Effect of virgin olive oil nanoemulsion combined with ajowan (Carum copticum)essential oil on the quality of lamb loins stored under chilled condition
Sjd Jfrini, Aziz A. Fllh,*, Sied Hiin Dehkordi
a Department of Food Hygiene and Quality Control, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord 34141, Iran
b Department of Pharmacology, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord 34141, Iran
Keywords:
Olive oil
Nanoemulsion
Carum copticum
Meat
Shelf-life
A B S T R A C T
This survey was conducted to evaluate the impact of virgin olive oil nanoemulsion (ONE) combined with ajowan (Carum copticum) essential oil (AEO) on quality of lamb loin under refrigerated condition. The treatments were control, ONE, ONE + 1% AEO, and ONE + 2% AEO. The treatments caused a delay in growth of mesophilic and psychrotrophic bacteria, Enterobacteriaceae, and lactic acid bacteria during chilled storage. The increasing rate of total volatile nitrogen content, lipid and protein oxidation, metmyoglobin formation, and color deterioration was more retarded in treatment groups. The treatments including ONE +1% AEO and ONE + 2% AEO were more efficient than ONE alone to delay microbial flora growth, slow down oxidative processes, and improve color in the loins. According to the results of microbial, chemical,and sensory parameters, shel flife of lamb loins was 4 days in control, 8 days in ONE, and at least 16 days in ONE + 1% AEO and ONE + 2% AEO groups. In conclusion, combination of ONE and AEO is appropriate as natural preservative to extend the shel flife of lamb loins stored under chilled condition.
1. Introduction
Owing to its biochemical composition, fresh red meat is susceptible to spoilage and has a limited shel flife. Therefore, effective storage methods should be applied to increase its shelflife and safety in fresh condition without adverse impacts on its consumer’s acceptance [1].
These days, application of nanotechnology-based methods has been increased in meat industry to improve the quality and safety of meat products. The applied nanoparticles can influence the color, flavor or texture, as well as the shelflife of meat and meat products [2,3].Among the nanoparticles, nanoemulsions are oil-in-water emulsions with small particle size ranging between 100–500 nm, which stabilized by a proper surfactant. The ingredients used for preparation of nanoemulsions, such as oil and surfactants, should be approved for human consumption and considered as “Generally Recognized as Safe” [4,5]. Comparing to conventional emulsions, nanoemulsions have some unique characteristics including high optical transparency,better physical stability, and improved bioavailability of encapsulated materials; these characteristics make nanoemulsions useful for food applications [6].
Ajowan (Carum copticum, syn:Trachyspermum ammi) with the Persian name “Zenyan” is a well-known medicinal herb belonging to the Apiaceae family that broadly distributed in Mediterranean region and southwest Asia [7]. The most important part of the plant is its brownish seeds that have several therapeutic effects and used as flavoring agent in muscle foods. The essential oil extracted from the seed of ajowan contains high amounts of monoterpenes and can be used as food preservative [8]. The essential oil has antimicrobial effect against some foodborne pathogens [9]. Moreover, it has remarkable antioxidative activityin vitroand in food models [10,11].
Olive oil consists a variety of bioactive components including free monounsaturated fatty acids, tocopherols, squalene hydrocarbons,and polyphenolic compounds [12]. The antimicrobial activity of polyphenolic compounds of olive oil against several foodborne pathogens has been proved. Moreover, these compounds have the potential to preventin vitroorin vivooxidative processes [13].Compared to re fined types of olive oil, its virgin type contains higher amount of bioactive substances including polyphenolic compounds as a result of the elimination or considerable reduction of these compounds during the re fining process [14].
In this research, we evaluated the impact of virgin olive oil nanoemulsion (ONE) alone and in combination with ajowan essential oil (AEO) on microbial, physicochemical, and sensory characteristics of lamb stored under chilled condition.
2. Materials and methods
2.1 Materials
Purified AEO was provided by Barij Essence Co. (Kashan,Iran). Virgin olive oil was purchased from a local producer. The microbial culture media were provided by Liofilchem (Roseto degli Abruzzi, Italy). Analytical grade chemical substances were used in our study.
2.2 Analysis of AEO
Chemical composition analysis of AEO was performed according to the gas chromatography-mass spectrometry method explained elsewhere [15].
2.3 Analysis of olive oil
Tocopherols content of virgin olive oil was assessed by the spectrophotometric method of Wong et al. [16]and expressed as mg/kg oil. Total polyphenols of the oil were assessed using Folin-Ciocalteu method and reported as gallic acid equivalent/kg oil [17].The carotenoids were evaluated by a spectrophotometric method according to Minguez-Mosquera et al. [18]and expressed as mg lutein/kg oil.
2.4 Nanoemulsion preparation
The ONE was made by using a two-step protocol as previously explained by Joe et al. [19]. The oil phase of nanoemulsion contains virgin olive oil (14%,V/V), ethanol (3%), and Tween 80 (3%)that forms 20% (V/V) of the emulsion. The mentioned ingredients were mixed and stored at 86 ºC for 1 h. Afterwards, the distilled water (80%) was added and mixed to form a coarse emulsion.An ultrasonic homogenizer (model UP400-A, TOP Sonics Co.,Tehran, Iran), at power of 400 W and frequency of 20 kHz,was employed to homogenize the coarse emulsion for 20 min.The beaker contained the emulsion was surrounded by crushed ice to control the emulsion temperature within homogenization process. Two replicates of ONE were prepared and each replicate analyzed twice for determining its particle size, stability, andin vitroantimicrobial and antioxidative activity. To prepare the formulations contained AEO, the essential oil was mixed with Tween 80 (0.15 g/mL of AEO) and added to ONE.
