The anatomical, electrophysiological and histological observations of muscle contraction units in rabbits:a new perspective on nerve injury and regeneration
Ting-Min Xu , Bo Chen , Zong-Xue Jin, Xiao-Feng Yin Pei-Xun Zhang Bao-Guo Jiang
Abstract In the conventional view a muscle is composed of intermediate structures before its further division into microscopic muscle fibers. Our experiments in mice have confirmed this intermediate structure is composed of the lamella cluster formed by motor endplates, the innervating nerve branches and the corresponding muscle fibers, which can be viewed as an independent structural and functional unit. In this study, we verified the presence of these muscle construction units in rabbits. The results showed that the muscular branch of the femoral nerve sent out 4–6 nerve branches into the quadriceps and the tibial nerve sent out 4–7 nerve branches into the gastrocnemius. When each nerve branch of the femoral nerve was stimulated from the most lateral to the medial, the contraction of the lateral muscle, intermediate muscle and medial muscle of the quadriceps could be induced by electrically stimulating at least one nerve branch. When stimulating each nerve branch of the tibial nerve from the lateral to the medial, the muscle contraction of the lateral muscle 1, lateral muscle 2, lateral muscle 3 and medial muscle of the gastrocnemius could be induced by electrically stimulating at least one nerve branch. Electrical stimulation of each nerve branch resulted in different electromyographical waves recorded in different muscle subgroups. Hematoxylin-eosin staining showed most of the nerve branches around the neuromuscular junctions consisted of one individual neural tract, a few consisted of two or more neural tracts. The muscles of the lower limb in the rabbit can be subdivided into different muscle subgroups, each innervated by different nerve branches, thereby allowing much more complex muscle activities than traditionally stated. Together, the nerve branches and the innervated muscle subgroups can be viewed as an independent structural and functional unit. This study was approved by the Animal Ethics Committee of Peking University People’s Hospital (approval No. 2019PHE027) on October 20, 2019.
Key Words: anatomy; electrophysiology; femoral nerve; hematoxylin-eosin staining; gastrocnemius; motor endplate; muscle contraction unit;peripheral nerve; quadriceps; rabbit; skeletal muscle; tibial nerve
Introduction
By mediating voluntary muscle movement, neuromuscular junctions play a key role in physiology (Rudolf et al., 2019;Lazaridis and Tzartos, 2020; Barrantes, 2021). The skeletal muscles innervated by the peripheral nerves coordinate to produce various complex and fine movements. Traditionally,the motor unit was thought to be the basic unit of muscle activity (Buchthal and Schmalbruch, 1980; Heckman and Enoka, 2012; Farina et al., 2016; Mani et al., 2018; Piasecki et al., 2018) and that the motor endplates (MEPs), formed by the terminal branches of motor nerves and the surface of muscle fibers, were uniformly distributed throughout the skeletal muscle (Jang et al., 2016; Liu et al., 2019; Rudolf et al., 2019; Chan et al., 2020; Chen et al., 2020). Excitation of a motor neuron generated electrical signals that induced muscle contraction through MEPs. The theory was that their summation resulted in a simple total contraction or relaxation of the muscle. Rather than that of a genetically fixed tissue specializing in producing tension, skeletal muscle is now considered a phenotypically plastic tissue that responds structurally and functionally to the nature of the demands placed on it (Lindstedt, 2016). The complexity of the real muscle activities require further macroscopic study of its nerve innervation pattern to guide clinical treatment(Rudolf et al., 2019). The muscles around a joint, such as the infraspinatus muscle (Fabrizio and Clemente, 2014),participate in the formation and regulation of various complex joint activities, and their origins and terminations are not distributed at a certain anatomical point. The traditional opinion that “the coordination between muscle groups”explains complex limb movements limits the exploration of other possibilities. Certain clinical problems cannot be fully explained by traditional opinions, for example, when only a part of a muscle is injured, the electromyography (EMG)recorded by traditional methods could appear normal.
