Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis
Yan Zhou , Lu-Lu Wen , Yan-Fei Li Kai-Min Wu Ran-Ran Duan Yao-Bing YaoLi-Jun Jing Zhe Gong Jun-Fang Teng Yan-Jie Jia
Abstract Mesenchymal stem cell (MSC) transplantation is a promising treatment strategy for spinal cord injury, but immunological rejection and possible tumor formation limit its application. The therapeutic effects of MSCs mainly depend on their release of soluble paracrine factors.Exosomes are essential for the secretion of these paracrine effectors. Bone marrow mesenchymal stem cell-derived exosomes (BMSC-EXOs)can be substituted for BMSCs in cell transplantation. However, the underlying mechanisms remain unclear. In this study, a rat model of T10 spinal cord injury was established using the impact method. Then, 30 minutes and 1 day after spinal cord injury, the rats were administered 200 µL exosomes via the tail vein (200 µg/mL; approximately 1 × 106 BMSCs). Treatment with BMSC-EXOs greatly reduced neuronal cell death,improved myelin arrangement and reduced myelin loss, increased pericyte/endothelial cell coverage on the vascular wall, decreased bloodspinal cord barrier leakage, reduced caspase 1 expression, inhibited interleukin-1β release, and accelerated locomotor functional recovery in rats with spinal cord injury. In the cell culture experiment, pericytes were treated with interferon-γ and tumor necrosis factor-α. Then,Lipofectamine 3000 was used to deliver lipopolysaccharide into the cells, and the cells were co-incubated with adenosine triphosphate to simulate injury in vitro. Pre-treatment with BMSC-EXOs for 8 hours greatly reduced pericyte pyroptosis and increased pericyte survival rate.These findings suggest that BMSC-EXOs may protect pericytes by inhibiting pyroptosis and by improving blood-spinal cord barrier integrity,thereby promoting the survival of neurons and the extension of nerve fibers, and ultimately improving motor function in rats with spinal cord injury. All protocols were conducted with the approval of the Animal Ethics Committee of Zhengzhou University on March 16, 2019.
Key Words: blood-spinal cord barrier; edema; exosome; pericyte; NOD1; pro-caspase 1; pyroptosis; spinal cord injury
Introduction
Spinal cord injury (SCI) is a life-threatening traumatic injury often accompanied by paraplegia, neurological complications,and reduced life expectancy (Rahimi-Movaghar et al., 2013).After the primary traumatic event, a cascade of secondary injury events start, which includes, but is not limited to,ischemia, hemorrhage, breakdown of the blood-spinal cord barrier (BSCB), edema, neuroinflammation and oxidative stress. These processes ultimately accelerate neuronal loss and axonal degeneration (Aslan et al., 2009; Haque et al., 2017). Among these, breakdown of the BSCB and neuroinflammation are key events in the pathogenesis of SCI,and make recovery of normal functioning of the spinal cord more difficult (Pan et al., 2019).
Pericytes are mural cells inserted in the basement membrane of microvessels throughout the body, including the spinal cord. Numerous preclinical studies have demonstrated that pericytes, a central component of the neurovascular unit,have critical functions, including the regulation of BSCB/blood-brain barrier permeability and capillary blood flow,the development and maintenance of the vascular system,clearance of toxic molecules, and the regulation of the entry of immune cells into the central nervous system (Berthiaume et al., 2018; Hashitani et al., 2018; Hashitani and Mitsui,2019). A reduction in pericytes contributes to increased BSCB permeability, microcirculatory disruption, and the leakage of harmful blood components into the central nervous system,thereby aggravating neurological dysfunction (Wu et al.,2014).
Previous studies have suggested that acute inflammatory responses after SCI cause severe tissue damage when cellular debris and released intracellular proteins stimulate inflammatory cells, including resident microglia and astrocytes,as well as blood neutrophils, macrophages, B lymphocytes and T lymphocytes recruited from the circulatory system(David and Kroner, 2011; Orr and Gensel, 2018; Ungerer et al.,2020). Some cell types such as pericytes and neurons can also secrete pro-inflammatory cytokines.
Pyroptosis is a recently identified form of programmed cell death that is dependent on the activation of caspases 1,4, 5 and 11, and induces the release of pro-inflammatory cytokines (Bergsbaken et al., 2009; Tan et al., 2021). Recently,Li et al. (2018) found that pericyte loss caused by pyroptosis,rather than apoptosis, leads to vascular leakage and mortality in an animal model of sepsis induced by cecal ligation and puncture. Previous studies indicate that inhibition of pyroptosis promotes motor functional recovery after SCI (Dai et al., 2019; Zheng et al., 2019). Thus, pyroptosis may also result in pericyte loss and BSCB permeability changes in SCI models. Further study is needed to clarify the role of pericytes in inflammation.
Mesenchymal stem cells (MSCs) derived from various sources have been reported to be a promising treatment strategy in preclinical and clinical studies of cell-based therapies (Huang et al., 2018; Mahmoudi et al., 2019; Yi et al., 2019; Wei et al.,2021). However, the therapeutic benefits of MSCs are limited by shortcomings, including poor survival rates, possible tumor formation, immunological rejection, and genetic variation(Hoogduijn et al., 2011; Moya et al., 2018). Previous studies have shown that the therapeutic effects of MSCs mainly depend on their release of paracrine soluble factors rather than their direct differentiation into injured cell types (Zhang et al., 2018; Yaghoubi et al., 2019). Exosomes (40–120 nm in diameter) are essential for the release of MSC-derived paracrine factors. Exosomes contain numerous key signaling molecules that are protected from degradation by the lipid bilayer packaging (Phinney et al., 2015). Emerging reports suggest that exosomes from MSCs promote recovery from traumatic brain injury, neuropathic pain and SCI (Kalani and Tyagi, 2015; Xiong et al., 2017; Gao et al., 2018). A novel study recently showed that embryonic stem cell derived-exosomes reduce inflammation-induced pyroptosis in doxorubicininduced cardiomyopathy (Tavakoli Dargani and Singla, 2019).However, the effects of bone MSC-derived exosomes (BMSCEXOs) on pyroptosis in spinal cord injury remain unknown.Therefore, in the present study, we test the hypothesis that BMSC-EXOs play a critical role in protecting the spinal cord through the inhibition of pericyte pyroptosisin vivoandin vitro.
