Influence of meteorological elements on chemical evolution of snow and ice of Urumqi Glacier No.1,eastern Tianshan Mountains
XiaoNi You ,ZhongQin Li ,LiXia Wang
1.Tianshui Normal University,Tianshui,Gansu 741001,China
2.State Key Laboratory of Cryospheric Sciences,Northwest Institute of Eco-Environment and Resources,Chinese Academy of Science,Lanzhou,Gansu 730000,China
ABSTRACT For most mountain glaciers,chemical components in snowfall are subject to the elution process under the influences of meltwater before they are preserved in ice,creating difficulties for interpreting ice core records.To understand the forma‐tion process of ice core records and analyze the influences of meteorological factors on the ice core resolution,we mea‐sured ion concentrations of snowpacks from 2003 to 2006 in the PGPI (Program for Glacier Processes Investigation) site of Urumqi Glacier No.1.The ion concentration variation in snowpack exhibits apparent seasonality.In summer,the high‐er snowmelt rates due to air temperature rise intensify dilution and lead to an exponential decrease in ion concentrations as the accumulated positive temperature increases.In winter,the snow ion concentrations are stable and low as a result of re‐duced temperature and rare precipitation.Many ions from summer precipitation are leached out by meltwater,and only the precipitation that occurs at the end of the wet season can be preserved.Through tracking the evolution of magnesium ion peaks in the snowpack,it is concluded that the ice core resolution is one year on Urumqi Glacier No.1,albeit 70% of the concentration information is lost.
Keywords:elution process;Urumqi Glacier No.1;temperature;precipitation;ice core resolution
1 Introduction
Ice cores are often used to explore historical cli‐mate change by researchers worldwide (Isakssonet al.,2001;Kotlyakovet al.,2004;Kaufmannet al.,2010;Lalurajet al.,2011;Thompsonet al.,2015;Graeteret al.,2018).However,summer ablation is likely to impact ice core records drilled in low lati‐tude mountain areas.Once the summer tempera‐tures are higher than the threshold value,the upper layer of snow begins to melt.Meltwater carries vari‐ous ions to mix with the bottom snow,which dis‐turbs the original ion information of the snow layer(Van de Walet al.,2002).For the ice cores drilled close to the firn line where melting is intense,their seasonal signals may be reduced or even removed(Koerneret al.,1997).Recognition of a climatic signal and development of time scales in these ice cores are difficult (Baleset al.,1989;Liet al.,2006).To resolve this problem,it is imperative to understand the effects of the elution process on the formation and resolution of ice core records under different meteorological conditions and further find out how to correct the meltwater influence on the climate and environmental information extracted from the ice cores.
Meltwater can lead to the migration of soluble and insoluble components in the snowpack,which greatly impacts the rivers and lakes mainly fed by glacier meltwater.There are already extensive stud‐ies on the seasonal variation in snow ice chemistry and its relationship with melting runoff (Marshet al.,1999;Anjaet al.,2001;Sunet al.,2018;Khod‐zheret al.,2019;Costaet al.,2020).With widely used analytical and mathematical models,more stud‐ies focus on quantitative relationships between postdeposition process and ice core records (Jeonghoonet al.,2008;Torstenet al.,2011) by predicting snow and ice melting runoff and relating air temperatures with the melting process (Franzet al.,2008;Woodset al.,2009;Yukiyoshiet al.,2011;Georget al.,2012).
The interpretation of ice core records is based on the hypothesis that the changes in atmospheric chemi‐cal composition are closely related to the variation in the chemical composition of ice and snow (Wolff,1996).As a critical wet deposition process,precipita‐tion brings the atmospheric components onto the sur‐face snow,which becomes an essential source of chemical ions in the snowpack (Lekhendraet al.,2013,2019;Conget al.,2015;Sharmaet al.,2020).
A research program,i.e.,the Program for Glacier Processes Investigation (PGPI),was launched in July 2002 to investigate the effects of depositional process‐es and meltwater-related post-depositional processes on chemical species in the snowpack by the Tianshan Glaciological Station (TGS),Chinese Academy of Sciences.In this study,taking advantage of the long time-series data from 2003 to 2006,we introduce me‐teorological parameters,including air temperature and precipitation,to explore the ions evolution characteris‐tics in snow and ice.The results could provide a refer‐ence for the revision of ice core records,the recon‐struction of climate information through ice cores,and further understanding of climate and environmen‐tal changes.