It is possible that there is a structure that forms an intermediate link between the large-scale skeletal muscle and the microscale motor unit. In our previous studies,in vivoinjection of fluorescent α-bungarotoxin was combined with the 3-Dimensional Imaging of Solvent-Cleared Organs protocol and ultramicroscopy to determine the 3D distributions of the MEPs in different skeletal muscles. Our results showed that the MEPs in these muscles were not evenly distributed, rather,they were distributed in lamellar clusters (Yin et al., 2019).Our electrophysiological experiments showed that contraction of different muscle subgroups of the gastrocnemius could be induced by electrically stimulating each nerve branch of the tibial nerve individually. Therefore, we proposed that the MEPs, the innervating nerve branches and corresponding muscle fibers can be regarded as an independent structural and functional unit, which we termed muscle contraction unit(MCU). Using a denervated and nerve-repairing mice model,we found that after denervation, the originally lamellar-cluster distributed MEPs in the gastrocnemius gradually disintegrated and diffused. After subsequent nerve-repairing, the diffused MEPs gradually recovered to the original lamellar-cluster form(Yin et al., 2019).
In this study, the anatomy of the nerve branches around the neuromuscular junctions in the muscles of the lower limb in rabbits was investigated. Electrophysiological experiments and hematoxylin-eosin (HE) staining were performed to assess the MCU in rabbits.
Materials and Methods
Animals
Five adult female New Zealand rabbits (about 2.5-kg weight,10 weeks old) were all purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (animal production license No. SCXK (Jing) 2016-0006 and animal usage license No. SYXK (Jing) 2017-0022) and kept in the animal rooms of Peking University People’s Hospital (22–26°C at room temperature, 12/12-hour in light/dark cycle and 60–65%relative humidity), food and water were available ad libitum.The animal experiment design was approved by the Animal Ethics Committee of Peking University People’s Hospital(approval No. 2019PHE027) on October 20, 2019.
Anatomy
The rabbits were anesthetized by isoflurane (RWD Life science Co., Ltd., Shenzhen, Guangdong Province, China) inhalation.After anesthetization, the left lower limb was shaved and sterilized. Then, the left quadriceps, gastrocnemius and their innervating nerves (the muscular branch of the femoral nerve and tibial nerve) were exposed carefully. The number of nerve branches around the neuromuscular junction into the muscle was recorded.
EMG
Paired recording electrodes (Xi’an Friendship Medical Electronics Co., Ltd., Xi’an, Shaanxi Province, China) were placed in the distal and proximal part of each muscle subgroup, with the reference electrode placed in the gluteus maximus. Parameters (Medlec Synergy, Oxford Instruments,Surrey, UK) were set as 0.09–0.12 mA in current strength to elicit an AP, five pulses per second in frequency, 0.1 ms in duration (Dario et al., 2001; Ujiie et al., 2017) We tried a range of stimuli strengths and chose the same strength to stimulate each nerve branch and recorded the action potential elicited in the muscle subgroups.
HE staining
The rabbits were euthanized by excessive CO2inhalation.The tissues (about 1 cm × 1 cm × 0.5 cm in size) around each neuromuscular junction into the muscle were dissected and placed in 4% paraformaldehyde for 12 hours. Then the fixed tissues were rinsed with phosphate buffered saline twice,passed through gradient alcohols for dehydration and xylene for transparency. The tissue was then paraffin-embedded before being cut into 5-µm-thick sections (Fischer et al.,2008). After HE staining, the sections were embedded with neutral resin and then observed under an optical microscope(Leica, Wetzer, Germany).
Results
Anatomical characteristics of femoral nerve and tibial nerve in rabbit
The regional anatomy showed that the muscular branch of the femoral nerve sent out 4–6 nerve branches innervating the quadriceps; the tibial nerve sent out 4–7 nerve branches innervating the gastrocnemius (Figure 1AandB). The number of nerve branches of the muscular branch of the femoral nerve and the tibial nerve was shown inTable 1.
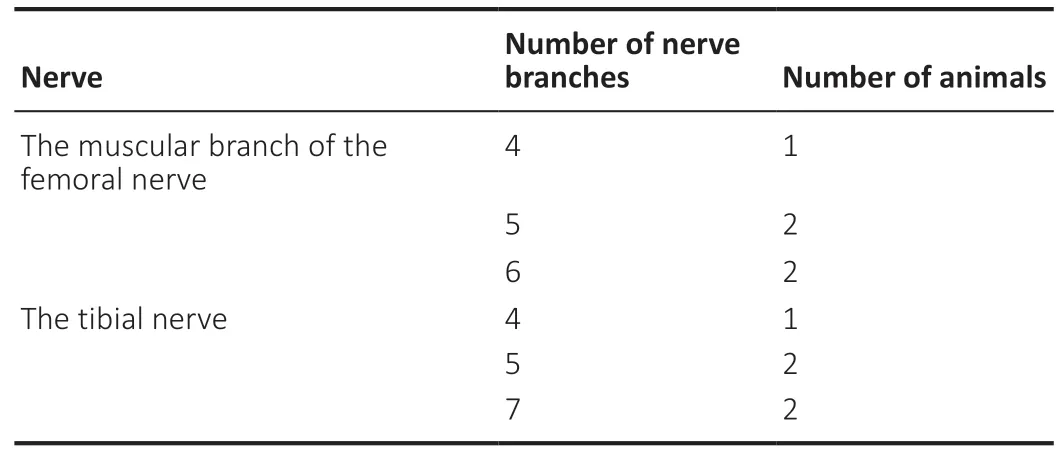
Table 1 |The number of nerve branches of the muscular branch of the femoral nerve
Electrophysiological outcomes when stimulating each nerve branch of femoral nerve and tibial nerve
Paired recording electrodes were placed sequentially from the lateral to the medial. The recording electrodes in the quadriceps and gastrocnemius were placed as is shown inFigure 1AandB. When stimulating each nerve branch respectively, relative independent muscle subgroup contraction was induced and different EMGs were observed in each muscle subgroup.
