A creatinine-based model for predicting recurrent bleeding after modified percutaneous transhepatic variceal embolization in patients with cirrhosis
Kun Ji, Xin Li, Hanlong Zhu, Si Zhao, Pngchao Zhan, Yang Shi, Shuwn Y,Bingcan Xi, Yuyuan Zhang, Png Yu, Zhigang Rn, Juan Ding, Xinwi Han, Zhn Li,*
a Hepatobiliary and Pancreatic Interventional Treatment Center,Division of Hepatobiliary and Pancreatic Surgery,The First Affiliated Hospital,Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China
b Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
c Department of Gastroenterology and Hepatology, Jinling Hospital, Medical School of Nanjing University, Nanjing, 210002, Jiangsu Province, China
d Department of Gastroenterology, Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, 210008, Jiangsu Province, China
e Department of Infectious Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
f Department of Quality Control, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
Keywords:Esophagogastric varices Percutaneous transhepatic variceal embolization Nomogram Prediction
ABSTRACT
1. Introduction
Esophagogastric variceal bleeding (EVB) occurs in 25%–40% of patients with cirrhosis, with a mortality rate of 25–30%, representing a leading cause of death in patients with portal hypertension.1,2Previous reports have found that patients who survive the first hemorrhage are at a high risk of recurrent variceal bleeding,which is significantly associated with an increased risk of death.3,4Therefore, research focusing on the various risk factors for rebleeding is of great significance in identifying high-risk patients.
Currently, endoscopic therapy, including sclerotherapy and band ligation, is recommended as the standard of care for EVB. Transjugular intrahepatic portosystemic shunt (TIPS) has emerged as an effective modality in the secondary control of variceal bleeding.5However, complications of TIPS,including a high risk of stent dysfunction and hepatic encephalopathy, restrict its application in clinical practice.6In patients for whom endoscopic therapy was unsuccessful, modified percutaneous transhepatic variceal embolization(PTVE)with cyanoacrylate remains a rescue therapy to achieve hemostasis. To date, only a few studies have identified independent predictors of rebleeding after cessation of initial EVB in patients with cirrhosis treated with PTVE.7,8The Model for End-Stage Liver Disease score (MELD) and Child–Pugh classification have been widely recognized as factors predictive of rebleeding after initial EVB.9However, the Child–Pugh classification only divides patients into high-,intermediate-and low-risk groups and fails to quantify the expected probability of rebleeding, and the MELD calculation is too complicated to obtain the score easily.To the best of our knowledge,this study is the first attempt to generate a predictive nomogram for patients with cirrhosis and EVB treated with PTVE that can simply predict the specific probability of rebleeding.
The present study aimed to identify the risk factors and establish a model to predict 3-month rebleeding in patients with cirrhosis and EVB treated with modified PTVE as rescue treatment after failing endoscopic therapy. Additionally, patients at a high-risk for rebleeding were identified on the basis of the nomogram in order to help implement aggressive treatments early and formulate intensive follow-up plans.
2. Materials and methods
2.1. Patients
Patients who were diagnosed with EVB and had undergone modified PTVE in our hospital between January 2015 and November 2020 were enrolled in this study. The inclusion criteria were as follows: (1) diagnosis of liver cirrhosis by clinical examination and imaging techniques,including ultrasound,CT,or MRI;(2)gastroscopic confirmation that the esophageal varices were the only potential source of bleeding;(3)PTVE conducted as rescue therapy in patients with uncontrolled severe or recurrent variceal bleeding after pharmacological and endoscopic therapy, such as sclerotherapy and band ligation failed; and (4) patients without catheterizable gastrorenal shunts who could not be treated with balloon-occluded retrograde transvenous obliteration. The exclusion criteria were as follows:(1)severe hypertension,coronary heart disease,cardiopulmonary insufficiency, or chronic renal insufficiency; (2) a history of TIPS creation, pericardial devascularization, or splenectomy for varices;(3)death during current hospitalization;and(4)incomplete data in medical records or follow-up.The flowchart of the enrolled patients is shown in Fig.1.This study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.Informed consent was obtained from all the participants.

Fig. 1. Flow diagram of the patient selection criteria.
2.2. Treatment
On admission,all patients were treated with an infusion of vasoactive drugs for 72 h,prophylactic antibiotics for 7 days,and endoscopy within the first 24 h of hospitalization. The enrolled patients refused or were unable to undergo TIPS because of the high cost of TIPS, fear of complications,concomitant hepatocellular carcinoma(HCC),and cavernous transformation of the portal vein. Therefore, PTVE with or without partial splenic embolization (PSE) was performed as rescue therapy in patients who had unsuccessful pharmacological and endoscopic treatment.All procedures were conducted by two interventional radiologists with 15 and 25 years of experience.