2.5 Physical characteristics of nanoemulsion
The mean particle diameter and polydispersity index (PDI) of ONE were assessed by using a dynamic light scattering instrument(Zetasizer Nano S, Malvern, UK). Stability of ONE was monitored for a period of 60-day storage period at room temperature (around 22 °C). To assess the stability, the ONE was centrifuged at 10 000 ×gfor 0.5 h. Before and after the centrifugation, ONE was visually inspected for phase separation or creaming.
2.6 In vitro antimicrobial assay
The agar disk diffusion assay was performed to assess the antimicrobial efficacy of ONE, ONE + 1% AEO, and ONE + 2% AEO against 4 bacterial pathogens includingListeria monocytogenesPTCC 1783,Staphylococcus aureusPTCC 1917,Escherichia coliO157:H7 PTCC 1860, andSalmonella typhimuriumPTCC 1709. The overnight bacterial suspension in Müeller-Hinton broth was adjusted to 0.5 McFarland turbidity standard (108CFU/mL) and then spread on the surface of Müeller-Hinton agar plate. The sterile blank disks(diameter 6 mm) were soaked in 20 μL of ONE, ONE + 1% AEO,ONE + 2% AEO, sterile pure water (negative control), or a positive control (streptomycin, 100 mg/L), dried for 10 min, and placed on inoculated plates. The plates were incubated at 35 °C overnight. The microbial inhibition was assessed by measurement of the inhibition zone diameter around the disk [19].
2.7 In vitro antioxidative assay
The antioxidative activity of ONE, ONE + 1% AEO, and ONE +2% AEO was assessed by the capability of the formulations to trap 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical according to the method of Rinaldi et al. [20]. Brie fly, 1.0 mL of each formulation was mixed with 4.0 mL of 0.10 mmol/L (0.004%) methanolic solution of DPPH and vortexed. The mixture was maintained in the dark at ambient temperature for 0.5 h. Finally, the mixture absorbance was read at 517 nm versus pure methanol. The following equation was used to evaluate the scavenging activity:
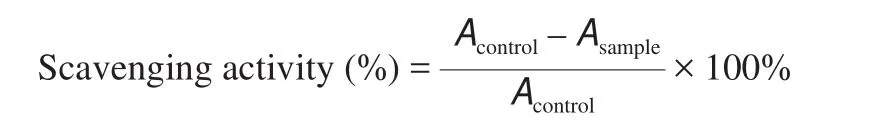
WhereAcontrolandAsampleare the absorbance of methanolic DPPH solution and sample, respectively.
2.8 Preparation of lamb loin samples
TheLongissimus thoracis et lumborummuscles (weighing 170–250 g each) from the right and left sides of lamb carcasses, after 12 h of slaughtering, were obtained from a local abattoir. The animals had the same breed and breeding conditions. The loin samples were carried to our lab inside an ice chest. In laboratory, the lamb loins were cut into (50 ± 10) g pieces; and then allocated to four equal parts randomly. The first part was immersed in double-distilled water and considered as control. The second part was immersed in ONE solution. The third and fourth parts were immersed in ONE +1% AEO (V/V) and ONE + 2% AEO (V/V) solutions, respectively.After 15 min of immersion, all loins were drained, wrapped in polyethylene pouches, and refrigerated at (3 ± 0.5) ºC. The microbial,physicochemical, and sensory parameters of loins were periodically analyzed at 0, 4, 8, 12, and 16 days of storage. The experiments were replicated twice with different samples of lamb loins. Duplicate samples were analyzed per each replicate.
2.9 Microbiological analyses
A portion of 10 g of lamb loin was transferred to a sterile plastic bag under aseptic conditions and mixed with 90 mL of sterile saline(0.85% sodium chloride) using a lab stomacher. Afterwards, suitable serial dilutions were made from this homogenate. Total mesophiles(TMs) and total psychrotrophes (TPs) were determined on plate count agar using pour-plate method following the incubation at 30 ºC for 72 h and 7 ºC for one week, respectively. Enterobacteriaceae were determined on violet red bile glucose agar using pour-overlay method after 24 h incubation at 35 ºC. Lactic acid bacteria (LAB)were enumerated on MRS agar using pour-plate method after 72 h incubation at 30 ºC [21,22].
2.10 Chemical analyses
Total volatile nitrogen (TVN) content of lamb loins was measured according to the procedure explained by Fallah et al. [23], and expressed as mg N/100 g of loin. In this experiment, volatile bases were extracted from meat by steam distillation with alkaline solution;and then recovered basic compounds were determined by titration.
The primary and secondary products of lipid oxidation were determined as peroxide value (PV) and thiobarbituric acid reactive substances (TBARS), respectively. PV was determined using an iodometry method and expressed as meq/kg of sample [24]. The TBARS was assessed using a spectrophotometric procedure described by Hasani-Javanmardi et al. [25]. In this method, malondialdehyde(MDA) and other aldehydes reacted with thiobarbituric acid (TBA)to form a pink colored compound. Absorption of the compound was determined at 532 nm; and TBARS content was expressed as mg MDA/kg of loin.
Protein oxidation was determined based on the formation of protein carbonyls and loss of sulphydryl groups, according to the procedures explained by Botsoglou et al. [26]. Protein carbonyls reacted with 2,4-dinitrophenylhydrazine and produced stable protein hydrazones. The absorption of hydrazones was measured at 370 nm; and the content of protein carbonyls was represented as nmol carbonyls/mg of protein using absorption coefficient of 21 000 L/(mol·cm). The free sulphydryl groups of proteins reacted with 5,5’-dithiobis(2-nitrobenzoic acid) to create a colored ionic compound. The absorption of the compound was determined at 412 nm; and the content of sulphydryls was represented as nmol sulphydryls/mg of protein using absorption coefficient of 13 600 L/(mol·cm).