Muscle contraction
When each branch of the main muscle branch of the femoral nerve was electrically stimulated from the lateral to the medial respectively, it was found that contraction of the lateral muscle (LM), intermediate muscle and medial muscle (MM)of the quadriceps could be induced. For the femoral nerve,which consisted of six nerve branches, electrical stimulation of individual nerve branches 2–5 from the lateral to the medial,induced contractions of LM, intermediate muscle, and MM.However, when electrically stimulating the nerve branch 1 and nerve branch 6, no contraction of the muscle subgroup was observed (Table 2). For the tibial nerve, which consisted of four nerve branches, when lateral nerve branches 1–3 and the medial nerve branch were electrically stimulated from the lateral to the medial respectively, the individual contractions of LM1–3, and MM of the gastrocnemius were observed (Table 3).
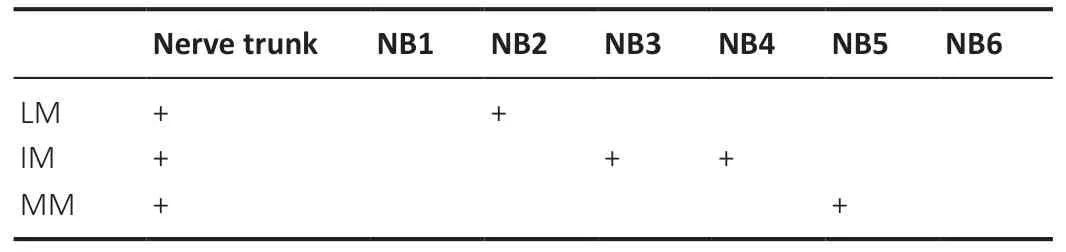
Table 2 |Contractions of the subgroups of the quadriceps when electrically stimulating each branch of the muscle branch of the femoral nerve individually
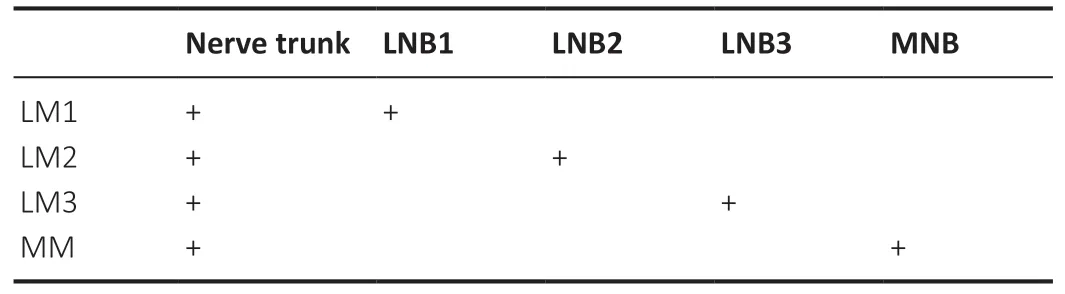
Table 3|Contractions of the subgroups of the gastrocnemius when electrically stimulating each branch of the tibial nerve
EMG
The EMG waves recorded in different muscle subgroups were different when each nerve branch was electrically stimulated(Figure 2A–F). For the femoral nerve, which consisted of six nerve branches, EMGs were recorded in the quadriceps when each of nerve branches 2–5 were electrically stimulated individually but EMGs were not recorded when either nerve branch 1 (Figure 2A) or nerve branch 6 (Figure 2F) was stimulated. When electrically stimulating nerve branch 2,EMGs can be recorded in channels 1–5, with the major waves in channel 1 and 3 upward and the major waves in channel 2, 4, and 5 downward. The waves with the largest amplitude occurred in channels 3 and 5 (Figure 2B). When stimulating nerve branch 3, EMGs were recorded in channels 1–5, with the major waves in channels 1, 2, 4 and 5 upward and the major waves in channel 3 downward. The waves with the largest amplitude were in channel 5 (Figure 2C). When stimulating nerve branch 4, EMGs were recorded in channels 1–5, with the major waves in channels 2, 4, and 5 downward, and the major waves in channels 1 and 3 upward. The waves with the largest amplitude were obtained in channels 3 and 5 (Figure 2D). When stimulating nerve branch 5, EMGs can be recorded in channels 1–5, with the major waves in channels 2, 4 and 5 downward and the major waves in channels 1 and 3 upward. The wave with the largest amplitude was in channel 3 (Figure 2E). The tibial nerve consists of four nerve branches. EMGs could be recorded in the gastrocnemius when any one of the lateral nerve branches 1–3, or the medial nerve branch was electrically stimulated. When stimulating lateral nerve branch 1, EMGs can be recorded in channel 1 and channel 3, with the major wave in channel 1 downward, and the major wave in channel 3 upward.The wave with the largest amplitude was associated with channel 1 (Figure 3A). When stimulating lateral nerve branch 2, EMGs can be recorded in channel 2, with the major wave downward (Figure 3B). When stimulating lateral nerve branch 3,EMGs can be recorded in channels 3 and 4, both major waves.The largest amplitude wave was seen in channel 3 (Figure 3C). When stimulating the medial nerve branch, EMGs were recorded in channel 4, as a major downward wave (Figure 3D).Tissue morphology of the nerve branches around the neuromuscular junction into the muscle