After a percutaneous transhepatic puncture of a branch of the portal vein under digital subtraction angiographic guidance (Artis zeego,Siemens,Munich,Germany),a 5-F Cobra catheter(Cook Medical, Bloomington, Indiana, USA) was introduced into the portal venous system,and splenoportography was performed to evaluate the location of the index varices,feeding vessels,draining veins,and possible presence of a gastrorenal shunt. The catheter was advanced into each of the main feeding vessels(e.g.,the left gastric vein,short gastric vein,or coronary gastric vein)to embolize the vascular trunk with coils(3–10 mm×5–12 cm; Cook Medical) or microcoils (2–3 mm × 2–3 cm; Cook Medical).Subsequently,cyanoacrylate was slowly injected to occlude the vascular bed until angiography confirmed that the blood flow in the varices was completely obstructed. Splenoportography was repeated to assess the extent of the variceal obliteration.If other feeding veins were available,the above steps were repeated until blood flow in the varices ceased completely. Ultimately, the catheter was withdrawn and the puncture tract was embolized with microcoils.
PSE was performed 5–7 days after PTVE in patients who were willing to undergo the procedure. 63 patients refused PSE after PTVE because they were worried about the postoperative complications of PSE.Briefly,splenic arterial angiography was performed using a transfemoral approach with a 5-F RH catheter (Cook Medical) to demonstrate the distribution of the splenic arteries. The catheter was inserted into the middle and lower branches of the splenic arteries, and 350–560 μm polyvinyl alcohol particles(ALICON Pharm SCI&TECH CO.,Hangzhou,China)mixed with contrast media were carefully injected into the splenic arteries through the catheter.Splenic arterial angiography was repeated to estimate the degree of splenic infarction in our cohort, which was found to be within 50–70%of the original splenic volume.10
Treatment after PTVE and PSE included the use of analgesics and antipyretic drugs, blood transfusion, and systemic prophylaxis with antibiotic agents for at least 7 days.
2.3. Data collection and follow up
All patients underwent a complete medical assessment at admission,including collection of demographic information, medical history,physical examination, clinical symptoms, ascites, concomitant HCC, degree of splenomegaly, encephalopathy, and portal vein thrombosis.Blood samples were also collected for laboratory testing[complete blood count, international normalized ratio (INR), total bilirubin, albumin,aspartate aminotransferase (AST), and serum creatinine]. A history of variceal bleeding (VB) indicated an earlier episode of VB before hospitalization. Prior history of endoscopic therapy was defined as that performed before the current hospitalization due to a previous episode of EVB. The Child–Pugh and MELD scores11were calculated from data recorded upon patient admission.
Rebleeding was defined according to the Baveno criteria12as recurrent hemorrhage proven by new melena or hematemesis,requirement for> 2 units of packed red blood cells in a 24 h period and hemodynamic instability after a 24 h period of stable vital signs and hemoglobin after PTVE.Time to rebleeding was defined as the time from the eradication of variceal hemorrhage to recurrent bleeding. Patients who experienced recurrent bleeding within 3 months after PTVE were classified into the rebleeding group, and the remaining patients into the non-rebleeding group. The patients were followed up in the outpatient clinic or by telephone at 1, 3, 6, and 12 months or until death, loss to follow-up, or the cut-off date for data analysis(February 2021).
2.4. Nomogram construction and validation
Univariate and multivariate logistic regression analyses were conducted to identify the independent risk factors that significantly affected rebleeding.Based on the identified independent risk factors,a nomogram was constructed to predict the probability of rebleeding within 3 months after PTVE.
