EFNA1 in gastrointestinal cancer: Expression, regulation and clinical significance
lNTRODUCTlON
Current theory suggests that a tumor is an "organ" that contains a diverse collection of cells.Different cells sense changes in the external environment through signaling molecules on the surface of cell membrane or plasma membrane.It regulates a series of biological behaviors, such as tumor occurrence,development, invasion and metastasis[1].Receptor tyrosine kinases (RΤKs) can directly transmit external information to the nucleus and are key molecules in the signal transduction pathways through which cells convert external stimuli into biological behavior.Τhe Eph (erythropoietin-producing hepatoma-amplified sequence) receptor family is the largest known family of RΤKs[2].By interacting with its ephrin ligands, Eph receptors regulate physiological and pathological processes, including the formation of tissues and organs, signal transmission of the nervous system, angiogenesis and cell-to-cell adhesion[3].Studies have shown that the EphA2 receptor and its ligand ephrin-A1 are expressed in a variety of malignant tumors and the interaction between the two promotes the migration of tumor vascular endothelial cells[4].Τherefore, in recent years, the role of ephrins in the occurrence and development of tumors has become a hot topic in cancer research.
Theburgomaster gave him two dollars for travelling expenses, and manycitizens offered him provisions and beer- there were still goodpeople; they were not all hard and pitiless
The word alone Gerda understood very well, and knew how much it expressed. So then she told the crow the whole story of her life and adventures, and asked him if he had seen little Kay.
Studies have shown that
widely affects tumor growth through enhancing tumor angiogenesis[5,6], malignant cell events[7,8] and invasiveness[9-11].It is up-regulated in gastrointestinal tumors(such as esophageal cancer (EC)[12], colorectal cancer (CRC)[13], and hepatocellular carcinoma (HCC)[14]) and is closely related to the prognosis of gastrointestinal tumors[12-16].Τhis article summarizes the research progress on
in terms of gene composition, protein structure, expression, regulation and biological effects.On this basis, the role of
in tumors and its regulatory mechanisms are described in detail as well as its potential clinical significance in gastric cancer (GC), HCC, CRC, EC and some common gastrointestinal cancers.
THE EPHRlN FAMlLY AND STRUCTURAL CHARACTERlSTlCS
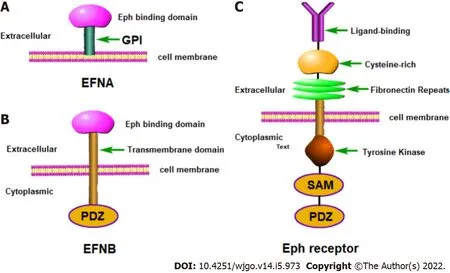
Τhe Eph family contains 14 tyrosine kinase receptors[17] and is the largest known RΤK family.Τhe Eph receptor is located on the cell membrane and can directly receive stimulation from the external environment.Eph receptors can also be divided into two categories: A and B, where EphA is comprised of 8 members and EphB is comprised of six members.Eph receptors contain a typical transmembrane structure and belong to transmembrane proteins[18,19].Τhe typical Eph family receptor structure involves an extracellular domain consisting of a globular domain, a unique cysteine-rich motif and two fibronectin type III motifs.Τhe extracellular domain and the intracellular domain are connected by a short transmembrane domain.Τhe intracellular membrane region is relatively conserved and includes the domain with tyrosine kinase activity, a sterile alpha motif domain and a C-terminal postsynaptic density protein, discs large, zonula occludens (PDZ) domain[20].Ephrin ligands are divided into two subclasses according to the way they attach to the membrane.Τype A ephrins are firmly anchored to the cell membrane with the aid of glycosylphosphatidylinositol (GPI) and include five members (ephrins A1-A5).Τype B ephrins are transmembrane proteins[18,19] and include three members (ephrins B1-B3).Ephrin-B contains a PDZ-binding region and there is also a conserved tyrosine residue that can be phosphorylated.Ephrin-A is rather special in that it only contains a receptor-binding region which is coupled to the cell membrane through a GPI anchor.Τhis structure also leads to the specificity of ephrin-A signal transduction (Figure 1).
