.4.1 Development of compressive strength and deterioration resistance coefficient of concrete in a mixed salt environment
Test results of compressive strength and deterio‐ration resistance coefficient of concrete in a mixed salt environment are provided in Figure 17 and Ta‐ble 13,respectively,where 'FY' represents mixed salt environment.
The influence of the content of GGBS on com‐pressive strength in a mixed salt environment is pro‐vided in Figure 17.It can be seen from Figure 17 that the compressive strength is in a state of growth from 7 days to 360 days after adding GGBS in the mixed salt environment.The result shows that the addition of GGBS is able to enhance the deterioration resis‐tance of non-dispersible underwater concrete to mixed salt.During the period of 7 days to 90 days,its compressive strength first increases and then de‐creases with increasing of GGBS content,and 40%slag powder is the optimal dosage.

Figure 17 Influence of GGBS addition on compressive strength in a mixed environment
The influence of GGBS addition on the deteriora‐tion resistance coefficient of concrete in a mixed salt environment is provided in Table 13.In the period of 7 days to 90 days,this coefficient fluctuates greatly with no obvious regularity,and then shows a down‐ward trend overall after 90 days.Also,the addition of GGBS is conducive to improving the coefficient effec‐tively,and the degree of improvement increases with an increase of its content.The presence of chloride ions in a mixed salt environment can weaken the cor‐rosion caused by sulphate on concrete,and this is be‐cause chloride ions have much greater permeability than sulphate ions and can react with the hydration products first to form Friedel's salt with less expan‐sion.The early stages are therefore dominated by the erosion of chloride salts,and an increase in strength may occur until the pores are gradually filled.
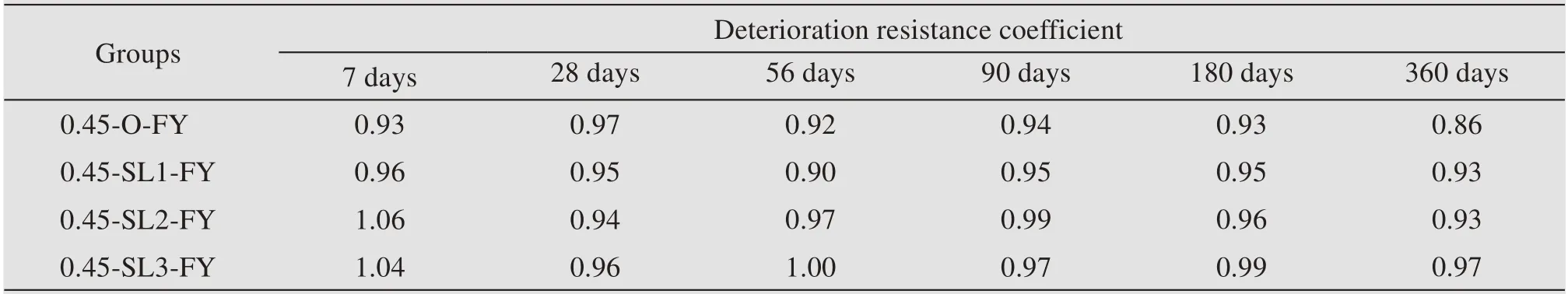
Table 13 Deterioration resistance coefficient of non-dispersible underwater concrete in a mixed salt environment
3.4.2 Influence of different corrosive environments on compressive strength of non-dispersible underwater concrete
The compressive strength of non-dispersible un‐derwater concrete in different corrosion environ‐ments is provided in Figure 18.According to Figure 18a,the compressive strength of concrete in differ‐ent environments is very close at 7 days without add‐ing GGBS.At 28 days,the compressive strength of concrete in a chloride environment is the highest,while that in a sulfate environment is the lowest.At that time,the compressive strength in chloride and mixed salt environments are 5.4% and 3.1% higher than that in a sulfate environment separately.The dif‐ference is more obvious with an increase of age,and increases to 22% and 7.3% at 360 days.After adding 60%GGBS,the compressive strength of non-dispers‐ible underwater concrete is almost the same in the period of 7 days to 180 days,which in chloride and mixed salt environments is only 6.3%and 1.3%high‐er than that in a sulfate environment at 360 days.