Materials and Methods
BMSC-EXO isolation
According to a previously published protocol (Li et al., 2019),male Sprague-Dawley rats (2 months old, specific-pathogenfree grade, purchased from Huaxing Company, Zhengzhou,China, license No. SCXK(Yu)2019-0002) were anesthetized by 4% isoflurane (RWD, Shenzhen, China) inhalation and sacrificed by decapitation (Table 1). The tibias were stripped to collect bone marrow cells. Then, the cell suspension wascentrifugated at 1000 ×gfor 5 minutes, and cell pellets were collected and resuspended in complete medium,consisting of Dulbecco’s modified Eagle medium (DMEM;Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific) and penicillin/streptomycin (Leagene Biotechnology, Beijing,China). After changing the complete medium 2 days later,the cells adhering to the culture dish were considered P0 BMSCs. At 80–90% confluence, the cells were digested with ethylenediaminetetraacetic acid-containing trypsin at 37°C for 1 minute. The digestion was terminated with complete medium, and the cell suspension was re-seeded into a dish.

Table 1 |The sex, age and number of each part of the rat
BMSCs at passages 3 to 6 were used to isolate exosomes.BMSCs at 80–90% confluence were cultured in exosomefree complete medium for 2 days, and the conditioned supernatant was collected into an ultracentrifuge tube and centrifuged (successively) at 300 ×gfor 10 minutes, 2000 ×gfor 10 minutes, and 10,000 ×gfor 30 minutes to eliminate cells and debris. Thereafter, filtration through a 0.22-µm filter(Millipore, Burlington, MA, USA) was performed to further reduce dead cells and particles in the supernatant. Exosomes were then separated by ultracentrifugation at 110,000 ×gfor 70 minutes. After discarding the supernatant, the pellet was resuspended in phosphate-buffered saline (PBS), and ultracentrifuged again at 110,000 ×gfor another 70 minutes.Finally, the supernatant was discarded, and the pellet was stored at –80°C until use in experiments. All of these procedures were conducted at 4°C. Nanosight technology(Particle Metrix, Meerbusch, Germany) was used to analyze the size distribution of exosomes.
Transmission electron microscopy examination
Exosomes from BMSCs were resuspended and observed by transmission electron microscopy (Tecnai 12, Philips,Amsterdam, Netherlands) (Wei et al., 2019). Specimens on copper grids were made using two methods: dripping and floating. In the dripping method, the exosome suspension was placed onto the copper mesh for a few seconds. In the floating method, the exosome suspension was placed onto parafilm,and the copper meshes were floated onto the droplet for 3 minutes. Subsequently, the excess liquid was absorbed by filter paper in both methods. All samples were floated in a 1% aqueous solution of phosphotungstic acid for 30 seconds,and air-dried before examination by transmission electron microscopy.
Nanoparticle tracking analysis
The size and concentration of exosomes were evaluated using Nanosight Zeta View PMX 110 (Particle Metrix). A size distribution plot with particle diameter (nm) on the x-axis and concentration (particles/mL) on the y-axis was made.
BMSC-EXO labeling
According to the manufacturer’s instructions, PKH26 dye(Sigma-Aldrich, St. Louis, MO, USA) was used to label exosomes. Briefly, the exosome suspension was incubated with 2 µL PKH26 for 20 minutes at room temperature. Then,the staining reaction was stopped by isovolumetric PBS containing 5% bovine serum albumin (Solarbio, Beijing, China).Labeled exosomes were isolated by ultracentrifugation, as described above.
SCI model and treatment
All experiments complied with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols were conducted with the approval of the Animal Ethics Committee of Zhengzhou University on March 16, 2019. A total of 160 adult male Sprague-Dawley rats (weighing 200–250 g, 3 months old, specific-pathogen-free grade, purchased from HuaXing Company, Zhengzhou, China, license No. SCXK (Yu)2019-0002) were divided into the following four groups at random: Sham, EXO (Sham + EXO), SCI, and SCI + EXOs (n=40/group). According to established protocol (Wang et al.,2018), rats were fixed on a fixing plate after anesthesia was induced by inhalation of 4% isoflurane. When the animals lost consciousness, the vertebral column was exposed by separation of the skin, muscle and ligament overlying the appropriate spinal cord region. A T10 laminectomy was conducted, and the exposed spinal cord was subjected to a contusive wound by applying an impact of 2 N (equal to 200 kilodyne) with a spinal cord impactor (IH Impactor; Precision Systems and Instrumentation, Lexington, KY, USA). The sham rats underwent laminectomy without a contusive wound.After surgery, all rats were treated with penicillin for 3 days,and the bladders were manually voided three times a day.In the SCI + EXO group, rats received 200 µL exosomes (200µg/mL, derived from ~1 × 106BMSCs) via the tail vein at 30 minutes and 1 day after SCI. Using the same administration route and at the same time points, rats in the EXO group received 200 µL exosomes (200 µg/mL), and rats in the SCI group received 200 µL PBS (Table 1).