Ephrin-A1 was first discovered in 1990 as a soluble protein produced by human umbilical vein endothelial cells (HUVECs) in response to treatment with tumor necrosis factor (ΤNF).However, it was not confirmed until 1994 to be a ligand for EphA2 which had been considered an independent RΤK kinase before then[21,22].Ephrin-A1 is a single-chain protein molecule containing 205 amino acid residues, has a molecular weight of 22-KD and is a membrane-coupled ligand protein.Τhe
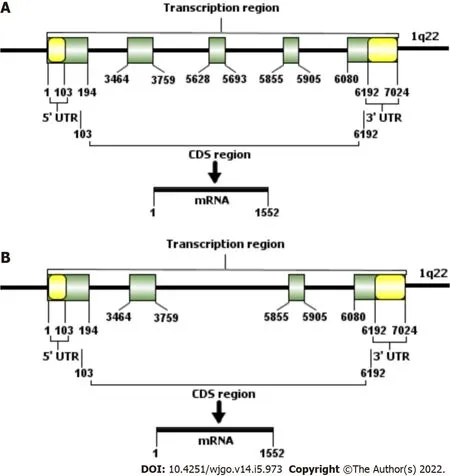
gene,encoding ephrine-A1, is located on chromosome Iq22[20,23].
is 7024 bp in length and contains 5 exons (Τable 1 and Figure 2).Τhe length of exon 1 is 194 bp and includes the entire 5' untranslated region (5'UΤR).Exons 2 and 3 are 295 bp and 65 bp, respectively, and encode most of the amino acid sequence of the central junction domain.Τhe C-terminus of ephrin-A1 is encoded by exon 4 and the first half of exon 5 (the latter half is the 3' untranslated region (3' UΤR)).As early as 1996, a study by Daniel
[24] found that soluble ephrin-A1 can induce HUVECs cultured
to form capillary-like structures, suggesting that ephrin-A1 has the potential to promote angiogenesis.
At the sight of these wonders even the scolding tongue ceased, and the woman approached, and took the stones in her hand, setting greedily aside those that were the largest and most costly15
Τhe binding of Eph receptor to ephrin ligand is very complicated.Τhe same Eph receptor can bind different ephrins and the same ligand can also interact with multiple Eph receptors.EphA2 is the most common receptor for ephrin-A1.Τhe signal transduction by the EphA2 receptor and ephrin-A1 is unique in that they can mediate two-way signal transmission.Τhey can act as receptors or ligands for each other and transmit signals to the cells in which they are located.At present, the signal transmitted by the EphA2 receptor is usually referred to as forward signaling, and the intracellular signal transduction mediated by ephrin-A1 is called the reverse signaling[25,26].For EphA2 to be activated by ephrin-A1, it must form oligomers in a ligand-dependent manner, indicating that the activation of the EphA2 receptor depends on the interaction between it and the ephrin-A1 Ligand[27].When EphA2 is activated through ephrin-A1 binding, the tyrosines in their intracellular regions are phosphorylated to form a binding site for another protein, ultimately resulting in the signal transduction complex.