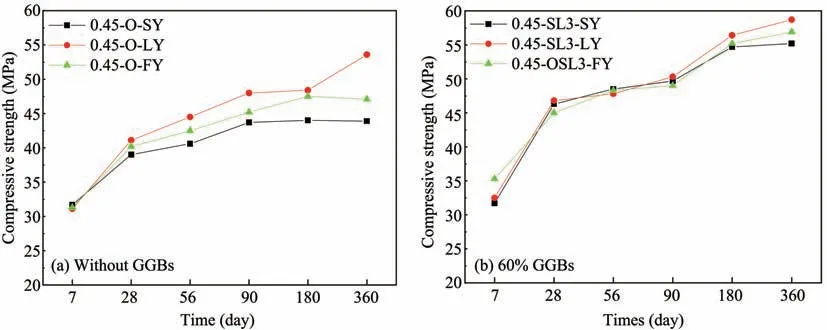
Figure 18 Compressive strength of non-dispersible underwater concrete in different corrosion environments
Through the aforementioned analysis,it turns out that without the addition of granulated blast furnace slag,the deterioration of non-dispersible underwater concrete in a sulfate environment is the most serious,followed by mixed salt environment,and there is no obvious deterioration phenomenon in a chloride envi‐ronment.Moreover,the deterioration in the first two environments begins to appear at 28 days,and be‐comes severe with an increase of age.By comparison,the obvious deterioration situation does not appear un‐til 360 days when 60%GGBS is added,which indicates that the mixing of GGBS can improve the deteriora‐tion resistance of non-dispersible underwater concrete.
The deterioration of non-dispersible underwater concrete in a mixed salt environment is weaker than in a sulfate environment for the reason that there is a competitive relationship between Cl−and SO42−,and the penetration rate of Cl−is faster than the latter,which is prior to the reaction of cement hydration products to form Friedel's salt with lesser expansibili‐ty,and set back the generation of ettringite.Also,ac‐tive SiO2and Al2O3in GGBS react with Ca(OH)2to produce calcium silicate hydrate and calcium alumi‐nate hydrate,which could reduce the porosity of con‐crete matrix,improve its compactness and hinder the infiltration of corrosive ions.At the same time,on ac‐count of the reduction of Ca(OH)2content,the amount of dihydrate gypsum in sulfate and mixed salt environments also greatly goes down,and thus the de‐terioration resistance is further improved.
3.4.3 Microscopic analysis and discussion
(1) SEM analysis
Figures 19 and 20 are SEM images of non-dis‐persible underwater concrete in a mixed salt envi‐ronment at 28 days and 360 days,respectively.It is clear from Figure 19a that a large number of needlelike ettringite crystals grow in the concrete pores,while the concrete matrix is relatively dense with‐out obvious corrosion products in Figure 19b.Also in Figure 20a,a large number of well-crystallized ettringite crystals are formed.From Figure 20b,ettr‐ingite and calcium hydroxide crystals are difficult to be found in the electron microscope pictures af‐ter 60%GGBS is added.

Figure 19 SEM images of non-dispersible underwater concrete in a mixed environment for 28 days
(2) XRD analysis
It can be seen from Figure 21 that there are obvi‐ous diffraction peaks at 9.12°,11.12° and 11.70° for sample 0.45-O-FY,showing that cement paste in a mixed salt environment is not only corroded by sul‐fate to produce ettringite and dihydrate gypsum,but also by chloride to produce Friedel's salt.