The nomogram performance was evaluated using the concordance index (C-index), receiver operating characteristic (ROC) curve, calibration curve,and decision curve analysis(DCA).The C-index is defined as the proportion of all evaluable patient pairs whose predictions are consistent with actual results.13The nomogram was subjected to bootstrapping validation(1,000 bootstrap resamples)to compute a relatively corrected C-index.14The nomogram's discrimination and clinical application values were measured using the area under the ROC curve(AUC)and DCA15to verify whether the nomogram was superior to the MELD and Child–Pugh models. The calibration of the nomogram was assessed using a calibration curve that compared the nomogram-predicted and actually observed estimates of rebleeding probability. A Kaplan–Meier(K–M) curve was constructed to analyze the difference in rebleeding between the high-and low-risk groups based on the nomogram.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software (version 21,IBM Corporation,Armonk,NY,USA)and the programming language R(version 3.6.2)for Windows.The Student's t-test and Chi-square test for continuous and categorical variables were used to evaluate the association between rebleeding and variables by comparing the rebleeding and non-rebleeding groups.Subsequently,the variables with a P-value below 0.05 in univariate analysis were selected for multivariate logistic regression analysis to ascertain the independent risk factors for rebleeding using the forward stepwise selection method. The optimized cutoff values for equally important sensitivity and specificity of INR,creatinine,aspartate aminotransferase, albumin, bilirubin, and the total scores calculated from the nomogram were determined using the ROC curve. The nomogram, CC-index, ROC curve, calibration curve, DCA curve and K–M curve were generated in R with packages“rms,”“Hmisc,”“ROCR,” “Survminer,” “survival,” and “rmda”. Statistical significance was set at P <0.05 in a two-sided test.
3. Results
3.1. Patient characteristics
Among the 122 patients, more than two-thirds had viral cirrhosis.HCC and portal vein thrombosis were present in 22.1%and 15.6%of the patients,respectively,and PSE was carried out in 48.4%of the patients.Hematemesis, melena, or a combination of both were the main clinical symptoms of hemorrhage in all cases. There were pure esophageal varices in 76 patients, type 1 gastroesophageal varices in 34 patients,type 2 gastroesophageal varices in 10 patients, and isolated gastric varices type 1 in 2 patients with no isolated gastric varices type 2.Of the patients enrolled in whom the endoscopic treatment was unsuccessful,47(38.5%) received endoscopic