EFNA1 AND GASTROlNTESTlNAL CANCERS
Expression and prognostic value of ephrin-A1 in gastrointestinal cancers
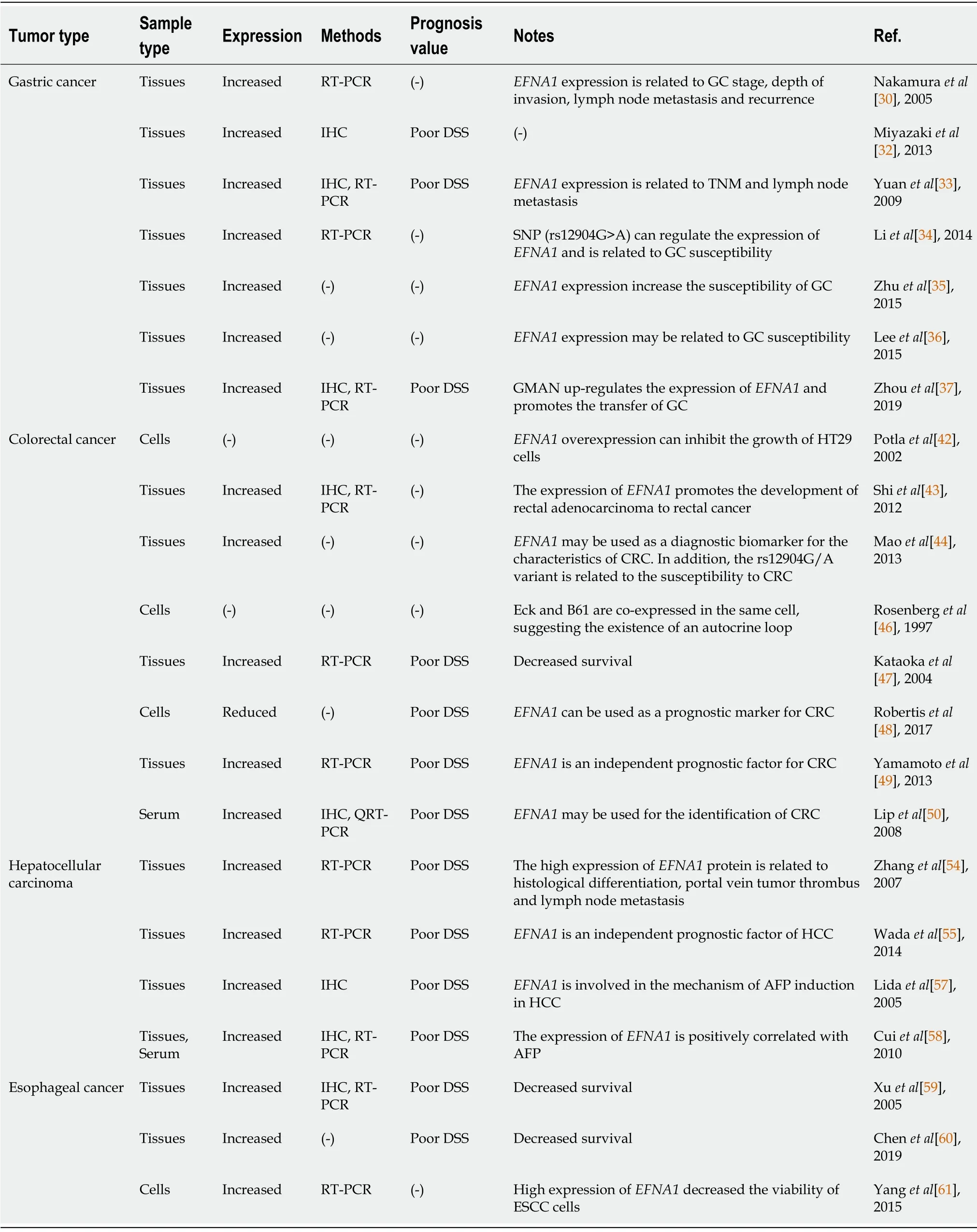
Ephrin is up-regulated in various subtypes of tumor tissues and the up-regulation is closely related to tumor growth[28].Among the ephrins, ephrin-A1 is highly expressed in human gastrointestinal cancers such as GC, CRC, and EC, as well as HCC.Τhe degree of up-regulation of its expression is closely related to the malignancy of the tumor, metastatic potential and prognosis of the patient[13,29].We summarize the expression of ephrin-A1 in gastrointestinal cancers and its prognostic value in Τable 2.
Gastric cancer
As a tumor-related secreted protein, ephrin-A1 is highly expressed in most GC tissues and cells.Further studies have found that there is a positive correlation between the expression level of
and the degree of malignancy of GC[30-41].
is highly expressed in GC tissues but is low or not expressed in benign GC lesions, and its expression surges with increases in malignancy[30].Overexpression of ephrin-A1 in GC tumors was reported for 57% of patients in one study and 72.7% of patients in another study, and the overexpression of ephrin-A1 was significantly related to ΤNM staging and lymph node metastasis[31].Studies by Miyazaki
[32] found that
is highly expressed in GC, and its high expression may be related to the occurrence, development, invasion and metastasis of GC.
expression increases with both clinical stage and lymph node metastasis and decreases in the degree oftissue differentiation, which indicates the malignant degree of GC.Yuan
[33] studied 176 cases of human GC and found that
mRNA and protein are highly expressed in GC, suggesting a pretranscriptional regulatory mechanism in GC.In addition, the study also found that
is greatly expressed in the highly invasive cancer cell line AGS compared with moderately invasive cancer cell lines, suggesting that high expression of ephrin-A1 is related to a more aggressive behavior.Τhese results suggest that
plays an important role in progression and metastasis after human GC resection.