Histological analysis
At 3 days post injury (dpi), rats were anesthetized by inhalation of 4% isoflurane (RWD Life Science) and perfused with physiological saline. The T10 spinal cords were embedded in paraffin and serially cut into 5-µm-thick coronal sections.Slides were then mounted and stored at 4°C for staining. For Nissl staining, the dewaxed tissue slides were dyed with 1%toluidine blue for 40 minutes at 60°C, dehydrated, and sealed with neutral gum. For Fluoro-Jade B (FJB) staining, slides were incubated with a 0.0004% solution of FJB (Millipore)for 20 min and washed with distilled water three times.FJB-stained cells were then captured on a fluorescence microscope (Olympus, Tokyo, Japan) at 200× magnification,and quantitatively analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). For Luxol Fast Blue staining (Amtul et al., 2021), slides were incubated in 0.1%Luxol Fast Blue stain overnight at 60°C. Thereafter, slides were washed with double-distilled water, and then incubated in 0.05% lithium carbonate for an additional 10 seconds.For hematoxylin and eosin staining, slides were successively stained with hematoxylin and eosin to observe the structural damage according to an established protocol (Xie et al., 2021).
Immunohistochemical analysis
For immunohistochemistry, paraffin sections of T10 spinal cord tissue at 3 dpi were incubated with anti-neurofilament 200 (NF-200) antibody (1:200; mouse, Cat# GB13141;Servicebio, Wuhan, China) overnight at 4°C. Then, the slides were washed with PBS, and goat anti-mouse IgG (1:200; Cat#G1214-100UL; Servicebio) was applied and incubated 1 hour at room temperature. The NF-200 signals were observed with a light microscope (Olympus).
Immunofluorescence analysis
For immunofluorescence of the spinal cord tissues at 3 dpi,tissue slides and round coverslips were blocked with 5%bovine serum albumin for 1 hour, and then incubated with primary antibody overnight at 4°C. The primary antibodies included mouse anti-platelet-derived growth factor receptor(Pdgfr; 1:250; Cat# ab69506; Abcam), mouse anti-caspase 1(1:250, Cat# sc-398715, Santa Cruz Biotechnology, Santa Cruz,CA, USA), rabbit anti-Nod1 (1:200; Cat# PA5-79747; Invitrogen,Carlsbad, CA, USA), and rabbit anti-CD31 (1:200; Cat# ab24590;Abcam). After washing with PBS three times, the tissues were incubated with the corresponding secondary antibody (IFKineTMGreen donkey anti-mouse IgG, 1:300, Cat# A24211, Abbkine,Wuhan, China; Dylight 594, rabbit anti-goat IgG, 1:300, Cat#A23430, Abbkine) for 1 hour at room temperature in the dark.Subsequently, the tissues were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 minutes, and images were acquired using fluorescence microscopy (Olympus).
Evans blue assessment
To assess the integrity of the BSCB at 3 dpi, the Evans blue (EB)extravasation assay was conducted as previously described (Xu et al., 2019). About 2% EB (10 mg/mL; Sigma-Aldrich) dissolved in PBS was injected into rats through the tail vein. Then, 2 hours later, the rats were anesthetized with 4% isoflurane and perfused with saline to clear the circulation of EB dye. The spinal cord was dissected, and the dye distribution was imaged using a digital camera. Then, the spinal cord was cut into two parts (T9–10 and T10–11 segments). The T9–10 segments were weighed and soaked in methanamide (Sigma-Aldrich) for 24 hours (80°C). After centrifugation at 20,000 ×gfor 20 minutes,the supernatant was collected. A spectrophotometer (Nanodrop One, Thermo Fisher Scientific) was used with excitation and emission wavelengths of 620 nm and 680 nm, respectively,to measure the absorbance of the supernatant. The EB dye content was analyzed using an EB standard curve. The T10–11 segments were frozen in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan), sectioned (20 µm),and stored in a dark container at –20°C before measurement of EB with fluorescence imaging (Olympus).
In Vivo Imaging System
The spinal cords, 3 days after SCI, were processed with 2% EB as mentioned above. Subsequently, the region of interest was manually defined, and images were captured using theIn VivoImaging System (IVIS) Lumina XR optical imager (Perkin Elmer,Waltham, MA, USA) with 620 nm excitation and 680 nm emission filters, auto exposure time, and a binning factor of 2. Fluorescence was expressed as the total or average radiant efficiency of selected regions of interest.
Spinal cord water content measurement
Rats were anesthetized by inhalation of 4% isoflurane, and then perfused with physiological saline. Water content was measured at 3 dpi as previously described (Li et al., 2014; Luo et al., 2015). A 1-cm section of spinal cord along the injury epicenter was obtained and weighed before and after drying for 48 hours at 80°C until a constant weight (dry weight)was achieved. The water content of the spinal cord (%) was calculated as: (wet weight – dry weight)/wet weight × 100.
Basso, Beattie and Bresnahan scores
To assess neurological function, the Basso, Beattie and Bresnahan (BBB) score (Basso et al., 1995) was calculated 1, 3,7, 14, 21 and 28 days after SCI in the open field. The BBB score ranges from 0 to 21, which mainly evaluates the joint function hind limb coordination and weight bearing ability; the higher score indicates better motor function.
Enzyme-linked immunosorbent assay
Following the manufacturer’s instructions, interleukin-1β (IL-1β) was quantified at 3 dpi with enzyme-linked immunosorbent assay kits. Spinal cord tissues were isolated,homogenized in PBS, and frozen at –20°C overnight. Then, the suspensions were centrifuged at 12,000 ×gfor 10 minutes at 4°C, and the supernatants were collected. The samples were analyzed with a rat IL-1β enzyme-linked immunosorbent assay kit (Multisciences Biotech, Hangzhou, China) at an absorbance of 450 nm on a spectrophotometer. IL-1β concentrations were determined from a standard curve.