2 Materials and methods
The snowpacks used in this study were sampled from a site at an altitude of 4,130 m on the east branch of Urumqi Glacier No.1 (43°06'N,86°49'E) at the headwater of Urumqi River in the Tianshan Moun‐tains,Central Asia.For this glacier,summer is a vital supplement period,during which about 90%of the an‐nual precipitation occurs.A complete snow profile was conserved at the study site due to short sun radia‐tion.The snowpacks were extracted from 20-40 cm be‐low the superimposed ice surface.Before sampling,it is necessary to remove the top 10-cm snow layer to ensure that the snow samples are not affected by solar radiation,wind,and man-made pollution.Clean poly‐ethylene sampling bottles were inserted into the snow layer to collect samples at 10 cm intervals from top to bottom.In hard snow and ice layers,samples were scraped into bottles with a pre-treated scraper (Youet al.,2015).From February 2003 to August 2006,186 snowpits were excavated at 1-week intervals,and 3,729 snow and ice samples were collected succes‐sively.All the samples were transported frozen to the State Key Laboratory of Cryospheric Sciences,North‐west Institute of Eco-Environment and Resources,Chinese Academy of Sciences.
The operators wore clean masks,hats,coats,and polyethylene gloves during the sampling process to prevent contamination.Blanks were made at each step in the process to ensure that the cumulative con‐tamination remained below the baseline of each mea‐sured chemical species (Liet al.,2006;Youet al.,2015).Samples were analyzed for major chemical ions,including Na+,K+,Ca2+,Mg2+,NH4+,Cl−,SO42−,and NO3−,by ion chromatography (Dionex DX-320).Detection limits (mg/L) of these ions are 0.0006(Na+),0.0009 (K+),0.00075 (Ca2+),0.0008 (Mg2+),0.0010 (NH4+),0.0006 (Cl−),0.00085 (SO42−) and 0.00065(NO3−)with precision ranges of 2%-12%.
The variation in snowpack ion concentration de‐pends on the relationship between deposition and elu‐tion process.Specifically,a decrease in ion concentra‐tion suggests more ions are leached out,and the snow‐pack is eluted.Conversely,more ions are deposited in‐to the snowpack when ion concentration increases.In this paper,snowpack ion concentration is calculated by averaging the measurements taken across the verti‐cal snowpack profile.
3 Results and discussions
3.1 Influence of temperature on elution process
3.1.1 Monthly average temperature and ion concentration
Figure 1 illustrates the variations in the monthly mean concentrations of chemical species in the snow‐pack and monthly mean air temperature from Febru‐ary 2003 to August 2006.Comparing the trends in the monthly mean ion concentration and air temperature indicates that the ion concentrations have an evident seasonality due to the influence of temperatures.The increase in the monthly average temperature is accom‐panied by the decline of ion concentration and vice versa.For instance,from January to June 2005,the to‐tal ion concentrations kept relatively high,ranging from 2,093 μg/L to 3,016 μg/L,while the daily aver‐age temperatures remained low,with an average of-13.0 ℃,and the maximum and minimum of 0 ℃and -26.2 ℃,respectively.With the increase in air temperature,the ion concentration decreased sharply and dropped to the lowest value of 985.4 μg/L in Au‐gust 2005,when the corresponding daily temperature averaged 0.3 ℃,with the highest and lowest tempera‐ture of 6.1 ℃and -5.9 ℃,respectively.By Septem‐ber,air temperature started to fall,and the ion concen‐tration rose and reached 2,363.2 μg/L in October and then stabilized.