sclerotherapy and 75 (61.5%) received endoscopic band ligation,which resulted in 16(13.1%)and 106(86.9%)patients having uncontrolled and recurrent EVB, respectively. The time interval between the endoscopic therapy and rebleeding was 4 (1–8)[Median(range)]days.There were 32 and 90 patients in the rebleeding and non-rebleeding groups,respectively.The median follow-up time was 6.7 months (3–12 months). More details on patient characteristics are shown in Table 1.
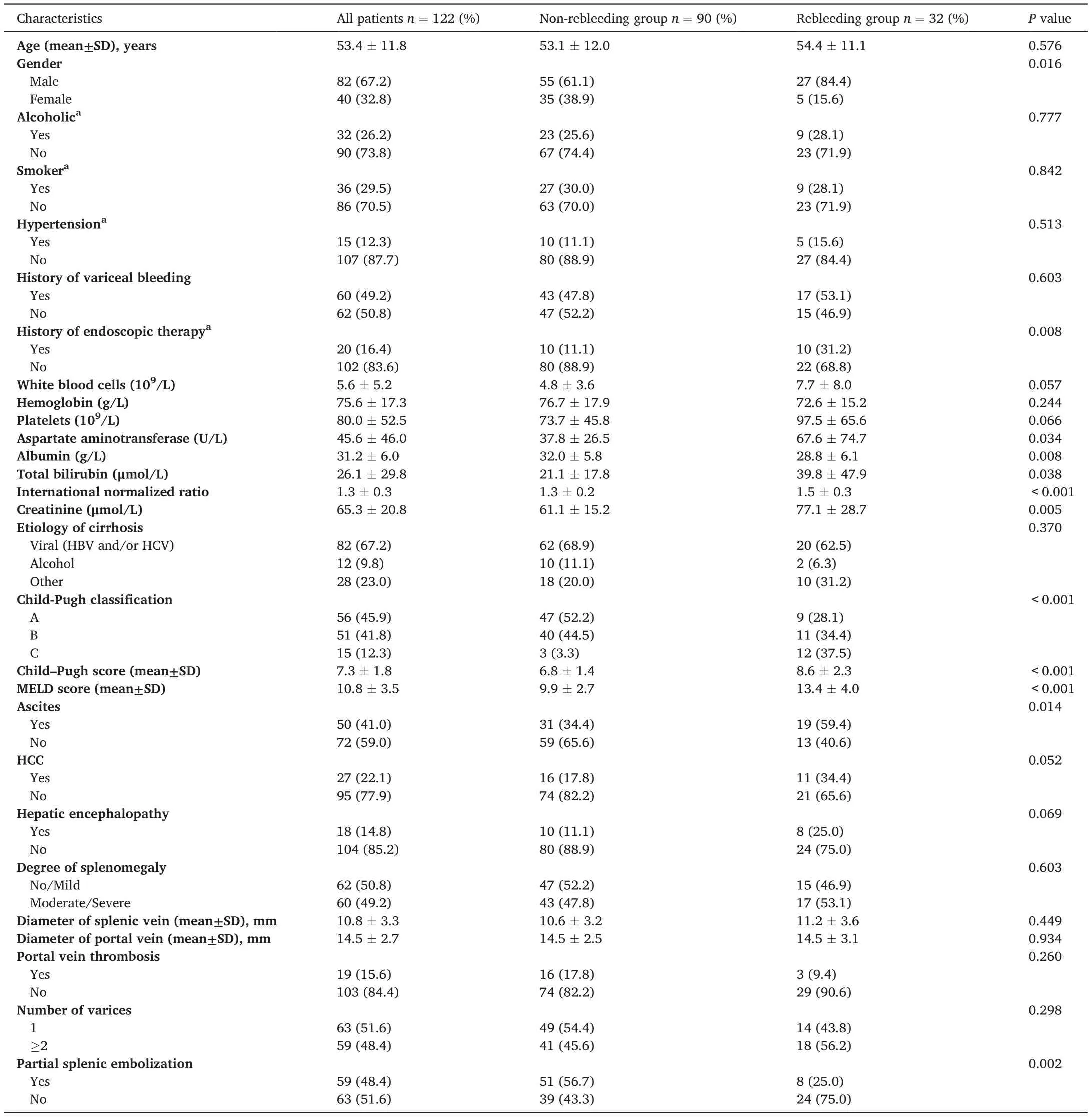
Table 1 Clinical and biological characteristics of patients on admission.
The mean number of coils and volume of cyanoacrylate used in PTVE were 3.8 ± 1.2 (range, 1–7) and 6.2 ± 1.9 mL (range, 4–15 mL),respectively. A total of 32 (26.2%) patients experienced adverse effects following PTVE or PSE including transient upper abdominal pain (n =23), low-grade fever (n = 6), and both transient upper abdominal pain and low-grade fever(n = 3), which were minor and alleviated by pharmacologic therapy.
3.2. Risk factors for rebleeding
Rebleeding occurred in 32 patients(26.2%)within the first 3 months after cessation of EVB. Recurrent bleeding was controlled by further pharmacological treatment (n = 6), endoscopic therapy (n = 13), and TIPS(n=4),while the remaining 9 patients died of massive bleeding(n=7)and hepatic or multiorgan failure(n=2).The aim of our study was to develop a nomogram to predict the probability of short-term rebleeding; therefore, we only focused on patient outcomes up to 12 months after PTVE. The cumulative probability of the presence of variceal rebleeding at 1,2,6,and 12 months was 9.0%,21.3%,37.4%,and 48.6%,respectively.As revealed by the univariable analysis,12 variables(sex,prior history of endoscopic therapy,INR,creatinine,AST,albumin,bilirubin, ascites, PSE, MELD score, Child–Pugh score, and Child–Pugh classification)were markedly correlated with the risk of rebleeding(all p< 0.05). Notably, the Child–Pugh classification and MELD were not included in the multivariate analysis. Child–Pugh classification was dependent on the Child–Pugh score. Both Child–Pugh score and creatinine levels were included in the multivariate analysis, and the most important difference between MELD and Child–Pugh score is that MELD incorporates creatinine.Therefore,MELD is similar to Child–Pugh score and creatinine together.In multivariable logistic analysis,a prior history of endoscopic therapy(OR=4.125;95%CI:1.208–14.084;P=0.024),Child–Pugh score(OR=1.792;95%CI:1.332–2.411;P <0.001),non-PSE(OR=0.258;95%CI:0.085–0.777;P=0.016),and a creatinine ≥78 μmol/L (OR = 7.960; 95% CI: 2.492–25.425; P <0.001) were independent risk factors for rebleeding within 3 months(Table 2).