Cell isolation, culture, and treatment
Rat spinal cord microvascular pericytes were isolated as previously described (Wu et al., 2015). Briefly, following decapitation (3–5 weeks of age, specific-pathogen-free grade, purchased from HuaXing Company, Zhengzhou, China,license No. SCXK (Yu) 2019-0002), the spinal column was isolated and immersed in pre-cooled PBS. After removing the meninges and large vessels, the gray matter of the spinal cord was separated and digested in DMEM containing collagenase type II (1 mg/mL; Worthington, Lakewood, NJ,USA) and DNase I (15 µg/mL; Sigma-Aldrich) for 1.5 hours at 37°C. The microvessel fragments were centrifuged in 20%bovine serum albumin/DMEM at 1000 ×gfor 20 minutes to adsorb the myelin. Collagenase/dispase (1 mg/mL;Roche, Basel, Switzerland) and DNase I (6.7 µg/mL) in DMEM were added and incubated for 1 hour at 37°C. The microvessel clusters were separated on a 33% continuous Percoll (GE Healthcare, Pittsburgh, PA, USA) gradient to remove endothelial cells. After washing twice in DMEM,the microvessels were collected in a culture dish containing complete medium, and the supernatant was replaced every 2 days. Pericytes in the microvascular fragment migrated out and proliferated. Pericytes with a purity > 90% were obtained by passage. The 3rdto 8thpassages were used.
Pericytes were divided into sham, EXO, IFN-γ + TNF-α + LPS+ Lipo + ATP, and IFN-γ + TNF-α + LPS + Lipo + ATP + EXO groups. Pericytes were pre-treated with interferon-γ (IFN-γ;100 ng/mL; PeproTech China, Suzhou, China) + tumor necrosis factor α (TNF-α; 10 ng/mL; PeproTech) for 3 hours, then transfected with lipopolysaccharide (LPS; 1 µg/mL; Sigma-Aldrich) using Lipofectamine 3000 (Invitrogen) and coincubated with or without adenosine triphosphate (ATP; 5 mM;Solarbio) for 4, 16 or 24 hours. Lipofectamine 3000 was used at a concentration of 5 µL/2 mL medium and premixed with LPS in DMEM for 15 minutes. When testing the protective effect of BMSCs-EXOs on pericytes, cells were co-incubated with or without BMSCs-EXOs (100 µg/mL) for 8 hours before exposure to a compound stimulus of IFN-γ + TNF-α + LPS + Lipo + ATP.
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
The viability of pericytes was evaluated with MTT (Amersco Grade, Shanghai, China) according to the manufacturers’protocols (Yu et al., 2019). Pericytes were seeded into 96-well plates at 5000 cells per well. After incubation, the supernatant was discarded and replaced with fresh culture medium. Thereafter, MTT, at a concentration of 5 mg/mL in PBS, was added to the medium, 20 µL per well, and incubated for 3 hours in the culture chamber. Next, dimethyl sulfoxide(Solarbio) was added at 150 µL per well for 10 minutes. Finally,optical absorbance was read at 562 nm using a SpectraMax Absorbance Reader (Molecular Devices, Silicon Valley, CA, USA).
Double staining with Hoechst 33342 and propidium iodide
Pericytes were co-incubated with propidium iodide (PI;5 µg/mL; BioLegend, Shanghai, China) and Hoechst 33342(1 µg/mL; Solarbio) for 15 minutes in a cell incubator, and then washed with PBS three times. Images were acquired using an inverted fluorescence microscope (BX53; Olympus).
Western blot assay
Total protein was extracted from pericytes using radio immunoprecipitation assay reagent (Beyotime Biotechnology,Shanghai, China) containing 100 µg/mL phenylmethylsulfonyl fluoride (Solarbio) and quantified using a bicinchoninic acid protein assay kit (Solarbio). Protein samples were denatured,separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene fluoride membrane (EMD Millipore Corp., Burlington, MA, USA).The membranes were then blocked with a blocking solution(QuickBlock, Beyotime Biotechnology) at room temperature for 30 minutes, and then incubated with primary antibody against pro-caspase-1 (1:1000, rabbit, Cat# ab179515,Abcam), Nod1 (1:500, mouse, Cat#sc-398696, Santa Cruz),IL-1β (1:500, rabbit, Cat# ab9722, Abcam), CD9 (1:2000,rabbit, Cat# ab92726, Abcam), CD81 (1:5000, rabbit, Cat#ab109201, Abcam), CD63 (1:500, mouse, Cat#sc-5275, Santa Cruz) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH;mouse, 1:1,0000, Cat# AC033, ABclonal, Wuhan, China) at 4°C overnight. The membranes were incubated with secondary goat anti-rabbit or mouse horseradish peroxidase-linked antibody (1:1000, Abbkine, Cat# A21220 or Cat# A25012)at room temperature for 1 hour. Finally, target bands were visualized using an enhanced chemiluminescence western blot kit (Thermo Fisher Scientific) and semi-quantified using ImageJ software.
Statistical analysis
Data are presented as mean ± standard deviation (SD).Group differences were determined by Student’st-test using SPSS 23.0 (IBM, Armonk, NY, USA).P< 0.05 was considered statistically significant.
Results
Characterization and tracing of BMSC-EXOs
To characterize the exosomes derived from BMSCs, they were isolated and purified from culture supernatants by ultracentrifugation, and then observed by transmission electron microscopy, which revealed numerous saucershaped vesicles (Figure 1AandB). Specimens prepared by the floating method were more stereoscopic and had a clearer morphology than those prepared by the dripping method.Nanoparticle tracking analysis revealed a particle size of approximately 100 nm in diameter (Figure 1C). In addition,western blot analysis confirmed distinctive exosome markers,including CD9, CD63 and CD81 (Figure 1D). For tracking,BMSC-EXOs were labeled with PKH26 dye. BMSC-EXOs were found at spinal cord lesion sites at 3 dpi and were assimilated by pericytes 6 hours after incubationin vitro(Figure 1EandF).