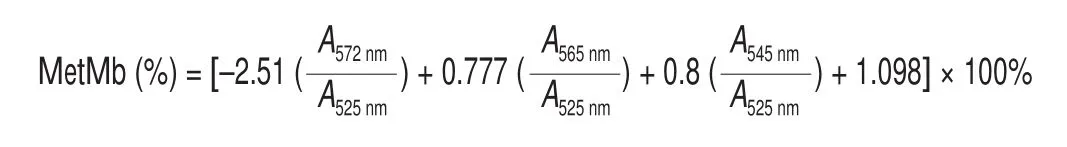
Metmyoglobin (MetMb) content of loins was determined according to the procedure described by Badr [27]. A portion of loin sample (5 g) was homogenised with 25 mL of ice-cold phosphate buffer (40 mmol/L, pH 6.8). The mixture was placed at refrigerator(4 ºC) for 60 min, and then centrifuged at 5 000 ×gfor 30 min at 4 °C. Afterwards, the upper layer was filtered via Whatman No. 1 filter paper followed by determination of its absorbance at 572 (A572nm),565 (A565nm), 545 (A545nm), and 525 nm (A525nm). The content of MetMb (%)was calculated as follow:
2.11 Instrumental color analysis
A portable digital colorimeter (model: CS-10; CHN Spec Technology Co., Ltd., Hangzhou, China) was used to determine the surface color of lamb loins. The color parameters includingL*(lightness),a* (redness), andb* (yellowness) were measured by the colorimeter at D65illuminant, observation angle of 10º, and instrument aperture of 8 mm. The hue angle (H*) was calculated as tangent−1(b*/a*). The measurements were carried out at 3 different parts of each loin. For each parameter, the average of 3 measurements was calculated for each loin replicate.
2.12 Sensory evaluation
Sensory attributes of lamb loins such as appearance (color), odor,texture, and overall acceptability were assessed during storage by a 15-member trained panel. A 9-point hedonic scale was employed, where scores 9 and 1 represented “like extremely” and “dislike extremely”,respectively. The acceptable scores were in the range of 6 to 9 [28].
In a preliminary study, loin samples were treated with ONE,and ONE + 1%, 2%, 3%, or 4% AEO, then sensory attributes were assessed in raw and cooked loins at days 0 and 4 of refrigerated storage. According to the panelists, the odor was unacceptable in raw samples treated with ONE + 3% or 4% AEO; while the odor of cooked samples treated with ONE + 3% AEO, and the odor and taste of cooked samples treated with ONE + 4% AEO were unacceptable.All sensory parameters of raw and cooked samples treated with ONE, and ONE + 1% or 2% AEO were highly acceptable. Therefore,concentrations of 1% and 2% AEO were used in this research.
2.13 Statistical analyses
3. Results and discussion
3.1 Composition of AEO
The chemical analysis of AEO demonstrated the presence of 18 compounds demonstrating 99.3% of total compounds identified in AEO. Thymol (62.5%),γ-terpinene (19.4%), andρ-cymene (10.7%)were the major compounds of AEO (Table 1). Our result is in accordance with the results of previous studies [29-31].

Table 1Chemical composition of AEO.
3.2 Characteristics of olive oil and its nanoemulsion
The virgin olive oil used in this study contained(436.1 ± 5.97) mg gallic acid/kg total polyphenols, (281.9 ± 3.78) mg/kg tocopherols, and (5.02 ± 0.79) mg lutein/kg carotenoids.
The average particle size of ONE was (181.2 ± 1.71) nm with the PDI of 0.301 ± 0.011. No phase separation or creaming was observed in ONE before and after centrifugation during 60-day storage at ambient temperature.
3.3 In vitro antimicrobial and antioxidative assays
The results ofin vitroantimicrobial activity of ONE, ONE + 1% AEO,and ONE + 2% AEO is shown in Table 2. The antimicrobial activity of the formulations against various foodborne pathogens ranked as follow: ONE + 2% AEO > ONE + 1% AEO > ONE. The most susceptible foodborne pathogen wasS. aureus, followed byL. monocytogenes,E. coliO157:H7, andS. typhimurium. The lower sensitivity of Gram-negative bacteria compared to Gram-positive bacteria can be explained by the presence of lipopolysaccharide in the outer membrane of Gram-negative bacteria. The antimicrobial efficacy of ONE and AEO is mainly related to their phenolic compounds that disintegrate the cytoplasmic membrane of microorganisms [32].
The results ofin vitroantioxidative activity of ONE, ONE + 1% AEO, and ONE + 2% AEO is depicted in Fig. 1. The DPPH free radical scavenging activity ranking of the formulations was as follow:ONE + 2% AEO > ONE + 1% AEO > ONE. The antioxidative activity of ONE is due to the polyphenols and vitamin E present in the virgin olive oil used for preparation of the nanoemulsion. Presence of the AEO in the formulations caused the enhanced antioxidative activity of the formulations in a dose-dependent manner. It has been determined that phenolic compounds are the main components involved in the antioxidative activity of plant essential oils [31].
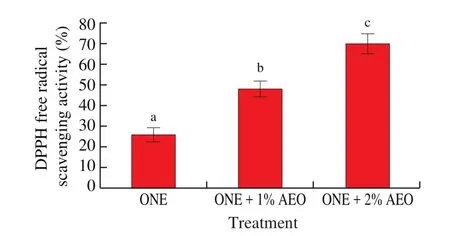
Fig. 1 In vitro antioxidative activity of ONE and AEO.
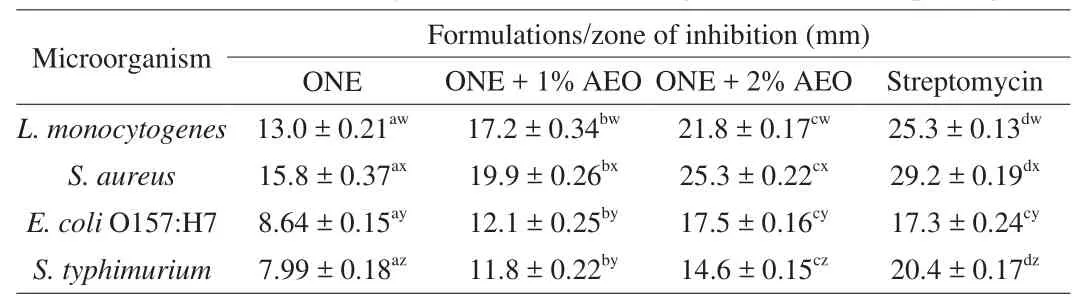
Table 2In vitro antimicrobial activity of ONE and AEO against foodborne pathogens.