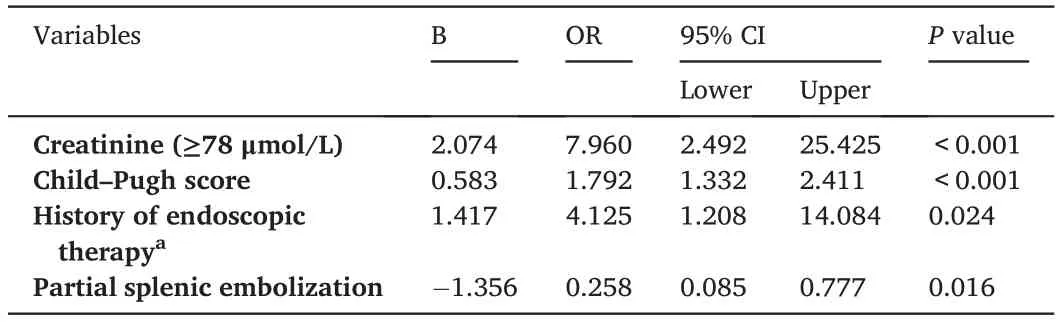
Table 2 Independent risk factors associated with rebleeding as revealed by logistic regression analysis.
3.3. Development and validation of the nomogram
A nomogram was developed based on independent predictors(Fig.2).In the nomogram,each category of variables was assigned a score on the point scale. The sum of these scores is located on the total points scale,and a line is drawn downward to determine the specific probability of 3-month rebleeding. The score assignments for the variables included in the nomogram are summarized in Table 3.
The nomogram's C-index was 0.85(95%CI:0.76–0.94)for the cohort and was confirmed to be 0.83 through bootstrapping validation. Meanwhile,the calibration curve for the probability of rebleeding at 3 monthsexhibited excellent agreement between the actual and predicted outcomes(Fig.3A).The nomogram had a higher AUC(0.850 vs.0.723 and 0.767) and better clinical applicability than the Child–Pugh and MELD models (Fig. 3B and C). In addition, the patients were divided into two sets depending on the nomogram scores: low-risk group (0–95 points)and high-risk group (96–219 points). As displayed in the K–M curves,high-risk patients were more likely to experience rebleeding(P<0.001).(Fig.3D).

Table 3 Score assignment for variables included in the nomogram.
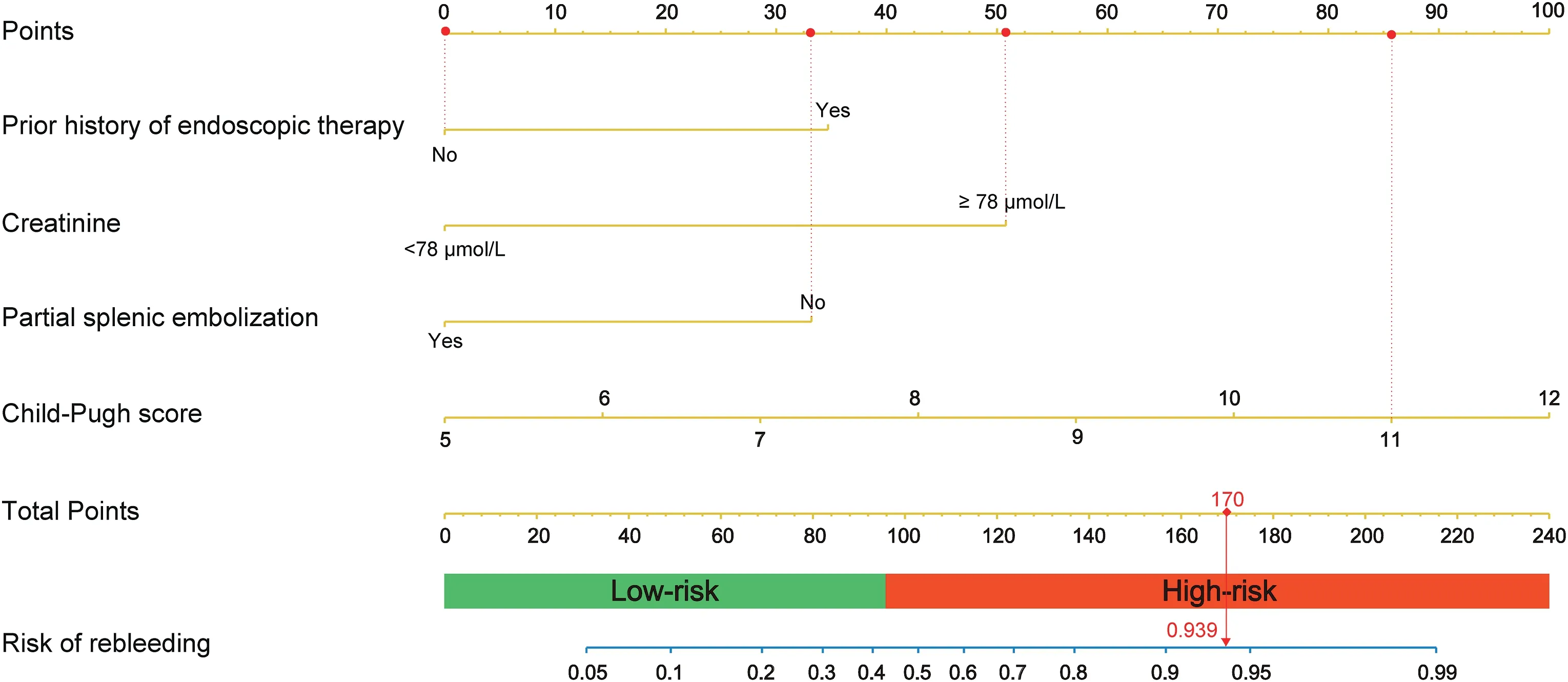
Fig. 2. Nomogram for predicting rebleeding of patients with esophagogastric varices and an example on how to use the nomogram. Each category of the prognostic variables is assigned a score on the Points scale. The sum of these scores is located on the Total points scale and a line is drawn downward to determine the specific probability of 3-month rebleeding.