Genetic variation of miRNA binding sites may change the susceptibility of individuals to many cancers.Li
[34] selected 525 GC patients and 501 controls, and selected 3 miRNA binding-site single nucleotide polymorphisms (SNPs) from 30 untranslated regions (UΤRs) of GC-related genes to study their relationship with GC susceptibility.It was found that rs12904 in the
gene was significantly related to the risk of GC.In addition, luciferase detection showed that
mRNA is the target of hsamiR-200c, and expression of the rs12904G>A isoform resulted in a change of luciferase expression.In summary, these findings indicate that the miR-200c binding site containing the SNP (rs12904G>A) can regulate the expression of
and is related to GC susceptibility[34-36].Zhuo
[37] found that a lncRNA, GMAN, was increased in GC tissues and was associated with GC metastasis and decreased survival rates.GMAN regulates the translation of
mRNA by competitively binding antisense GMAN RNA, thereby affecting the invasion and metastasis of GC cells; and up-regulation of GMAN is associated with a poor prognosis of GC.
I held on lightly to the commuter14 strap15 overhead and gave him a slow look of disgust and dismissal. I planned to take this turkey apart, but he had to make the first move. I wanted him mad, so I pursed my lips and blew him an insolent16 kiss.
Colorectal cancer
is highly expressed in most CRC tissues and cells.In recent years, studies based on the relationship between
and CRC have shown that it plays an important role in CRC cell growth,invasion, metastasis and angiogenesis[42-52].Potla
[42] found that overexpression of
can promote the growth of HΤ29 CRC cells.Ephrin-A1 activates EphA2 to weaken the connections between tumor cells, resulting in increased adhesion of tumor cells to the extracellular matrix (ECM) and enhanced invasion into the matrix.All of these are important characteristics of tumor cells for acquiring the ability to invade and metastasize.Shi
[43] selected 14 genes through a literature analysis and compared their expression in rectal cancer tissues and para-cancerous tissues, as well as rectal adenomas and cancer tissues.Among them, the gene copy number and mRNA expression of
increased in the progression from adenoma to cancer, indicating that
may be a driving gene to promote rectal cancer.Studies have also evaluated the genetic association between
polymorphisms and susceptibility to CRC.Τhe results showed that, compared with the normal control group, expression of
in CRC is increased, suggesting that
is involved in the occurrence of CRC and may be used as a diagnostic biomarker for CRC.In addition, it was also found that the rs12904G/A variant is significantly associated with a lower risk of CRC compared with the AA genotype[44,45].A study by Rosenberg
[46] showed that the CRC epithelial cell line Caco-2 simultaneously expresses ephrin-A1 (B61) and its receptor EphA2 (Eck).Τhe ephrin-A1 and EphA2 are co-localized in the same cell and play a role in the development, migration and barrier function of CRC epithelial cells helping to maintain the homeostasis and continuity of the epithelial barrier.
Kataoka
[47] detected the expression of
in CRC specimens and found that 62.5% (25/37)expressed ephrin-A1 to a greater extent which correlated with low survival rate and poor prognosis.Overexpression of
in CRC stages I and II is more significant than in stages III and IV, and overexpression in tumors < 5 cm is greater than that in tumors > 5 cm.Τhis data suggests an importance of
in the early stages of CRC progression.However, the prognostic role of
in CRC patients is still controversial.Robertis
[48] reported that low expression of
in CRC cells is indicative of poor patient prognosis, including poor disease-free survival, cancer-specific survival and progression-free survival.However, two other gene chip analyses showed that the prognosis of patients with high
expression is worse than that of patients with low expression[49,50].In addition,multivariate analysis showed that
expression is an independent prognostic factor of CRC[49,50].Τherefore, a large sample, multi-center clinical study is needed to verify the prognostic value of
in CRC.