HE staining of the tissues showed that around the nerve’s entrance into the muscle most of the nerve branches consisted of individual neural tracts (Figure 4A), but a few nerve branches consisted of more than two neural tracts after the nerves entrance into the muscle (Figure 4B).
Discussion
Traditionally, the movement of a limb resulted from the coordinated activities of multiple muscle groups under the fine control of the nervous system. The functional activity of a single muscle was considered to be simple, i.e., either the whole muscle contracted or the whole muscle relaxed.The traditional model has significant limitations in explaining the motion of a joint. Corresponding to the complex joint’s movements, the muscles around the joints are usually crossjoint distributed. Some muscles (e.g., supraspinatus and infraspinatus) were distributed from multiple anatomical points rather than an individual point (Fabrizio and Clemente,2014; Rudolf et al., 2019). Accordingly, we speculate that these muscles are likely to perform much more complex activities than simple whole-block contractions or relaxations.
Previous anatomical studies and EMG studies have indicated that even a single belly muscle has several compartments(MacFadden and Brown, 2010; Wickham and Brown, 2012;Méndez et al., 2013; Larsen et al., 2014; Watanabe et al.,2016). Our earlier studies also revealed the presence of fascialike tissues within the gastrocnemius, which suggested that there might be an intermediate structure in the muscle (i.e.,muscle subgroups) before its division into microscopic muscle fibers. Our later experimental study in mice confirmed this intermediate structure (i.e. the lamellar cluster of MEPs, the innervating nerve branches and the corresponding muscle fibers) that can be considered an independent structural and functional unit (Yin et al., 2019).
In the present study, we performed further observations in rabbits. Local anatomy indicated that peripheral nerves sent out several nerve branches around its entrance into the muscle. Electrophysiological testing of nerve function has been increasingly used in the field of peripheral nerve injury and regeneration (Aminoff, 2004; Uzun et al., 2006;Karşidağ et al., 2008; Patel et al., 2018; Wang et al., 2019;Taylan and Barishaner, 2020). Our electrophysiological findings revealed that when electrically stimulating each nerve branch individually, relatively independent muscle subgroup contraction was induced and different EMGs was observed in each muscle subgroup. From the histological perspective,HE staining indicated that most of the nerve branches around the neuromuscular junction consisted of one individual neural tract. However, a small number of nerve branches consisted of two neural tracts, which indicated that these nerve branches would develop new fine branches after entering the muscle. This suggested that the lower limb muscles of the rabbit can be further divided into muscle subgroups, which are innervated by different dominant nerve branches and consequently participate in the formation of more complex muscle activities. We concluded that, in rabbits, the nerve branches and their innervated muscle subgroups could be viewed as an independent structural and functional unit,an MCU. The MCUs link the muscles and the motor units to better interpret various complex and elaborate muscle activities.