Fig.3. The validation of the nomogram.(A)calibration curve;(B)ROC curves of the nomogram,MELD and Child-Pugh models;(C)DCA of the nomogram,MELD and Child-Pugh models; and (D) Kaplan-Meier curves of the low-risk and high-risk patients.ROC: receiver operating characteristic curve; AUC: areas under the ROC curve; MELD: the Model for End-stage Liver Disease; DCA: decision curve analysis; PTVE:percutaneous transhepatic variceal embolization.
3.4. Development of webserver
To facilitate clinical use of the nomogram, an online version is provided at https://jikun.shinyapps.io/rebleeding_of_ev(Fig.4), which can not only predict personalized rebleeding but also prevent manual measurement errors.
4. Discussion
Conventional PTVE was introduced in the 1970s for the treatment of EVB, yet it has not attracted sufficient attention because of its high rebleeding rate.However,modified PTVE with cyanoacrylate has started a new chapter in the management of EVB,since it has a higher degree of hemostasis and lower rebleeding rate due to a more extensive obliteration area and permanent embolization of variceal veins as opposed to conventional PTVE.16,17A study that compared the modified PTVE and TIPS revealed that variceal rebleeding rates were comparable(30.2%in TIPS group vs.20.8%in PTVE group),and PTVE had a lower incidence of encephalopathy than TIPS18. A similar result was reported by Zhang et al.16
In the present study, 122 patients with EVB who had unsuccessful endoscopic therapy underwent modified PTVE with cyanoacrylate as rescue therapy. Because variceal embolization of PTVE only occludes portosystemic shunts but fails to eliminate cirrhosis,new esophagogastric varices arise as a result of worsening portal hypertension.19Our study showed that 32 patients (26.2%) developed variceal rebleeding within 3 months after the cessation of the initial hemorrhage,which is similar to the 6-week rebleeding rate of 20.8%after PTVE reported by Zhao et al.7
Most previous studies regarded 6 weeks as an interval for rebleeding according to the Baveno criteria and excluded seriously ill patients with advanced cirrhosis, HCC, and portal thrombosis.12,20In our study, a period of 3 months for rebleeding was chosen in view of studies evaluating the long-term efficacy of PTVE. Furthermore, the average waiting time on a list for liver transplantation is approximately 3 months.21Risk factors, such as advanced cirrhosis, HCC, and portal thrombosis, may result in a continuous increase in portal hypertension, making patients more susceptible to early rebleeding.22Therefore,we analyzed a group of unselected patients with cirrhosis to quantify the risk of rebleeding and help identify patients who may need early TIPS or liver transplantation and an intensive follow-up plan.