Figure 1 Variations in the monthly mean concentrations of all and each chemical species in the snowpack and monthly mean air temperature from February 2003 to August 2006
The influence of air temperatures on ion species is discriminatory.The annual variations in the concentra‐tions of all analyzed ions except for K+and Mg2+were similar to that of the total ion concentration.Based on various field and laboratory studies,ions are leached from snow and firn with different efficiencies (Eichleret al.,2001;Iizukaet al.,2002;Liet al.,2006),which are controlled by snowmelt dynamics and snowpack's physical and chemical properties (such as snowmelt metamorphism,refreezing of meltwater,and ion ex‐clusion from snow crystals) (Costaet al.,2019,2020).On the other hand,previous studies show that snowpack K+ion is derived from local rocks and gla‐cial sediments (Williamset al.,1991;Houet al.,2002;Youet al.,2015),and potential sources of Mg2+ion include mineral dust and evaporated aerosols en‐trained by the air masses (Liet al.,2006;Youet al.,2011).Both Mg2+and K+ions are relatively immobile in the snowpack compared to the other ion species,which is probably the result of their resistance to transport.
The change of ion concentration in the snowpack is mainly affected by two processes:one is the depo‐sition process dominated by wet(precipitation)depo‐sition and dry deposition;the other is the post-depo‐sition process controlled by wind blowing snow,sub‐limation,and eluviation (Houet al.,2002;Wanget al.,2006;Youet al.,2015).In winter (December to February),the precipitation at the Urumqi River headwater is rare,and the temperature is low.The snowpack ion concentration is mainly affected by blowing snow,sublimation,dry deposition,etc.,pro‐ducing a limited effect.From spring to early summer(March to June),the temperature and precipitation begin to rise,and the snowpack ion concentrations increase slightly,which may be attributed to frequent dust events,cold front,and low-pressure activities.In spring,under the westerly updraft,a large amount of dust from the sand source navigates through the Tian‐shan Mountains to the east.Meanwhile,abundant steam moves eastward under the effect of westerlies,bringing frequent precipitation to the eastern part of the Tianshan Mountains (Liet al.,2006;Wanget al.,2006).As a result,the total ion concentration in early summer reaches the maximum.In July,the higher temperature leads to the snowpack melting,resulting in more ions leaching out by meltwater and a sharp drop in the ion concentration.In September,the fall‐ing temperature gradually ends the snowpack melting,weakening the eluviation process and allowing the ion concentration to rise again.
3.1.2 Accumulative temperature and ion concentration
Considering that the elution process is more closely related to the temperatures above zero,we further compare the relationship between weekly ac‐cumulative temperature,weekly positive accumula‐tive temperature,and ion concentration during the study period,as depicted in Figure 2.Also,the snow depth from September 2003 to September 2006 is shown in Figure 2 for comparison.The intense elu‐tion period is characterized by periods when the total ion concentration decreases from the maxima to the minima.These periods perfectly overlap the terms when the snow depth shrinks from a largest to a smallest;it is also consistent with the interval when the positive accumulative temperatures are signifi‐cant.That means,once the positive accumulative temperatures are significant,the ion concentration and snow depth fluctuate considerably and decrease to the minimum rapidly.The elution process might stop or weaken around August even though the posi‐tive accumulative temperatures are still above zero,which is demonstrated by the gradually increased ion concentrations and snow depth.It is worth men‐tioning that the elution process may occur before the strong elution period,even though it does not affect the total ion concentration of the snowpack signifi‐cantly.Generally,a small amount of meltwater car‐ries chemical ions to the lower part of the snow layer at the early ablation stage.They might be blocked by the dense snow layer or ice layer and refreeze due to the lower temperature in the snow (Baleset al.,1989;Zhanget al.,2010).Although such a process may alter the ion distribution in the snowpack and contaminate the annual ion information,the amount of the total ions does not decrease.However,the re‐freezing process tends to form ion-rich quasi-liquid layers at the surface of growing ice crystals due to the low solubility of many ions,leading to a rapid re‐lease of these ions during the early snowmelt stages(Wanget al.,2006;Lilbæket al.,2010).