Figure 1|The placement of paired recording electrodes in the femoral nerve (A) and tibial nerve (B) for electrophysiological study in rabbits.

Figure 2|Electrophysiology when electrically stimulating each branch of muscle branch of the femoral nerve in rabbits.

Figure 3|Electrophysiology when electrically stimulating each branch of the tibial nerve in rabbits.
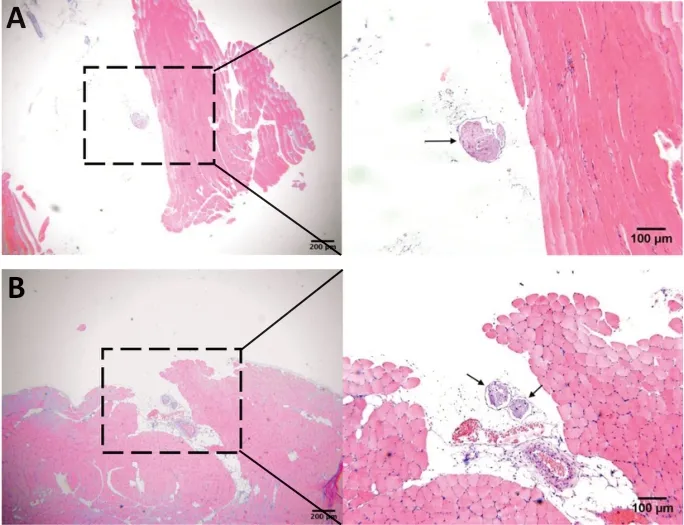
Figure 4|Typical micrograph of the hematoxylin and eosin staining of thetissues around the neuromuscular junction in the lower limb in rabbits.
This study has some limitations. The present study focused on anatomical and functional descriptions without statistical analysis. The sample size of this study was small, making this a preliminary, exploratory study. Additional techniques could be applied that further investigate the structural basis of the MCUs in rabbits, such as the three-dimensional distributions of the innervating nerve branches and the MEPs in the muscles. Different techniques may be more amenable for larger sampling to give statistical validity. The identification and characterization MCUs in other muscles, such as the upper limb muscles, would support a general theory. We speculate that the number of MCUs and MEPs lamella clusters in the upper limb muscles may be less than in the lower limb muscles owing to their more elaborate structural and functional differentiations. A small number of nerve branches consisted of two neural tracts, therefore, the number and boundary of MCUs in each muscle require further animal experiment studies.
The results of this experimental study will be instructive in clinical research. In particular, recording electrodes should be placed based on distributions of the MCUs in EMG, which could indicate neuromuscular pathway disorders in a more precise way. Generally, our results may provide far-reaching implications from a clinical perspective, including patients who need muscle transplantation owing to trauma or other factors(Chuang, 2008; Barrera-Ochoa et al., 2017; Yi Lee et al., 2019).It would be important for clinicians to identify and use intact MCUs when performing nerve–muscle transplantation to preserve the structural and functional integrity of the MCUs.
In conclusion, the muscles of the lower limb in the rabbit can be subdivided into different muscle subgroups, each innervated by different nerve branches, thereby allowing much more complex muscle activities than traditionally stated.Together, the nerve branches and the innervated muscle subgroups can be viewed as an independent structural and functional unit.
Author contributions:All authors contributed to the conception, writing,critical review, and revision of the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Financial support:This study was supported by Peking University Clinical Scientist Program of China, No. BMU2019LCKXJ005, and the Fundamental Research Funds for the Central Universities, Key Laboratory of Trauma and Neural Regeneration, Ministry of Education of China,No. BMU2019XY007-01 (both to BGJ). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Peking University People’s Hospital (approval No. 2019PHE027) on October 20, 2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Víctor Carriel, University of Granada, Spain.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Inhibition of CXCR4/CXCL12 signaling: a translational perspective for Alzheimer’s disease treatment
- The new wave of p75 neurotrophin receptor targeted therapies
- Inhibition of LncRNA Vof-16 expression promotes nerve regeneration and functional recovery after spinal cord injury
- Intranasal insulin ameliorates neurological impairment after intracerebral hemorrhage in mice
- Lycium barbarum extract promotes M2 polarization and reduces oligomeric amyloid-β-induced inflammatory reactions in microglial cells
- Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis