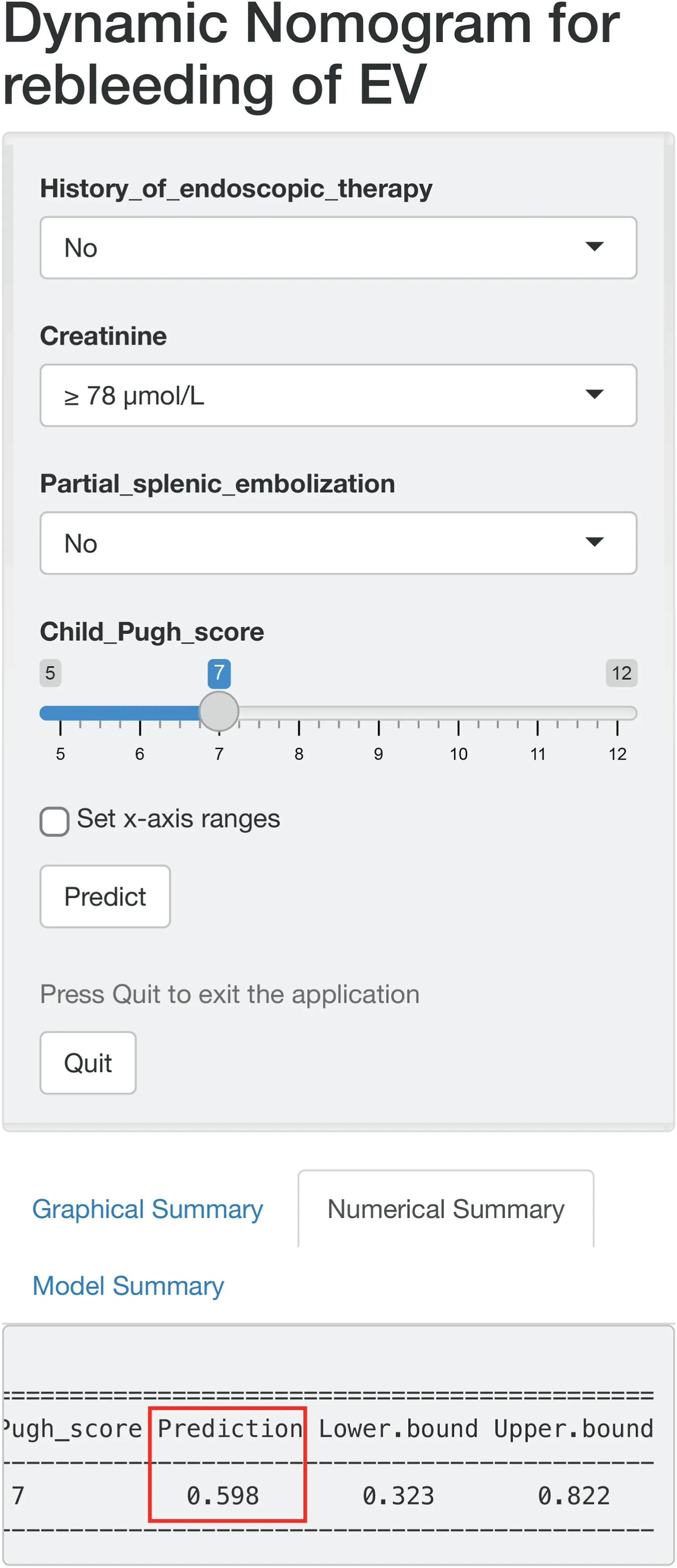
Fig. 4. Layout of an online version of the developed nomogram (https://jikun.shinyapps.io/rebleeding_of_ev).
Our findings revealed that rebleeding was noticeably related to a prior history of endoscopic therapy, Child–Pugh score, PSE, and creatinine,which is consistent with previous reports.3,17The Child–Pugh classification only stratifies risk as high, intermediate, and low levels;therefore, we selected it to quantify the expected probability of rebleeding.The Child–Pugh score is a vital factor reflecting the severity of liver disease in patients with cirrhosis,with every 1-point increase in the Child–Pugh score conferring a 79% risk increase of rebleeding at 3 months in our study.The present study also revealed that patients with a history of endoscopic therapy were more likely to experience rebleeding within 3 months after PTVE. Coincidentally, a retrospective study conducted by Lee et al.23found that 58.3% of patients with cirrhosis had early recurrent hemorrhage after banding ligation as well. This relationship could be explained by the extensive mucosal injury surface area and post-banding ulcers caused by the endoscopic procedure.23This is to be expected since a patient with a prior history of endoscopic therapy,as a sign of previous EVB,would be at a higher risk of repeated bleeding.