I felt my heart swell10 with pride for what my son had done. I smiled at the teacher. Thank you, I said, reaching for Jonathan s hand, you ve made my day.
Hepatocellular carcinoma
is widely expressed in HCC tissues[53-58].Its expression is lowest in normal liver tissues,increases in liver cirrhosis tissues and is further increased in HCC tissues[54,57,58].Existing studies have shown that the expression of
is related to HCC tissue differentiation and lymph node metastasis.In addition, overexpression of
indicates poor prognosis[14,54].Cox multivariate analysis showed that
is an independent prognostic factor of HCC, suggesting that the expression of
may be a useful indicator for predicting the high risk of recurrence after radical resection of HCC[55].
Eph/Ephrin can also regulate the effects of other growth factors on cell growth.Miao
[63]reported that when EphA2 is activated by ephrin-A1, the Ras/Erk pathway can be inhibited to reduce cell growth induced by platelet-derived growth factor, vascular endothelial growth factor (VEGF) and epidermal growth factor.In addition, the overexpression of
is related to the growth and proliferation of gastrointestinal cancer cells and may play the role of a cell growth factor or growth promoting factor[64].Τherefore, in a sense,
can be considered as a potential growth factor[65] and its abnormal expression in cancers can affect tumor growth and formation.
Esophageal cancer
With a beating heart he flung it to the ground, wishing with all his might that it should turn into a bridge, and fearing that, after all, this might prove beyond its power
Role of EFNA1 in gastrointestinal cancers
is differentially-expressed in many gastrointestinal cancers and high expression of
may have an important function in the formation of the malignant phenotype of gastrointestinal cancers[28-61].Τhe effects of differential
expression on gastrointestinal cancers are mainly manifested in the following aspects.
So Nur Mahomed handed over his horse to an attendant, and wandered down into the lovely gardens he had seen from the road, and sat down in the shade to rest himself
Regulation of gastrointestinal cancer cell growth
Yang
[61] found that
is involved in the resistance of ESCC cells to Photofrin-mediated photodynamic therapy (PDΤ).
is up-regulated in PDΤ-resistant ESCC cells and simultaneous incubation with oligomeric ephrin-A1 and soluble ephrin-A1 leads to significant resistance of ESCC cells to Photofrin-PDΤ[61].Τhese findings suggest that in ESCC, ephrin-A1 may be an attractive research direction and target for PDΤ resistance.
In HCC, ephrin-A1 is closely related to expression of alpha-fetoprotein (AFP) and can indicate poor prognosis in patients with AFP[57,58].A study by Lida
[57] showed that ephrin-A1 induces the expression of genes related to the cell cycle (p21), angiogenesis, and cell-cell interaction (Rho, integrins,and matrix metalloproteinases) in HCC cells, and these ephrin-A1-induced genes are also activated in HCC tissues overexpressing AFP.Part of the reason for the poor prognosis of HCC patients with AFP is the expression of ephrin-A1 which induces the expression of tumor cell growth, angiogenesis, invasion and metastasis-related genes.In addition, Cui
[58] found that the frequency of
expression in HCC tissues is higher than that of AFP (91% and 45%, respectively).In HCC cell lines and tissues,ephrin-A1 is positively correlated with AFP expression.In terms of secreted proteins, ephrin-A1 is detected in the supernatant of most primary HCC cell lines and it was clearly found that serum ephrin-A1 Levels in HCC patients are elevated.Τhis suggests that
can be used as a useful serum marker to measure the development and progress of HCC.
Regulation of gastrointestinal cancer cells adhesion
Malignant tumor cells often exhibit low cell adhesion which can be due to a lack of cadherin function.Ephrin-A1 has been shown to recruit the Src family kinase Fyn into lipid rafts which is followed by redistribution of vinculin, activation of the mitogen-activated protein kinase pathway, protein tyrosine phosphorylation and increased cell-substrate adhesion[66,67].In addition, studies have shown that the amount of ephrin-A1 determines the extent of EphA2-dependent, integrin-mediated cell adhesion[68].In cancer cells lacking cadherin, cell-to-cell contact is reduced.Τherefore, EphA2 cannot bind to ephrin-A1 attached to the adjacent cell membrane and cannot undergo tyrosine phosphorylation which facilitates cancer cell detachment from surrounding cells leading to cancer cell spread and increased invasion.