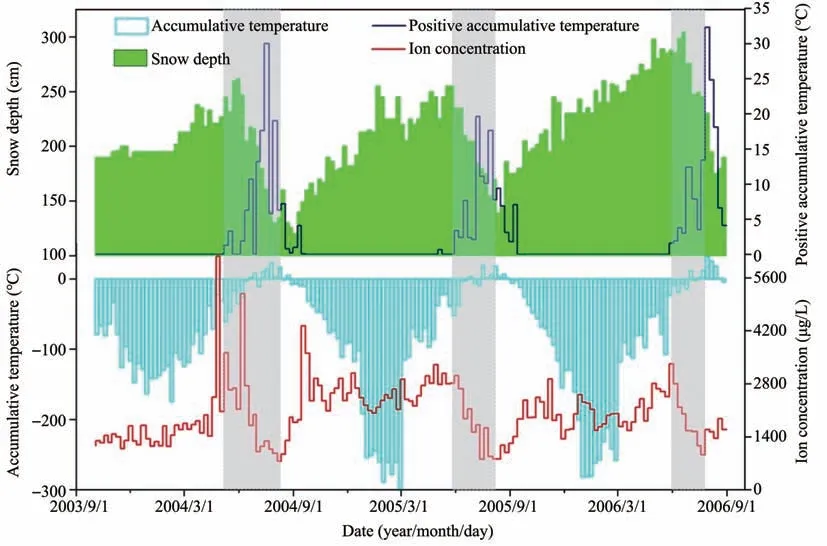
Figure 2 Comparison of the total ion concentration in the snowpack,accumulative temperature,positive accumulative temperature,and snow depth.The shaded zones indicate the intense elution period that ion concentrations decrease from the maxima to the minima
3.2 Influence of precipitation on chemical evolution
Chemical components are removed from the atmo‐sphere and deposited on the surface snow by precipita‐tion.Hence,precipitation is presumably a crucial mete‐orological parameter that affects the snowpack ion con‐centration.To explore the mechanisms of precipitation influence on chemical evolution,we defineR-value as the ratio of concentration in the surface snow to that in the snowpack and analyze the relationship betweenR-value and precipitation.Here the ion concentration in the snowpack is defined as the average value of ion concentrations in snow layers (including the concen‐trations in surface snow) across the vertical snowpack profile.Based on theR-value,a complete seasonal cy‐cle from March 2005 to March 2006 is divided into four periods marked by T1,T2,T3,and T4,as illustrat‐ed in Figure 3.During T1(roughly from the beginning of March to the middle of June),theR-value mainly fluctuates between 0.3 and 4.5.With frequent precipi‐tation totaling 108.7 mm,the ion concentrations in the snowpack gradually rise and reach the maximum.Likewise,ion concentrations in the surface snow vary sharply at a higher level with an average concentra‐tion of 3,788.8 μg/L.T2(from the middle of June to the beginning of September) is characterized by lower R-values (less than 1).The ion concentrations in the snowpack and surface snow also abruptly decline to the average values of 1,305.5 μg/L and 824.6 μg/L,re‐spectively.Although approximately 60% of the annu‐al precipitation occurs in this period,the ion concen‐tration in the surface snow remains low and stable,suggesting that they are taken away easily during the elution process.Furthermore,the smallR-values(mostly less than 1)also demonstrate that the ion con‐centrations in the surface snow are less than that in the snowpack,revealing a significant effect of the downward leaching process.Owing to precipitation,theR-values and ion concentrations in the surface snow fluctuate again during T3(from the beginning of September to the beginning of December),suggesting that the ions could be left in the surface snow due to the lower temperatures.Although the total precipita‐tion in this period is 99.1 mm,accounting for approxi‐mately 18% of the annual precipitation,it is mostly preserved in snow layers.During T4(from the middle of December to the beginning of March of the next year),scarce precipitation (a total amount of 15.7 mm)and the lowest temperatures keep the ion concentra‐tions in both the surface snow and snowpack relative‐ly stable.

Figure 3 The ratio of ion concentrations in the surface snow to that in the snowpack with corresponding precipitation.Ion concentrations in the wet season(from April to September)are shown in the box,with the orange triangles and the fitting curve indicating the total ion concentrations in the snowpack

Table 1 Variation in average total ion concentration and precipitation in different periods
The variation in the snowpack ion concentrations during the wet season (here defined as the period from April to September) is shown in Figure 3.It can be well fitted by a Gauss Function with correlation co‐efficients (R2) of 0.89 (n=26,P<0.01).Although fre‐quent precipitation occurs,ion concentration in the snowpack decreases sharply at the early ablation peri‐od (from April to the middle of August),demonstrat‐ing that many chemical ions carried by precipitation during this period are leached out.In the latter half of the wet season (from the middle of August to the end of September),ion concentration rapidly rises because the decreasing meltwater allows preservation of the chemical components brought by local wind and pre‐cipitation.It is concluded that only the chemical infor‐mation from the precipitation at the end of the wet sea‐son can be retained in the snow layer(Youet al.,2015).