Acute renal failure is a severe complication of cirrhosis and a possible harbinger of death.24Serum creatinine is a sensitive marker of renal function, and therefore is a key component of the MELD model. As exhibited in our study, a creatinine level of ≥78 μmol/L was an independent predictor of rebleeding.Augustin et al.proposed creatinine as a crucial marker to identify patients at a high risk of acute variceal hemorrhage.25There are reports that 24%–55% of patients with end-stage renal disease have increased bleeding complications attributed to the inhibitory effect of uremia on platelet function.26It has been demonstrated that PSE appears to be efficient in reducing episodes of variceal bleeding by reducing the increased portal pressure,as well as improving leukocytopenia and thrombocytopenia caused by hypersplenism.10,27In accordance with a previously conducted controlled study,8our findings indicated that rebleeding occurred more frequently in those treated only with PTVE than in those treated with a combination of PSE and PTVE(38.1%vs.13.6%,p<0.05),and no severe complications were observed because we achieved a limited embolization of part of the middle or lower pole of the spleen, and a limited ratio of 50–70% of the original splenic volume. A meta-analysis also demonstrated the dramatic superiority of PSE in preventing recurrent variceal hemorrhage and prolonging the overall survival.28
To date,only a few studies have identified the value of risk factors in predicting rebleeding after cessation of initial EVB in patients with cirrhosis treated with PTVE.7,8However,these studies failed to establish a simple model that could conveniently and accurately predict the probability of rebleeding after PTVE.To the best of our knowledge,this study is the first attempt to develop a predictive nomogram based on unselected patients with cirrhosis and EVB treated with PTVE.Although the MELD model has been proven to be superior to the Child–Pugh model as an index of liver disease severity,11the MELD calculation is too complex for obtaining a score in clinical practice.Therefore,we did not take it into account for the nomogram construction. The nomogram exhibited an accurate prediction for rebleeding with a high C-index of 0.85, and had superior predictive accuracy and clinical applicability to the Child–Pugh and MELD models.
One concern worth highlighting is that our proposed nomogram could be used for the early identification of patients with EVB who are at a high risk of rebleeding.The rational therapeutic approach should adapt to the different expected risks of rebleeding for each patient. In other words, more aggressive treatments should be administered early for high-risk patients, and unnecessary procedures should be reduced for low-risk patients.As presented in the K–M curves,there was a significant correlation between high-risk patients and the increased probability of rebleeding.We recommend that a high-risk patient can be a candidate for early TIPS or liver transplantation and an active follow-up plan.29
The limitations of the present study include its retrospective nature and single-center design.Additionally,hepatic venous pressure gradient(HVPG)greater than 20 mmHg has been previously demonstrated to be predictive of rebleeding,30whereas HVPG was not available in our study because direct measurement of portal hypertension is invasive and inconvenient. Fortunately, Child–Pugh classification could be regarded as an alternative factor because an HVPG above 20 mm Hg is equivalent to Child–Pugh grade C in more than 85% of patients.30The third limitation is the lack of a large cohort of patients from other institutions to verify our nomogram. Finally, the sample size was insufficient to a certain degree. This was an exploratory study, and our nomogram achieved excellent prediction ability with a high C-index of 0.85.Although the model requires continued refinement and improvement, its current form may be useful for assisting clinicians in identifying high-risk patients, selecting optimal treatment protocols, and making clinical decisions and follow-up strategies.
5. Conclusions
In conclusion,we developed and validated a creatinine-based model to predict the 3-month rebleeding probability in unselected patients with cirrhosis with EVB after modified PTVE.Compared with Child–Pugh and MELD scores, our nomogram showed better prediction ability and clinical applicability.Risk stratification may help identify high-risk patients and lead to earlier treatments via TIPS or liver transplantations,as well as implementation of intensive follow-up plans.
Funding
This study was supported by the Key Scientific Research Project of Colleges and Universities in Henan Province, China (grant number 17A320011).
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors would like to thank all participants who were enrolled in this study.
 Journal of Interventional Medicine2022年2期
Journal of Interventional Medicine2022年2期
- Journal of Interventional Medicine的其它文章
- Onyx embolization of a spinal epidural hemorrhage caused by thoracic spinal epidural arteriovenous fistula: A case report and literature review
- Intravascular treatment for abnormal catheter positioning of port-a-cath system in the subclavian vein: A single-center study
- Irreversible electroporation versus radiofrequency ablation for malignant hepatic tumor: A prospective single-center double-arm trial
- Safety and efficacy of transcatheter arterial embolization for management of refractory hematuria of prostatic origin
- Intrahepatic flow diversion prior to segmental Yttrium-90 radioembolization for challenging tumor vasculature
- Preparation and investigation of a novel iodine-based visible polyvinyl alcohol embolization material