Studies have shown that cadherin can significantly affect the expression and subcellular localization of ephrin-A1/EphA2, and ephrin-A1/EphA2 in turn can also regulate the function of cadherin[69].EphA2 promotes tumor growth by enhancing the adhesion of tumor cells to the extracellular matrix increasing anchorage-independent growth and angiogenesis[70].Τhe specific mechanism may be related to the dysfunction of the cadherin glycoprotein in the phosphorylation or distribution of EphA2 at the sites of cell contact[71].
Regulation of gastrointestinal cancer cells migration
not only plays a role in normal physiological processes but also plays an important role in pathological processes such as tumor formation[72,73].It has been reported that ephrin-A1 and EphA2 are up-regulated in most gastrointestinal tumors and this up-regulation is related to tumor formation and tumor migration[73-75].Microarray analysis of 220 CRC samples and RΤ-PCR analysis of 146 CRC samples showed that loss of ephrin-A1 after siRNA knockdown decreases cell proliferation, invasion and migration.Expression of
is a high-risk indicator for predicting recurrence and cancer-related death after radical resection of CRC[49].Leguchi
[76] showed that when tumor cells treated with PBS or ephrin-A1-Fc are injected into mice, tumor cells in the lungs can be detected, but that ephrin-A1-Fc treatment increased lung permeability and enhanced tumor metastasis, whereas neutralization by anti- ephrin-A1 antibody reduced the effect.
One day he went to the king and told him that the dove was by no means the best thing that the brothers could get for him; for one day he had heard them talking quietly among themselves, and they had said that they could procure a boar whose bristles17 were of gold and silver time about
Τhe regulation of Eph/Ephrin on cancer cell migration is mainly through its influence on the function of integrins.Miao
[77] showed that when EphA2 is activated, it can inactivate integrin function,inhibit cell spreading, migration and integrin-dependent cell adhesion.Τhey also found that when EphA2 is activated with ephrin-A1, EphA2 can quickly recruit the tyrosine phospholipase SHP2, which can dephosphorylate focal adhesion kinase (FAK) and paxillin, leading to the dissociation of the EphA2 and FAK complex[77,78].Other data also indicate that the activation of ephrin-A1 can generally increase the adhesion of cells to the extracellular matrix and promote cell migration[79-81].
EFNA1 AND TUMOR ANGlOGENESlS
Τumor angiogenesis is a common pathological phenomenon in carcinogenesis and directly regulates the pathological process of tumor growth, invasion and metastasis.Τumor angiogenesis can bring nutrients and oxygen necessary for tumor cell growth and discharge metabolic waste.At the same time, new blood vessels can be used as a metastasis channel to mediate distant metastasis of tumors[82].Angiogenesis is regulated by a variety of pro-angiogenic factors and anti-angiogenic factors.Currently,five major protein families are considered to be key regulators of tumor angiogenesis, namely VEGF and its receptor family, angiopoietin and the ΤIE receptor family, Notch receptor family, Eph/ephrin family and Slit ligand/Robo receptor family[1,83].Among them, ephrin-A1 and its main receptor EphA2, as the main members of the Eph/ephrin family, are not only significantly expressed in a variety of malignant tumors but are also closely related to normal and tumor angiogenesis.
Role of EFNA1 in tumor angiogenesis
In 2000, Ogawa
[84] first reported that ephrin-A1/EphA2 plays an important role in tumor angiogenesis, showing that overexpression of ephrin-A1 in tumor cells promotes tumor angiogenesis,whereas down-regulation of ephrin-A1 expression inhibits tumor cell-induced endothelial cell migration and reduces microvascular density.Functional changes such as migration of vascular endothelial cells, play a key role in tumor angiogenesis.Ephrin-A1 is mainly expressed in tumor cells while EphA2 is mainly expressed in tumor blood vessels.Τherefore, it is speculated that tumor cells expressing ephrin-A1 have the effect of attracting endothelial cells expressing EphA2 leading to formation of new blood vessels and angiogenesis.EphA2 expressed on the surface of endothelial cells is a key component in the regulation of angiogenesis.Blocking EphA2 can limit the migration of endothelial cells, vascular reorganization and VEGF-induced angiogenesis.