BMSC-EXOs reduce neuron death, decrease neural fiber degeneration, and improve locomotor functional recovery after SCI
To examine the effects of the injection of BMSC-EXOs on SCIrelated pathology, a series of histological analyses at 3 dpi were conducted. We first performed Nissl staining to assess the survival of neurons on adjacent paraffin sections of the spinal cord. Structurally intact and deeply stained Nissl bodies were observed in the Sham and EXO groups. In the SCI group,a number of neurons exhibited karyopyknosis and even loss of Nissl bodies. The morphology and number of Nissl bodies in the SCI + EXO group was dramatically ameliorated compared with the SCI group (Figure 2A). Next, FJB staining was used to detect neuronal death in the spinal cord lesion core. Few FJBpositive cells were observed in the Sham and EXO groups. In the SCI group, the lesion region contained many FJB-positive cells.Notably, the numbers of FJB-positive cells were reduced after BMSC-EXO treatment (P< 0.05,vs. SCI group;Figure 2BandF).
Luxol Fast Blue staining was carried out to evaluate the demyelination of neural fibers (Fan et al., 2020). The Sham and EXO groups exhibited blue staining of the regular structure of the myelin sheath. Severe perturbation of myelin arrangement along with myelin loss was observed in the SCI group. These defects were improved in the BMSC-EXOs group compared with the SCI group (Figure 2C).
NF-200 is an important neurofilament found specifically in neuronal cell bodies and axons, and its expression indirectly reflects the degree of axonal damage and repair after SCI (Trojanowski et al., 1986). Therefore, we used immunohistochemical staining to observe the changes in NF-200-positive axons. Significant differences were observed between the Sham and SCI groups. Furthermore,the expression of NF-200 was increased in the SCI + EXO group compared with the SCI group, suggesting that BMSCEXOs inhibit axonal degeneration (Figure 2D). In addition,hematoxylin and eosin staining was used to assess overall damage. As shown inFigure 2E, the Sham and EXO groups exhibited normal motor neurons and Nissl bodies. Severe structural damage was observed in the SCI group, which was ameliorated by treatment with BMSC-EXOs.
Following SCI, the rats displayed obvious behavioral deficiencies. To further examine the effects of BMSC-EXOs on locomotor functional recovery after injury, BBB scores were recorded. BMSC-EXOs treatment improved locomotor function from 2 weeks post-SCI, compared with the SCI group (Figure 2G). These results demonstrate that BMSC-EXOs accelerate locomotor functional recovery in rats with SCI.
BMSC-EXOs attenuate BSCB leakage and reduce edema after SCI by inhibiting pyroptosis in pericytes
Studies have shown that traumatic SCI induces microvascular dysfunction and persistent BSCB disruption (Yu et al., 2016;Li et al., 2020). BSCB permeability was evaluated using the EB extravasation assay at 3 dpi. As shown inFigure 3, the infiltration of EB, fluorescence intensity of tissue and water content were low in the Sham and EXO groups. Compared with the high level of infiltration in the SCI group, EB infiltration was significantly reduced in the SCI + EXO group (P< 0.05;Figure 3A,BandD). Consistent with this, IVIS images showed evident increases in the fluorescence intensity of the surrounding tissue in the SCI group, which was lessened by BMSC-EXO administration (Figure 3C). In addition, the water content of spinal cord tissues was decreased in SCI rats administered BMSC-EXOs (P< 0.05;Figure 3E). These results suggest that BMSC-EXOs help maintain BSCB integrity.
To clarify the molecular mechanisms underlying the protective effects of BMSC-EXOs on BSCB integrity, we performed immunofluorescence staining for Pdgfr-β/caspase 1.Compared with the sham group, the co-localization of Pdgfr-β and caspase 1, as well as immunoreactivity for caspase 1,were increased in spinal cord tissue in SCI rats (Figure 4A).Notably, caspase 1 immunoreactivity was weak in pericytes in the SCI + EXOs group compared with the SCI group. The innate immune response is the first line of defense against microbial pathogens and endogenous stress, and pattern recognition receptors are the key to this nonspecific response. Nod1 is a pattern recognition receptor localized in the cytoplasm, and can activate caspase 1 and the cell death pathway (Clarke and Weiser, 2011). Western blot analysis showed that levels of Nod1 and pro-caspase 1 were significantly increased in spinal cord lesions compared with the sham group, and that BMSC-EXO treatment could reverse these changes (P< 0.05;Figure 4BandC). Moreover, IL-1β in the SCI + EXOs group was markedly lower than in the SCI group (P< 0.05,Figure 4D). Thus, we reasoned that pyroptosis-induced pericyte loss might be responsible for BSCB disruption.
To further elucidate the mechanisms of BSCB disruption,double immunofluorescence staining for Pdgfr-β and CD31(pericyte/endothelial cell) was carried out. As shown inFigure 4, a marked loss of pericyte coverage on the vascular wall was observed in the spinal cord of SCI rats at 72 hours postsurgery. Notably, pericyte/endothelial cell coverage was evidently elevated in the SCI + EXO group compared with the SCI group (P< 0.05;Figure 4EandF). Taken together, these findings provide strong evidence that BMSC-EXO treatment results in decreased pericyte loss on the microvessels, thereby reducing BSCB leakage and edema after SCI.