3.4 Microbiological analyses
The microbiological quality of lamb loins in different groups is depicted in Fig. 2. The primary count between 3.23 and 3.47 (lg (CFU/g))for TMs and 2.87 and 3.26 (lg (CFU/g)) for TPs was found in experimental groups, which indicated acceptable microbial quality of lamb loins. At days 0 and 4, the enumeration of TMs and TPs did not show significant difference (P> 0.05) among the groups. In all groups, TMs and TPs significantly increased (P≤ 0.05) during chilled condition; whereas their increasing speed was faster in control than that in treatment groups. The counts of TMs in lamb loins exceeded the value of 7.0 (lg (CFU/g)), indicated as the microbial acceptable level of fresh red meat, at day 8 in control and day 12 in ONE group; whilst ONE + 1% AEO and ONE + 2% AEO groups did not reach the level during 16 days of refrigerated storage. At day 16, TMs count of ONE, ONE + 1% AEO, and ONE + 2% AEO groups was 1.27, 2.63, and 3.02 (lg (CFU/g)) lower than the control group; and TPs count of the mentioned experimental groups was 1.22,2.35, and 2.54 (lg (CFU/g)) lower than control group, respectively(P≤ 0.05; Fig. 2). Özogul et al. [33]found that TMs and TPs counts of fish fillets treated with cooking oil-based nanoemulsions exceeded the value of 7.0 (lg (CFU/g)) after 10 days while the control group exceeded the value after 8 days of chilled storage. Farahmandfar et al. [34]also found that AEO exerted inhibitory effect on TMs and TPs of refrigerated fish surimi.
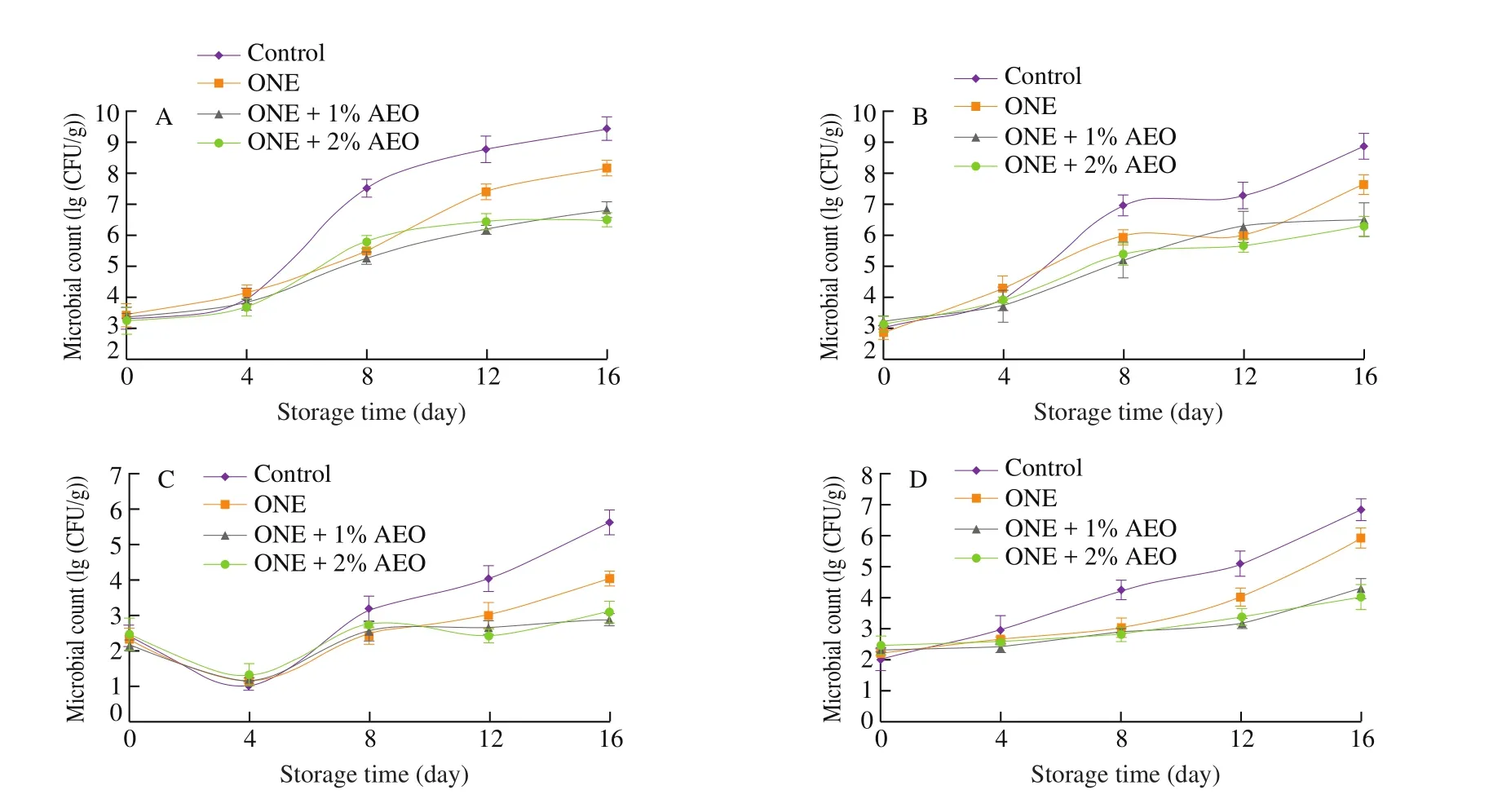
Fig. 2 Changes in counts of (A) TM, (B) TP, (C) Enterobacteriaceae, and (D) LAB in lamb loins treated with ONE and AEO during refrigerated storage.