Figure 20 SEM images of non-dispersible underwater concrete in a mixed environment for 360 days
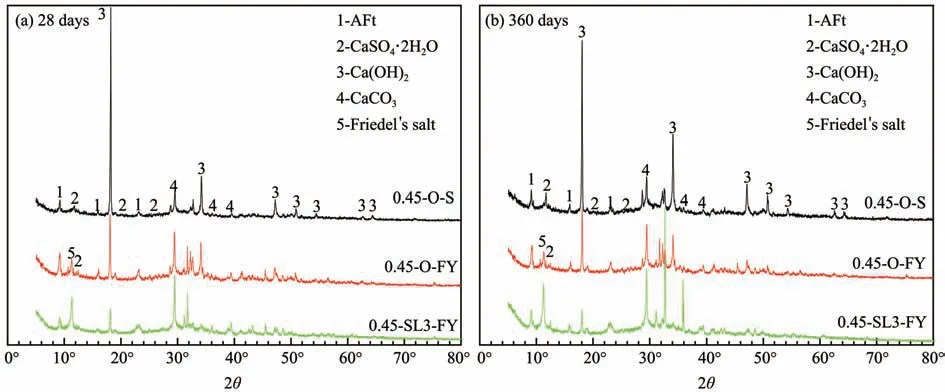
Figure 21 XRD patterns of cement pastes in a mixed salt environment
Compared with Tables 10,12 and 14,we find that,in a mixed salt environment,the diffraction peaks of samples 0.45-O-FY and 0.45-SL3-FY at 9.12° and 11.70° are generally smaller than those in a sulfate environment,and their diffraction peaks at 11.12° are both smaller than those in a chloride envi‐ronment.The results signify that the content of ettrin‐gite and dihydrate gypsum produced in a mixed salt environment are lower than those in a sulfate envi‐ronment,and the content of Friedel's salt produced in the cement paste under a mixed salt environment is lower than that in a chloride environment.There‐fore,it can be inferred that the presence of chloride salt can contribute to weakening the corrosion effect of sulfate on concrete,and the presence of sulfate can also weaken the formation of Friedel's salt.When chloride ions and sulfate ions exist at the same time,there will be competition and restriction between them.

Table 14 Peak intensity of main phase of cement pastes at ages of 28 days and 360 days
(3) TG/DTG analysis
TG/DTG analysis of cement paste samples in a mixed salt environment for 180 days is provided in Fig‐ure 22.It can be found that Figure 15 is similar to Fig‐ure 22,both of which have obvious absorption peaks in the range of 50−200°C,250−350°C,420−480°C,and 700−800 °C,indicating that the cement paste un‐der a mixed salt environment also produces Friedel's salt,the new hydration product.In Figures 22a and 22b,the weight loss rate of Friedel's salt is 2.07% and 2.99%,respectively,both lower than that in Figure 15.This shows that compared with a chloride environ‐ment,the mixed salt environment produces less Frie‐del's salt as sulfate ions will weaken the production of Friedel's salt(Luoet al.,2003).
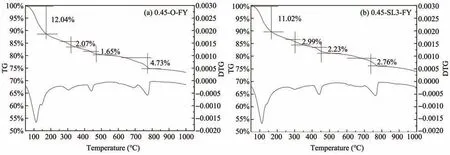
Figure 22 TG/DTG analysis of cement paste samples in a mixed salt environment for 180 days
(4) FT-IR analysis
Figure 23 shows FT-IR images of cement paste at 28 days and 360 days in a mixed salt environment.Compared with sample 0.45-O-FY in Figures 23a and 23b,it can be found that at 360 days,the diffrac‐tion peak of sample 0.45-O-FY at 1,103 per cm is more obvious,showing that ettringite is produced in cement paste under a mixed salt environment,and its amount increases with an increase of corrosion time.Comparing sample 0.45-O-FY in Figure 23 and sam‐ple 0.45-O-SY in Figure 10,it can be concluded that the diffraction peak at 1,103 per cm of cement paste under a sulfate environment is stronger than in a mixed salt environment,which indicates that ettring‐ite,the corrosive product generated by cement paste in a sulfate environment is more than in a mixed salt environment,and the presence of chloride salt can weaken the corrosion of sulfate on concrete.There is no obvious characteristic peak of ettringite at 1,103 per cm of sample 0.45-SL3-FY in Figure 23,mean‐ing that adding 60% GGBS can hinder the formation of corrosion product ettringite,and improve the resis‐tance of cement paste to mixed salt attack.