A compound stimulus induces pyroptosis in pericytes in vitro
A recent study showed that pyroptosis in pericytes is activated by intracellular LPS (Nyúl-Tóth et al., 2017), distinct from the activation of other cell types such as astrocytes and BV2 cells (Adamczak et al., 2014; Nyúl-Tóth et al., 2017; Wilson et al., 2017). Double fluorescence staining with Hoechst and PI was performed to assess cell death. In the Sham and ATP groups, the cells were healthy, with only a few dead cells. We examined the effects of a combined stimulus of IFN-γ, TNF-α and LPS delivered using Lipofectamine 3000.This caused pro-caspase 1 activation in pericytes. However,after stimulation for 24 hours, cell viability was reduced only slightly and cell death was induced only weakly, which does not conform with the low rate of pericyte/endothelial cell coverage in SCI models. ATP is released by bacteria and the host during bacterial infection and sterile tissue damage, and may promote cell pyroptosis through excitation of the AMPK pathway and inflammasome activation (Zha et al., 2016).On the basis of previous studies, we primed pericytes with IFN-γ + TNF-α for 4 hours. When delivering LPS into pericytes using Lipofectamine 3000, ATP was added simultaneously for different intervals. We observed a marked decrease in cell viability and an increase in cell death at 16 and 24 hours,corresponding to a higher expression of pro-caspase 1 (P<0.05;Figure 5). Thus, we chose this model to examine the protective effect of BMSC-EXOs on pericyte injuryin vitro.
BMSC-EXOs provide cytoprotection against pyroptosis in pericytes in vitro
To further clarify whether BMSC-EXOs participate in cytoprotection against pyroptosis in pericytesin vitro, and to elucidate the underlying molecular mechanisms, pericytes were coincubated with BMSC-EXOs before exposure to the combined stimulus. The cells appeared healthy, with only a few dead cells in the Sham and EXO groups. BMSC-EXOs reversed the cell viability reduction, and decreased the number of PI-positive cells in rats with SCI (P< 0.05;Figure 6A,BandD). Nod1 and caspase 1 co-localization is an indication of inflammasome activation. BMSC-EXOs returned the inflammasome to a relatively low activation state (Figure 6C).Western blot analysis showed that the compound stimulus elevated the levels of Nod1, pro-caspase 1 and cleaved-IL-1β,reflecting the pyroptosis of pericytes. BMSC-EXO treatment reversed these changes (P< 0.05;Figure 6EandF).
Discussion
Accumulating evidence shows that exosomes derived from various stem cells exert neuroprotective effects in various disease models (Perets et al., 2018; Singla et al.,2019). Direct stem cell transplantation shows no significant differences in therapeutic effectiveness compared with transplantation of exosomes (Zhang et al., 2018; Xian et al.,2019). Exosomes have thus become a potential alternative to stem cell transplantation, and accordingly, BMSC-EXOs were investigated as a treatment for SCI in this study. Our findings clearly demonstrate that treatment with BMSC-EXOs reduces pericyte pyroptosis and improves survival following a compound stimulus challengein vitro. Furthermore, BMSCEXO administration not only maintained BSCB integrity by inhibiting pericyte pyroptosis, but also promoted neuronal survival, fiber regeneration, and functional recovery in the SCI model.

Figure 1|Characterization and tracing of BMSC-EXOs.
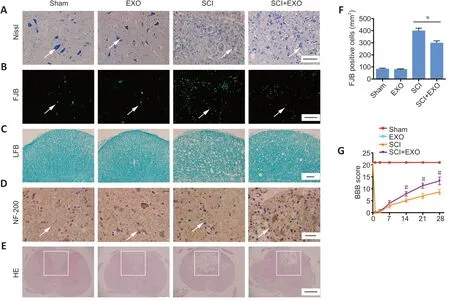
Figure 2|BMSC-EXOs reduce the lesion and improve locomotor functional recovery after SCI.
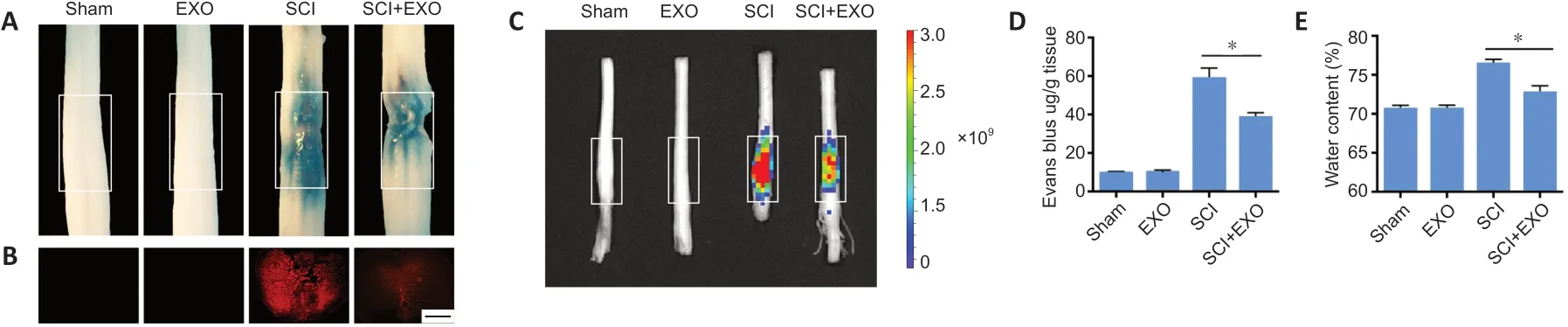
Figure 3|BMSC-EXOs preserve blood-spinal cord barrier integrity and reduce edema post-SCI.