The initial count of Enterobacteriaceae was in the range of 2.21 (lg (CFU/g)) to 2.44 (lg (CFU/g)) in experimental groups (P >0.05).The Enterobacteriaceae counts significantly reduced (P≤ 0.05) at day 4 because of the sensitivity of Enterobacteriaceae to low temperature.After day 4, the count of Enterobacteriaceae significantly increased(P≤ 0.05) during storage period; whereas the increasing speed was faster in control group. At day 16, a significantly lower (P≤ 0.05)Enterobacteriaceae count was found in treatment groups than in control (Fig. 2). Similarly, Yazgan et al. [35]reported that sun flower oil-based nanoemulsion inhibited the growth of Enterobacteriaceae inflsh fillets during refrigerated storage. Moreover, it was found that chitosan films containing AEO decreased coliform counts in chicken fillets during chilled storage [36].
6.2 青海三江源、青海湖流域、祁连山等重大生态保护工程生态监测项目的开展积累了包括原始监测数据、衍生数据、基础地理数据、专题数据、站网观测数据、遥感监测数据和社会经济统计数据等大量的连续的基础性监测数据,但如何对监测数据进行深入的挖掘分析,提交科学的和有说服力的监测分析报告,是我们专业技术人员面临的巨大挑战。
Initial count of LAB was in the range of 2.07 (lg (CFU/g)) to 2.45 (lg (CFU/g)) in the groups (P> 0.05). LAB counts significantly increased (P≤ 0.05) over the storage time; whereas the increasing speed was faster in control group. At day 16, LAB counts of ONE,ONE + 1% AEO, and ONE + 2% AEO groups were 0.92, 2.51, and 2.82 (lg (CFU/g)) lower than in control group (Fig. 2). In agreement with our result, Joe et al. [37]reported a remarkable reduction of LAB counts inflsh fillets treated with sun flower oil nanoemulsion during 72 h of storage at 20 ºC. Moreover, Jebelli Javan et al. [38]found the inhibitory effects of chitosan coating containing AEO on LAB in chicken fillets during chilled storage.
It has been proved that oil-based nanoemulsions have antimicrobial effects against Gram-negative and Gram-positive bacteria [39].They are also self-preserving antimicrobial agents because water,as the main portion of nanoemulsions, is efficiently bound and is not accessible by the microorganisms. Moreover, nanoemulsions cause instability in lipid envelope of organisms; therefore, they have harmful effects on structure and function of various microorganisms including bacteria [33,37]. The antimicrobial effect of AEO is due to the presence of thymol as the most abundant component in AEO. Our result demonstrated that ONE + 1% AEO and ONE + 2% AEO were more efficient than ONE alone to slow down the growth of examined bacteria during storage. It might be due to the combined antimicrobial activities of ONE and AEO.
3.5 Chemical analyses
The chemical quality of lamb loins in different groups is demonstrated in Table 3. The primary TVN level of different groups was ranging from 7.64 mg N/100 g to 8.60 mg N/100 g, indicating an acceptable quality of lamb loins. At day 0, the level of TVN did not show significant difference (P> 0.05) among the groups. In all groups, TVN level significantly increased (P≤ 0.05) over the storage time; whereas its increasing speed was faster in control group.The TVN level of the lamb loins was higher than permissible level of 25 mg N/100 g [40]at day 12 in control and day 16 in ONE group; whilst ONE + 1% AEO and ONE + 2% AEO groups did not reach the level even after 16 days of chilled storage. At day 16, the TVN level of ONE +2% AEO group was significantly lower (P≤ 0.05) than the other groups (Table 3). The TVN is associated to breakdown of proteins and non-protein nitrogenous components by proteolytic enzymes mostly produced by microorganisms and formation of volatile amines as well as ammonia [41,42]. It has been proposed that TVN measurement has the potential to replace microbial and/or sensory analyses to determine meat freshness, due to the positive relation between TVN content and counts of spoilage microorganisms [43].Owing to their antimicrobial effects, ONE and AEO can slow down the increasing rate of TVN in treated lamb loins.