3.3 Ice core resolution and its influencing factors
Aiming to explore the formation of ice core re‐cording on Urumqi Glacier No.1 and its influenc‐ing factors,we track the position of Mg2+ion peaks in snow layers from December 2003 to August 2006 until they are preserved in granular ice (Figure 4).Generally,three Mg2+ion peaks exist in the snow‐pack,separately located in the upper,middle,and bottom (Youet al.,2005;Wanget al.,2006).How‐ever,only two peaks could be retained after sum‐mer ablation,including the one in the middle or lower part of the snowpack and the other near the ice surface.At the end of the ablation period,i.e.,December 12,2003,only two concentration peaks(P1and P2) occurred at 45 cm and 85 cm above the ice surface,respectively (Figure 4).On April 3,2004,P3started to appear in the upper snowpack at 153 cm above the ice surface.After the three abla‐tion periods from 2004 to 2006,P3reached the ice surface on August 10,2006 and never emerged af‐terward,suggesting that it had been preserved in granular ice.
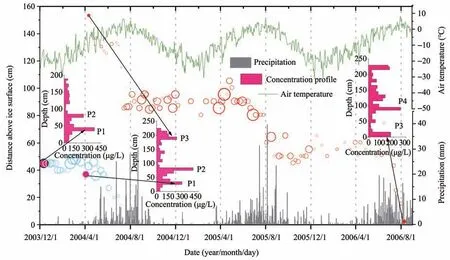
Figure 4 The evolutional process of Mg2+ion peaks(P1and P3),along with air temperature and precipitation.The blue circles and red circles stand for P1 and P3,respectively.Their diameter is proportional to the concentration.The three concentration profiles indicate the locations of Mg2+concentration peaks in the snowpack on December 12,2003,April 3,2004 and August 10,2006
From December 2003 to April 2004,the position of P1was relatively stable due to the low temperature and the infrequent precipitation during this term.When frequent dusty weather dominates in the Tian‐shan area,Mg2+ion is carried by westerly circulation and deposited on the surface snow through long-dis‐tance transportation (Youet al.,2015),which initiated the growth of P1concentration in spring 2004.From late May to August,with further increased air temper‐ature,the magnesium ion peak became smaller,moved downwards to the ice surface rapidly,and was finally preserved in the granular ice.It is speculated that P1was formed in spring 2002 and went through three ablation seasons from 2002 to 2004.The com‐plete annual data of P3could reflect the ice core recording formation.In spring 2004,P3was formed in the upper snowpack,with an initial concentration of 134.3 μg/L.Meanwhile,many small peaks appeared in the surface snow due to the frequent dry or wet de‐position events.Then,meltwater acted as a carrier,transferring many ions downwards to merge into P3.Until August 2004,P3became stable and more prom‐inent with its concentration increased to 381.9 μg/L at 90 cm above the ice surface.Since frequent dust and precipitation events in spring 2005,the concen‐tration of P3quickly rose and reached the maximum of 502.6 μg/L.Upon the arrival of the second ablation period,the concentration of P3dropped to 263.1 μg/L and remained stable at 40 cm above the ice surface by the end of August 2005.The process repeated in the next seasonal cycle,and P3eventually entered the granular ice on August 10,2006 with a concen‐tration of 176.7 μg/L.
In summary,despite the influence of ablation,the magnesium ion peaks are still preserved in granular ice every year,suggesting that the environmental in‐formation could be preserved in ice core records with a one-year resolution on Glacier No.1.However,the concentration peaks are weakened to a great extent due to the influence of the elution process.Taking P1and P3as examples,the concentrations decrease by nearly 70%on average,which means only 30%ion in‐formation is left.Additionally,it is challenging to dis‐tinguish seasonal variation since the ions deposited in the early wet season are contaminated or leached out by the elution process.