The BSCB is of critical importance in maintaining the fluid microenvironment of the spinal cord within narrow limits(Albayar et al., 2019). Pericytes wrap around the capillaries and share a common basement membrane with microvascular endothelial cells (Bang et al., 2017). The interaction between pericytes and neighboring cells is essential for angiogenesis,angioarchitecture, vascular stability, and BBB/BSCB formation and maintenance (Bernacki et al., 2008; Bhowmick et al., 2019;Courtney and Sutherland, 2020). A large number of studies have advanced our understanding of the detrimental effect of pericyte loss on BSCB permeability in diverse diseases,including multiple sclerosis, amyotrophic lateral sclerosis and SCI (Wu et al., 2014; Garbuzova-Davis et al., 2019; Uchida et al., 2019). Previous studies have shown that traumatic insults to the spinal cord invariably lead to pericyte injury, disruption of microvascular stability, and a dramatic increase in BSCB leakage (Figley et al., 2014). Therefore, to improve motor functional recovery, it is imperative to protect pericytes from pathological insults and preserve BSCB integrity. Consistent with the results of others, we found increased permeability of the BSCB and severe tissue edema after SCI. Furthermore,our findings demonstrate that BMSC-EXOs can reverse BSCB leakage and reduce edema by inhibiting pericyte pyroptosis,thereby improving pericyte/endothelial cell coverage and resulting in better functional recovery after injury.
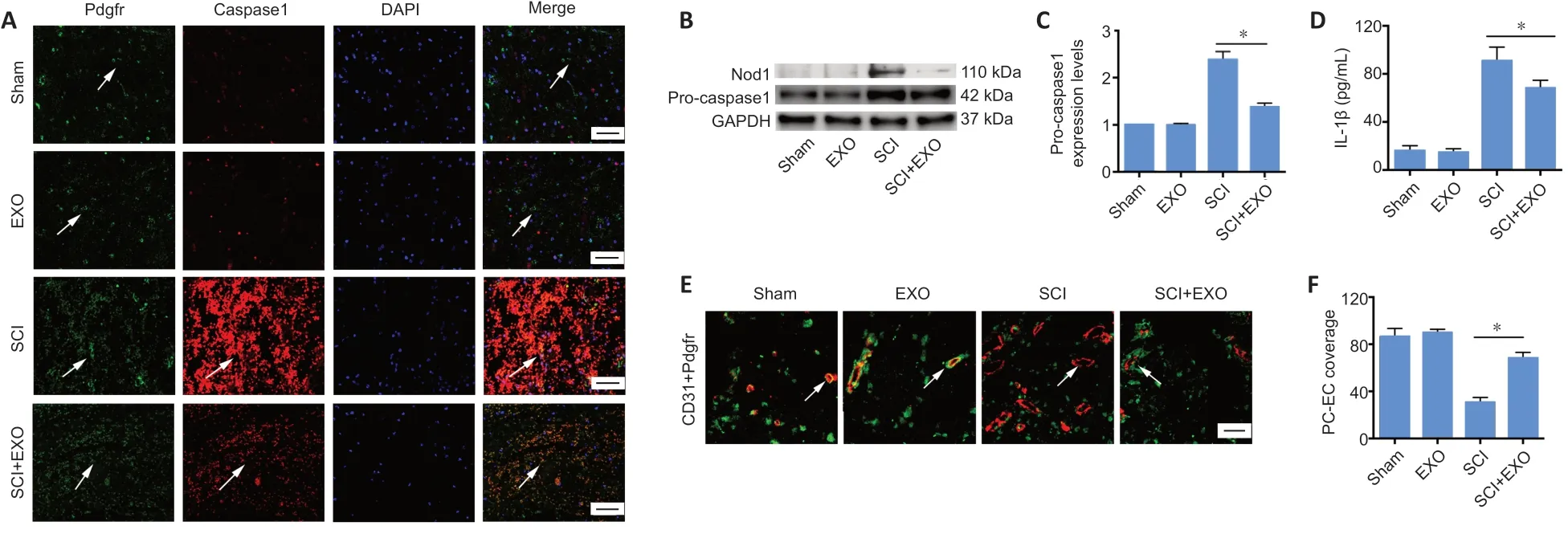
Figure 4|BMSC-EXOs decrease pyroptosis in pericytes and increase pericyte coverage in vivo.
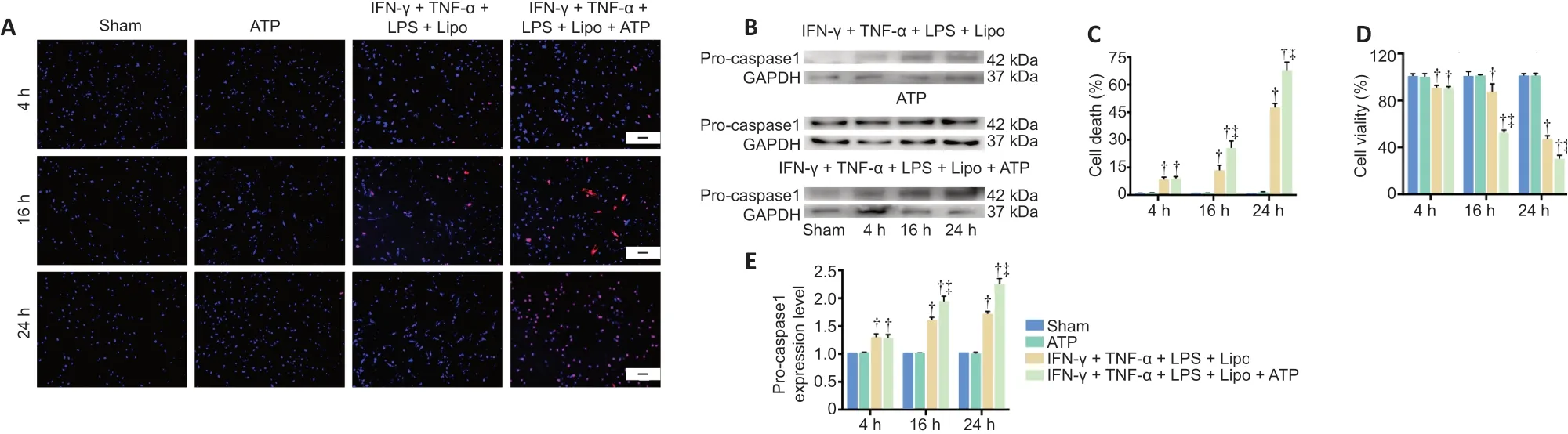
Figure 5|Compound stimulus induces pyroptosis in pericytes in vitro.