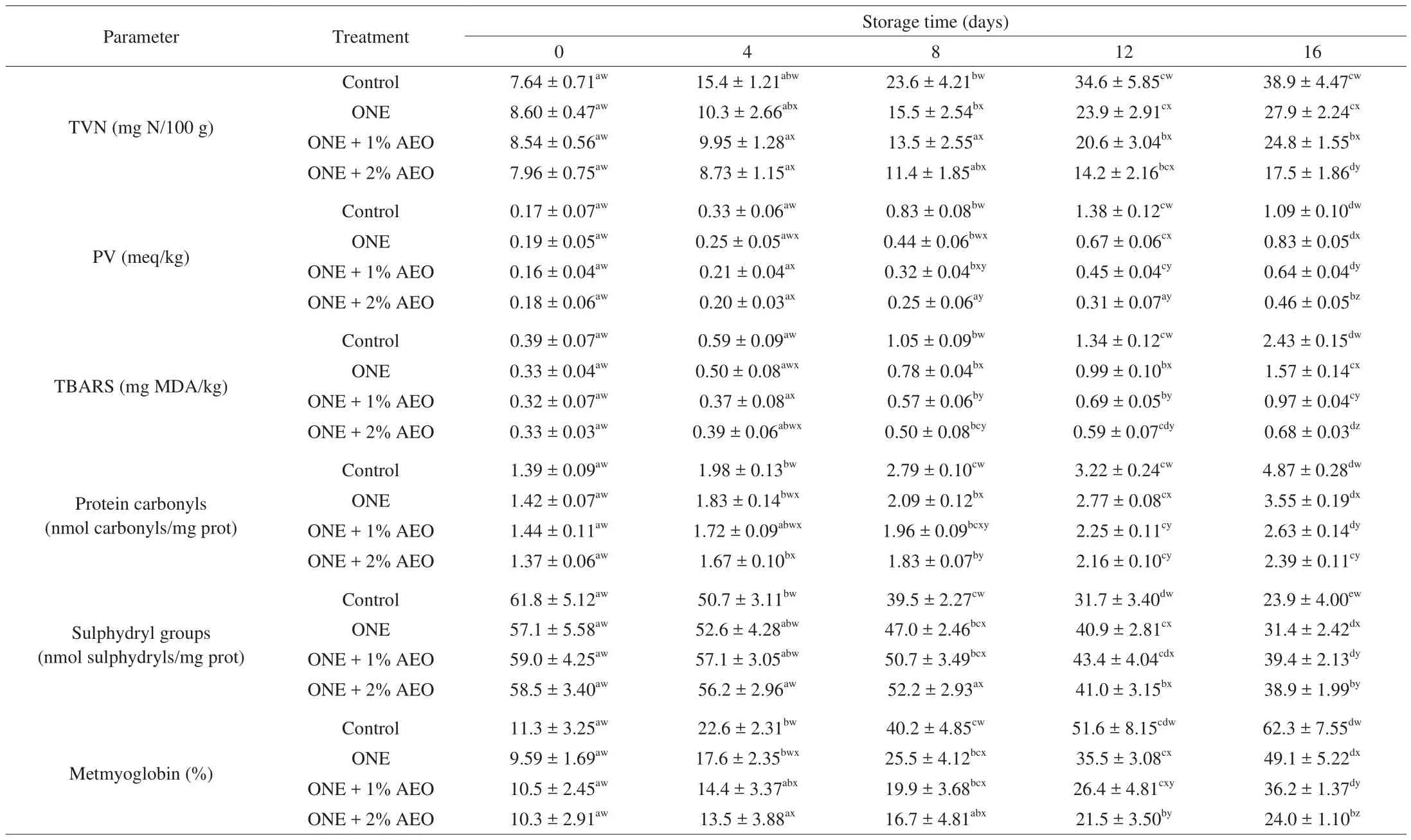
Table 3Changes in chemical parameters of lamb loins treated with ONE and AEO during refrigerated storage.
The PV determines the content of lipid peroxides and hydroperoxides as the primary products of lipid oxidation [44]. The initial PV content in control group was 0.17 meq/kg, reached to maximum level of 1.38 meq/kg at day 12, and afterwards significantly decreased (P≤ 0.05) to 1.09 meq/kg at the end of storage (Table 3).This sudden decrease in PV content of control group at day 16 might be due to the higher break down rate of peroxides and hydroperoxides than their formation rate. At day 16, PV content of treatment groups was significantly lower (P≤ 0.05) than that of the control group; and ONE + 2% AEO group had the lowest PV content (Table 3). Result of this research is consistent with the study of Shadman et al. [45]found that trout samples treated with sun flower oil-based nanoemulsion +Zataria multifloraessential oil had lower PV content than control samples during 15 days of chilled storage. In another study, chitosan coating containing AEO significantly decreased PV content in chicken fillets under chilled condition. [38]
Using of PV as the only indicator of lipid oxidation can lead to bias in measurement of lipid oxidation. It is due to the fact that peroxides and hydroperoxides break down to secondary products such as hydrocarbons, alcohols, carbonyls, and aldehydes, which cause the flavor deterioration of foods. The TBARS test measures the amount of aldehydes as the secondary products of lipid peroxidation [46,47].In our research, primary TBARS level was in the range of 0.32 mg MDA/kg to 0.39 mg MDA/kg in different groups (P> 0.05).TBARS level significantly increased (P≤ 0.05) over the storage time;whereas its increasing speed was faster in control group. At day 16,TBARS level of treatment groups was significantly lower (P≤ 0.05)than that of the control group; and the lowest TBARS level was found in ONE + 2% AEO group (Table 3). Verma et al. [48]reported an acceptable level of 1 mg MDA/kg for meat, beyond which offodor can be developed. In this research, the TBARS level was in the acceptable range (≤ 1 mg MDA/kg of meat) for 4 days in control, 12 days in ONE, and at least 16 days in ONE + 1% AEO and ONE + 2% AEO groups (Table 3). Shadman et al. [45]observed that dipping of trout fillets in sun flower oil-based nanoemulsion + 1%Z. multi floraessential oil retarded the production of TBARS in the fillets under chilled storage. Results of previous surveys also indicated lower TBARS content in fish fillets treated with commercial oil-based or essential oil-based nanoemulsions compared to controls [33,35,49,50].
Virgin olive oil is a good source of antioxidants such as tocopherols, polyphenols, and carotenoids. It has been determined that antioxidative activity of polyphenols is higher than the other compounds found in virgin olive oil [51,52]. The virgin olive oil used in this study was a good source of mentioned antioxidants; hence the nanoemulsion prepared from the oil can have antioxidative effects to retard lipid oxidation and other oxidative processes in lamb loins under chilled condition. It has been indicated that the antioxidative activity of AEO is related to the oxygenated monoterpenes and monoterpene hydrocarbons such as thymol,γ-terpinene, andρ-cymene [10].In this study, ONE + 1% AEO and ONE + 2% AEO were more effective than ONE alone to retard lipid oxidation in lamb loins during storage. It may be due to the combined antioxidative activity of ONE and AEO.