Figure 23 FT-IR analysis of non-dispersible underwater concrete in a mixed salt environment
4 Conclusions
In this paper,the influence of granulated blast fur‐nace slag on compressive strength and deterioration resistance coefficient of non-dispersible underwater concrete in sulfate,chlorine salt and mixed salt envi‐ronments was analyzed.We also studied the deteriora‐tion mechanism of non-dispersible underwater con‐crete in different corrosion environments and the mi‐croscopic mechanism of granulated blast furnace slag powder in non-dispersible underwater concrete.The conclusions are as follows:
(1)The deterioration of non-dispersible underwa‐ter concrete is the strongest in a sulfate environment,followed by mixed salt environment,and the weak‐est in a chloride environment.Adding granulated blast furnace slag powder can effectively improve the compressive strength of non-dispersible under‐water concrete.
(2) In a sulfate environment,SEM images show that a large number of needle-like ettringite crystals are produced in the non-dispersible underwater con‐crete,and the compactness of concrete matrix increas‐es after adding 60% granulated blast furnace slag powder;XRD,TG-DTG and FT-IR spectra show that the content of calcium hydroxide,corrosion product ettringite,as well as dihydrate gypsum in the non-dis‐persible underwater concrete significantly reduces af‐ter adding 60% granulated high furnace slag powder in a sulfate environment.These results indicate that the addition of granulated blast furnace slag powder is conducive to improving the sulfate resistance of nondispersible underwater concrete.
(3)The compressive strength of concrete in a chlo‐ride environment is close to that in fresh water,indi‐cating that chloride has no adverse effect on concrete strength;According to SEM images,there are similar micro-morphology between non-dispersible concrete in chloride and fresh water environments.Also,XRD,TG-DTG and FT-IR spectra show that the corrosion product Friedel's salt are produced in a chloride envi‐ronment,and its content will be increased by adding granulated blast furnace slag.
(4) SEM images show that a large amount of ettr‐ingite is produced in non-dispersible underwater con‐crete under a mixed salt environment.Also,XRD,TG-DTG and FT-IR spectra show that the content of ettringite and dihydrate gypsum in a mixed salt envi‐ronment is significantly lower than that in a sulfate en‐vironment,and the content of Friedel's salt is also evi‐dently lower than that in the chloride environment.The addition of granulated blast furnace slag powder is able to consume the content of calcium hydroxide,reducing the production of ettringite and dihydrate gypsum,increasing the content of Friedel's salt,and improving the resistance of non-dispersible underwa‐ter concrete to mixed salt corrosion.
The deterioration of non-dispersible underwater concrete in saline soil environments takes place over a very long period and due to time constraints the age of corrosion in the test is only 360 days.Subsequent research will therefore explore the deterioration of non-dispersible underwater concrete at a longer age.In addition,the test method is full solution immersion here,but given that dry-wet alternation areas are where the concrete suffers most from corrosion in practice,the semi-submersion test will be carried out to investigate the erosive effects of saline soil environ‐ments on non-dispersible underwater concrete.
Acknowledgments:
This work is supported by the National Natural Sci‐ence Foundation of China (51878116 and 51902270);Liaoning Province Key Project of Research and De‐velopment Plan (2020JH2/10100016);Dalian Science and Technology Innovation Fund Project (2020JJ26 SN060);the Open Research Fund of State Key Labo‐ratory of Simulation and Regulation of Water Cycle in River Basins (China Institute of Water Resources and Hydropower Research),Grant No.IWHR-SKL- 201910;the Special Fund for the Launch of Scientific Re‐search in Xijing University (XJ21T01);the Youth In‐novation Team of Shaanxi Universities.
 Sciences in Cold and Arid Regions2022年2期
Sciences in Cold and Arid Regions2022年2期
- Sciences in Cold and Arid Regions的其它文章
- Editor-in-Chief Yuanming Lai
- Estimating snow depth or snow water equivalent from space
- Influence of meteorological elements on chemical evolution of snow and ice of Urumqi Glacier No.1,eastern Tianshan Mountains
- Coarse fragment content influences estimates of soil C and N stocks of alpine grassland on the northeastern edge of Qinghai-Tibetan Plateau,China
- Diversity and composition of culturable fungi in Horqin Sandy Land
- Simulation assessment and prediction of future temperatures in Northwest China from BCC-CSM Model