Figure 6|BMSC-EXOs provide cytoprotection against pyroptosis in pericytes in vitro by inhibiting Nod1 inflammasome activation.
Pyroptosis is a unique type of cell death, characterized by the activation of caspase 1, the formation of discrete pores in the cytomembrane, and the maturation and release of inflammatory cytokines such as IL-1β. When cells are subjected to pathological insult, such as ischemia-reperfusion and microbial infection, pyroptosis is induced. Several major cell types in the central nervous system have been reported to undergo pyroptosis, including microglia, endothelial cells and astrocytes (Abulafia et al., 2009; Minkiewicz et al., 2013; Adamczak et al., 2014). Furthermore, previous studies have shown that caspase 1 downregulation inhibits inflammasome assembly and pyroptosis in several disease models, including SCI, epilepsy, Alzheimer’s disease and multiple sclerosis, which are associated with neuronal injury and neuroinflammation (Tan et al., 2015; McKenzie et al.,2018). Hence, targeting the regulation of cellular pyroptosis may allow us to regulate disease pathological processes. Our results suggest that compared with the IFN-γ + TNF-α + LPS +Lipo group, a combined stimulus of IFN-γ + TNF-α + LPS + Lipo+ ATP significantly enhances the expression of caspase 1 and robustly induces pericyte death, which may better simulate the internal environment of the body under pathological conditions. Furthermore, pericyte death caused by caspase 1-mediated activation of the classical pyroptosis pathway aggravated BSCB breakdown in the SCI model. Strikingly, these pathological changes and motor dysfunction after SCI were ameliorated by administration of exosomes.
Emerging evidence shows a protective effect of Nod1 in the central nervous system. A previous study demonstrated that Nod1 protects mice from infection by L. monocytogenes and inhibits intracellular bacterial growth in different cell types,including astrocytes and fibroblasts (Mosa et al., 2009). A recent study showed that activation of infiltrating dendritic cells may participate in the pathologic changes in experimental autoimmune encephalomyelitis via RIP2-, NOD1- and NOD2-mediated pathways (Shaw et al., 2011). Additionally, previous studies reported that there is no canonical activation of NLRP1, NLRP2, NLRP3 or NLRC4 inflammasomes, but only non-canonical activation of Nod1, Nod2 and TLR2 in human brain pericytes (Nyúl-Tóth et al., 2017). Thus, we chose to detect Nod1 expression in pericytes after treatment with the various drugs.
In our study, Nod1 in pericytes was markedly upregulated after exposure to IFN-γ + TNF-α + LPS + Lipo + ATP. Furthermore,the upregulation of pro-caspase 1 and the release of IL-1β inhibited the survival of pericytes. Our findings indicate for the first time that exosome treatment effectively inhibits pyroptosis in pericytes by inhibiting the Nod1 inflammasome.These findings suggest that BSCB integrity is improved by exosomes post-SCI by the inhibition of pericyte pyroptosis.
There are limitations to this study. Exosomes derived from BMSCs contain DNA, mRNA, miRNA, lncRNA, proteins and lipomolecules, which can be transferred and subsequently influence signaling pathways in recipient cells (Raposo and Stoorvogel, 2013). A recent study showed that exosomes,extracted from the supernatant of miR-21-modified MSCs,improved the survival of neurons and promoted functional recovery after SCI via the miR-21/PTEN/PDCD4 signaling pathway (Kang et al., 2019). Further study is needed to identify the exosomal components involved in regulating pericyte pyroptosis. However, it would have been highly informative for using exosomes derived from a non-BMSC source, for comparison. It is possible that the protective effects of the BMSC-derived exosomes could be mimicked by exosomes from another cell type, such as placental mesenchymal stem cells.
Our novel findings collectively suggest that BMSC-EXOs significantly promote the survival of pericytes by modulating NOD1-related signaling pathwaysin vitro. BMSC-EXOs effectively suppress pericytes pyroptosis and maintain BSCB integrity, thereby enhancing recovery following SCI. Thus,BMSC-EXOs may have therapeutic potential in neuroprotection in SCI.
Author contributions:Study design: LLW, YZ, YJJ; SCI model establishment and BBB score assessment: KMW, RRD; molecular biology: YBY, LJJ; data collection and statistical analysis: LLW, ZG; manuscript writing: ZG, JFT. All authors reviewed and approved the manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, No. U1604170 (to YJJ). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Zhengzhou University on March 16, 2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Inhibition of CXCR4/CXCL12 signaling: a translational perspective for Alzheimer’s disease treatment
- The new wave of p75 neurotrophin receptor targeted therapies
- The anatomical, electrophysiological and histological observations of muscle contraction units in rabbits:a new perspective on nerve injury and regeneration
- Inhibition of LncRNA Vof-16 expression promotes nerve regeneration and functional recovery after spinal cord injury
- Intranasal insulin ameliorates neurological impairment after intracerebral hemorrhage in mice
- Lycium barbarum extract promotes M2 polarization and reduces oligomeric amyloid-β-induced inflammatory reactions in microglial cells