The oxidation of side chains of different amino acids caused to production of protein carbonyls [26]. In our research, the primary level of protein carbonyls was not significantly different (P> 0.05)among the groups. The content of protein carbonyls significantly increased (P≤ 0.05) over the storage time; whereas its increasing speed was faster in control group. At days 12 and 16, the contents of protein carbonyls were significantly lower (P≤ 0.05) in treatment groups than that of the control group; and ONE + 1% AEO and ONE + 2% AEO groups had the lowest contents of protein carbonyls (Table 3).
The usage of protein carbonyls as the only indicator of lipid oxidation can lead to bias in measurement of protein oxidation. It is due to the fact that existence of lipid carbonyls and artifacts may cause an overestimation of protein oxidation. As a result, use of another method based on loss of sulphydryl groups can be helpful to assess the actual development of protein oxidation [26]. The initial level of sulphydryl groups was in the range of 57.1 nmoL sulphydryls/mg prot to 61.8 nmol sulphydryls/mg prot in different groups (P >0.05). The level of sulphydryl groups significantly decreased (P≤ 0.05) over the storage time; whereas its decreasing speed was slower in treatment groups. At day 16, level of sulphydryl groups was significantly higher (P≤ 0.05) in treatment groups compared to control; and ONE + 1% AEO and ONE + 2% AEO groups had the highest levels of sulphydryl groups (Table 3).
It has been found a powerful correlation between lipid and protein oxidation in meat. Oxidative processes can easily be transferred from lipids to proteins due to the reaction of primary and secondary lipid oxidation products with proteins [53]. Oxidation of proteins can lead to reduction of nutritional quality of meat because of the lower bioaccessibility of oxidised compounds [54].
The primary content of MetMb in loins of different groups was ranging from 9.59% to 11.3% (P> 0.05), which is in agreement with the study of Lauzurica et al. [55]. The content of MetMb significantly increased (P≤ 0.05) during storage and reached to 62.3%, 49.1%,36.2%, and 24.0% in control, ONE, ONE + 1% AEO, and ONE+ 2% AEO groups, respectively, at the end of storage period(P≤ 0.05; Table 3). It has been reported that there was a positive relation between MetMb content and level of lipid oxidation in meat [56].Indeed, lipid oxidation products enhance the alteration of red oxymyoglobin into brown MetMb [57]. This study demonstrated that antioxidative activity of ONE and AEO retarded the lipid oxidation,hence delayed the accumulation of MetMb in treated loin samples.
3.6 Instrumental color analysis
The changes in color parameters of lamb loins during chilled storage are shown in Fig. 3. In our research, the primary lightness(L* value) did not differ significantly (P> 0.05) among the groups.Moreover,L* value did not significantly change (P> 0.05) in control and treatment groups until day 4 and 8, respectively. At day 16,L* value of treatment groups was significantly lower (P≤ 0.05) than that of the control; and ONE + 1% AEO and ONE + 2% AEO groups had the lowestL* value. It has been found that meatL* value is not related to alterations in chemical state of myoglobin, but related to the structural changes of proteins. In this regard, oxidative processes can cause alterations in protein structure in such a way that increase lightness [58-60]. In our study, antioxidative effects of ONE and AEO retarded oxidative processes, hence decreasedL* value in treated lamb loins.
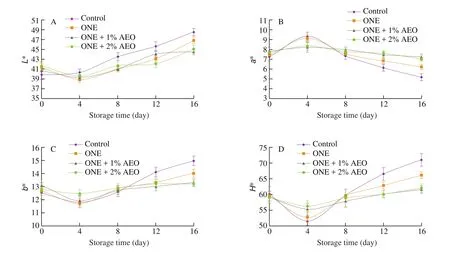
Fig. 3 Changes in instrumental color parameters (A) L*, (B) a*, (C) b*, and (D) H* of lamb loins treated with ONE and AEO during refrigerated storage.
Owing to the fact that consumers prefer red-colored meat, redness(a* value) determination is essential to assess meat oxidation. At day 0,a* value did not differ significantly (P> 0.05) among the groups. At day 4, the value was increased in all groups, but the changes were significant (P≤ 0.05) only in control and ONE groups. After day 4,a* value was significantly reduced (P≤ 0.05); whereas its reduction speed was slower in treatment groups. At day 16,a* value was significantly higher (P≤ 0.05) in treatment groups than in control group; and ONE + 1% AEO and ONE + 2% AEO groups had the highesta* value (Fig. 3). Our result is consistent with Zhang et al. [43]reported that pork loins sprayed with black pepper essential oil had highera* value than control due to retardation of myoglobin oxidation by the essential oil.
In our research, the primary yellowness (b* value) did not differ significantly (P> 0.05) among the groups. At day 4,b* value decreased in all groups, and the changes were significant in all groups except ONE + 2% AEO. After day 4,b* value was significantly increased (P≤ 0.05); whereas its increasing speed was slower in treatment groups. At day 16,b* value was significantly lower(P≤ 0.05) in treatment groups than in control group; and ONE + 1% AEO and ONE + 2% AEO groups had the lowestb* value (Fig. 3).In this study highera* value and lowerb*value of treatment groups compared to control group might be due to the retardation of MetMb accumulation.
The primary hue angle (H* value) did not differ significantly(P> 0.05) among the groups. At day 4, the value decreased in all groups,but the changes were significant only in control and ONE groups.After day 4,H* value significantly increased (P≤ 0.05); whereas its increasing speed was slower in treatment groups. At day 16,H* value was significantly lower (P≤ 0.05) in treatment groups compared to control; and ONE + 1% AEO and ONE + 2% AEO groups had the lowestH* value (Fig. 3). TheH* value is a good indicator of meat browning.It was reported that there is positive relation betweenH* value and content of MetMb; therefore, determination ofH* value is highly recommended to evaluate meat discoloration [61].