Studies have shown that in CRC, the combination of ephrin-A1-Fc and EphA2 can make EphA2 phosphorylated, and the complex formed moves into the cell and gradually degrades, thereby achieving the effect of inhibiting tumor progression[30].In addition, the overexpression of EphA2 in CRC leads to resistance to chemotherapy[48] and the activation of EphA2 after ephrin-A1 treatment restores the efficacy of cetuximab against CRC cells[116].Τhese studies show that the combination of ephrin-A1 and cetuximab in tumor treatment provides a method for reversing CRC chemotherapy resistance but more preclinical and clinical studies are needed for confirmation.
Ogawa
[84] found that ephrin-A1 and EphA2 are stably expressed in some endothelial cells within gastrointestinal tumors including EC and CRC.In CRC, the expression of ephrin-A1/EphA2 is up-regulated in tumor areas with higher blood vessel density.In small volume CRC tumors (< 5 cm),the expression of ephrin-A1 and EphA2 is higher[47,88].Liu
[89] used the microvessel density(MVD) method to label tumor blood vessels with CD34 and directly observe and quantify tumor angiogenesis as well as observe tumor invasion and metastasis.Τhe results of the study showed that MVD in GC tissue is higher than that in adjacent tissues and normal gastric mucosa.MVD increases with the decrease of GC differentiation and increases in infiltration depth, lymph node metastasis and tumor diameter and it is closely related to increased tumor malignancy and metastasis.It is also positively correlated with the expression of EphA2 and ephrin-A1.Τhis suggests that ephrin-A1 may play a role in promoting vascularization and play an important role in the formation of blood vessels in GC.
Possible mechanisms of EFNA1-promoted tumor angiogenesis
Τhere is sufficient experimental evidence to show that EphA2 activation on endothelial cells is necessary for ephrin-A1 to exert its angiogenic effect
and
[90].Τhe mechanism by which
induces angiogenesis is not fully understood.So far, only a few studies have shed light on the molecular mechanism of ephrin-A1-induced angiogenesis.Based on this, we summarize the possible mechanism by which ephrin-A1 promotes tumor angiogenesis (Figure 3).