3.4 Migration of chemical ions at snow-ice interface
Urumqi Glacier No.1 is a typical warm ice forma‐tion glacier mainly characterized by melting and re‐freezing processes.Therefore,the snow-ice interface,where complex material migration and energy conver‐sion occur,plays a crucial role in preserving chemical ions.The snow-ice ratio,defined as the ratio of ion concentration at the snow layer bottom to that at the ice layer top,is presented in Figure 5 to reveal the ion migration process at the interface.Contrary to the dry season (the period from October to March),the average value of the snow-ice ratio in the wet season (the period from April to September) is less than 1,suggesting that the ion concentrations at the snow layer bottom are lower than that at the ice layer top.The results reveal that the wet season is a main ice formation period,during which many ions are preserved in the uppermost ice layer.This enrich‐ment can be attributed to ion exclusion during re‐freezing of meltwater into basal ice (Lilbæket al.,2008).At some point,refreezing meltwater releases enough latent heat to increase the temperature of the surrounding snow to the melting point and initiates runoff.The runoff may take many ions away along the ice surface and trigger ion pulse phenomenon,or refreeze again if the temperature drops below zero.Both of them lead to the ion concentration decrease in snow layers.
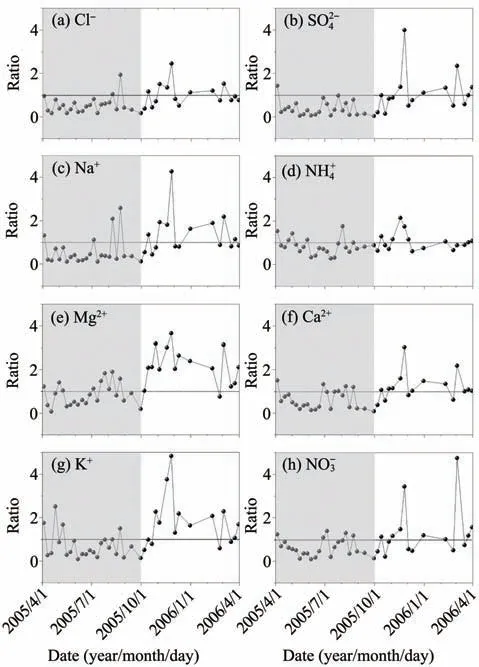
Figure 5 Variations in the snow-ice ratio of ion concentration at the snow-ice interface with time.The shaded and unshaded parts indicate the wet and dry seasons,respectively
4 Conclusions
This study analyzes the chemical evolution in snow and ice based on the ion concentration data of successive multi-year snowpacks on Glacier No.1 at the headwater of Urumqi River and discusses the ef‐fects of the meteorological elements on this process.It is demonstrated that ion concentrations in the snow‐pack have an apparent seasonality due to air tempera‐ture variation.The total ion concentration fluctuates significantly with a declining trend in summer and re‐mains stable at a lower level in winter.Once the posi‐tive accumulative temperatures are significant,the ion concentration rapidly decreases to a minimum value as a result of intense elution.Most of the ions brought by precipitation during the intense elution period are leached out,and only the ions in the precipitation at the end of the wet season could be preserved in the snow layers due to the decrease of snowmelt.Results suggest that the environmental information can be pre‐served in ice core records with a one-year resolution on Glacier No.1,even though the concentration peak values are weakened 70% on average by the elution process.
Acknowledgments:
This work was supported by the National Natural Sci‐ence Foundation of China (41761017,41261017) and the Natural Science Foundation of Gansu Province(18JR3RE247).The authors sincerely thank all the contributors for obtaining field monitoring data.
 Sciences in Cold and Arid Regions2022年2期
Sciences in Cold and Arid Regions2022年2期
- Sciences in Cold and Arid Regions的其它文章
- Editor-in-Chief Yuanming Lai
- Estimating snow depth or snow water equivalent from space
- Coarse fragment content influences estimates of soil C and N stocks of alpine grassland on the northeastern edge of Qinghai-Tibetan Plateau,China
- Diversity and composition of culturable fungi in Horqin Sandy Land
- Effect of GGBS on performance deterioration of non-dispersible underwater concrete in saline soil
- Simulation assessment and prediction of future temperatures in Northwest China from BCC-CSM Model