3.7 Sensory evaluation
The results of sensory appraisal (color, odor, texture, and overall acceptability) of control and treated lamb loins under chilled condition are shown in Fig. 4. At day 0, the scores of all sensory attributes did not differ significantly (P> 0.05) among the groups.According to panelists, AEO had a desirable effect on the odor of lamb loins. Loin samples with a score equal or higher than 6 were acceptable. The sensory attributes were admissible in loin samples for 8 days in control and 12 days in ONE group, whilst the attributes were admissible in samples of ONE + 1% AEO and ONE + 2% AEO groups during 16 days of storage (Fig. 4). In this study, when the loin samples were rejected by panelists, the MetMb content was approximately 50%. The obtained result is consistent with previous studies found that MetMb content equal or above 40% caused meat rejection by sensory panel [27,56].
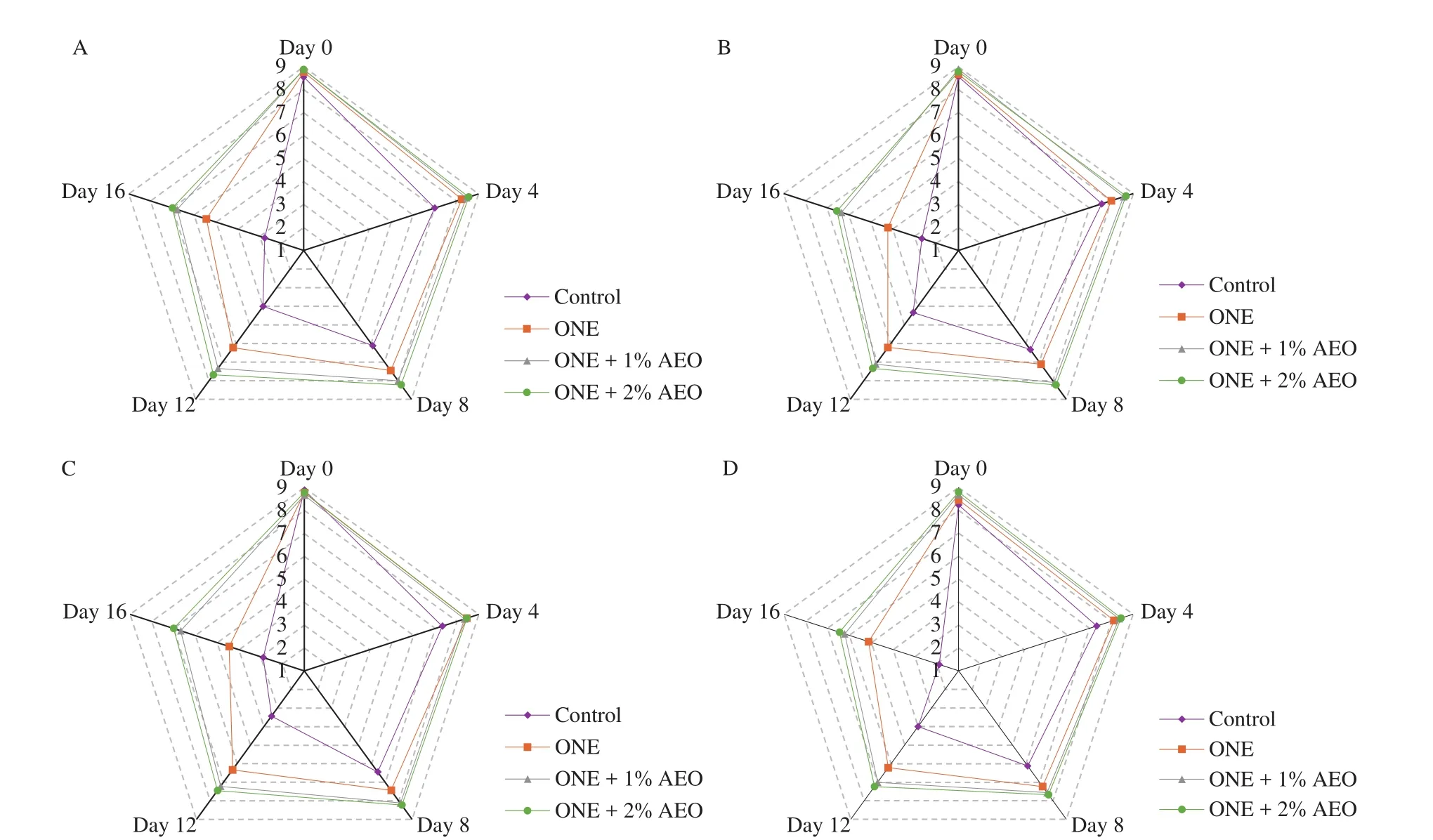
Fig. 4 Changes in sensory attributes of lamb loins treated with ONE and AEO during refrigerated storage. The scores of (A) appearance, (B) odor, (C) texture,and (D) overall acceptability.
Based on various parameters (i.e. TMs, TVN, TBARS, and overall acceptability), the shel flife of lamb loins was 4 days in control,8 days in ONE, and at least 16 days in ONE + 1% AEO and ONE + 2% AEO groups. We found that the obtained results for sensory analyses were not well correlated with microbiological analyses in control and ONE groups. The shel flife of lamb loins in the mentioned groups based on TMs was 4 days less than that based on sensory analyses.
4. Conclusion
The results of this survey showed that ONE alone or combined with AEO enhanced the quality of lamb loins during refrigerated storage. Application of ONE and ONE + 1% or 2% AEO increased the shelflife of loins to 8 and 12 days, respectively; moreover, the treatments enhanced microbiological, physicochemical, and sensory attributes during chilled condition. The ONE + 1% AEO or ONE +2% AEO were more efficient than ONE alone to delay the growth of microbial flora, slow down oxidative processes, and improve color in lamb loins. In conclusion, combination of ONE and AEO is suggested as a natural preservative for lamb loins stored under chilled condition.
Conflict of interest statement
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
This research is a part of MSc dissertation approved and financially supported by the Shahrekord University, Iran.
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