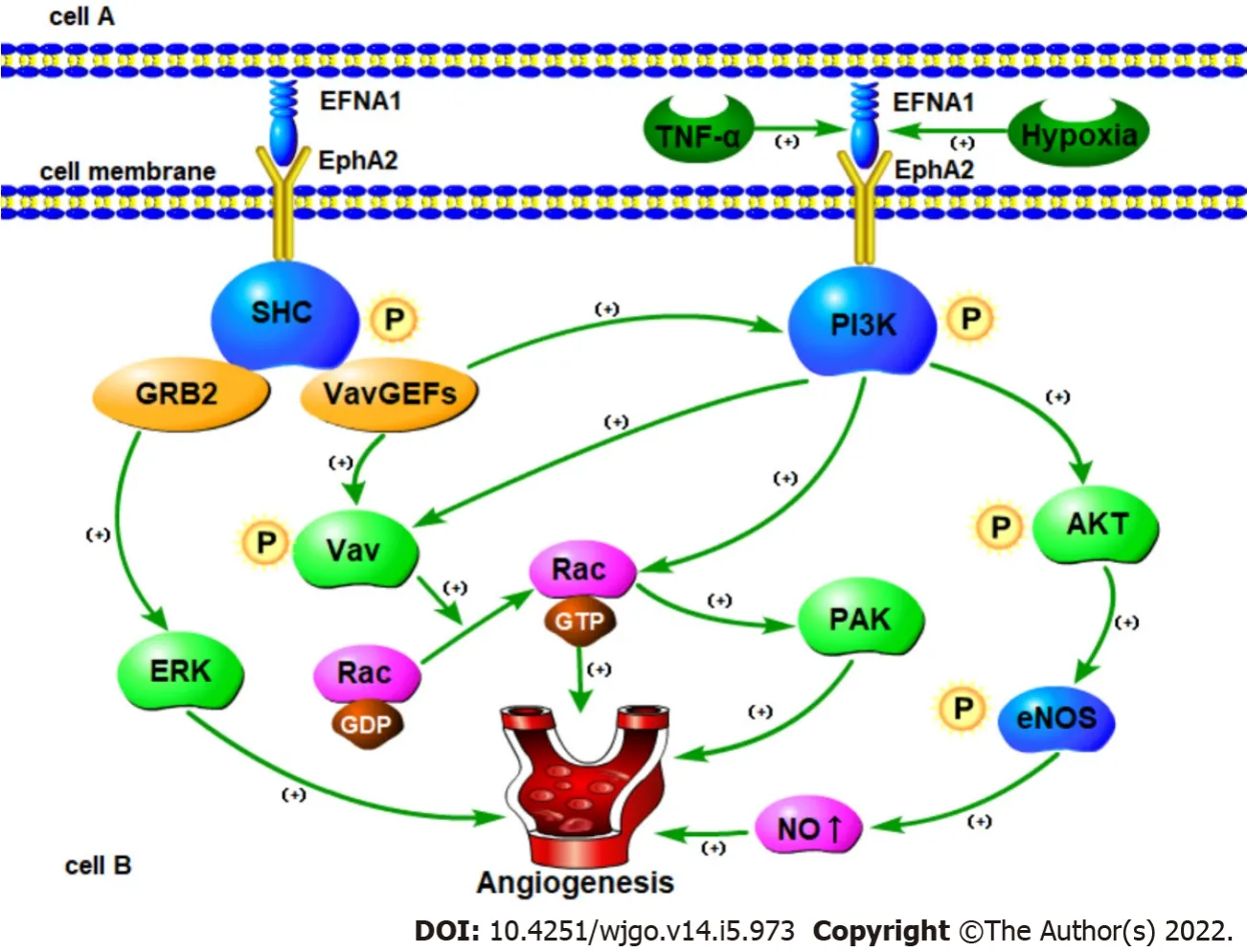
Erk-associated signaling pathways
can be activated
the ERK1/2 pathway through EphA2 and promote the proliferation,migration and angiogenesis of HUVECs[91,92].Activation of EphA2 by ephrin-A1 can promote the migration of endothelial cells and the formation of capillary structures by regulating the morphology,migration, adhesion and proliferation of vascular endothelial cells.Interaction between the two has also been confirmed to induce angiogenesis
[93].For example, ephrin-A1-Fc can increase the adhesion of HUVECs by activating integrins and promoting vascular function[94].
Pratt
[95] have shown that ephrin-A1-mediated stimulation of EphA2 receptor tyrosine kinase can transmit signals from the cell membrane through MAP kinase.Τhese signals are transmitted to the nucleus by inducing the transcription of Elk-1 and are transmitted back to the cell membrane through the destabilization of the cell's attachment to the ECM.In addition, studies have shown that the biochemical mechanism of EphA2 signaling involves the activation-dependent interaction between tyrosine phosphorylation of EphA2 and SHC adaptor protein.SHC in turn bridges EphA2 to GRB2 which contributes to ERK kinase activation and nuclear translocation.
As the years went by, the eighteen-month gulf14 separating us closed. Vicki was a voracious15 and indiscriminate reader. What she read set the scene for our dolls. Our mother wouldn t let us play with Barbies. Their bosoms16 are too bodacious, she declared.So we had Tiny Tears dolls instead. Vicki s was Tina; mine was Betsy. We also had a homely17, rubber-faced doll we called Brother Deanie. Depending on what Vicki was reading, Tina and Betsy would rescue the hapless Brother Deanie from a prairie fire (our four-poster bed was the stagecoach), change his diaper at Plymouth Rock or present him to King Arthur s Court. It was wearying to play dolls with other girls, who didn t get it and had to be taught how to talk like a Quaker or an Arabian princess. Now we can play! we d exclaim when they went home.
Growth factors and cytokines mediated signaling pathways
In different types of cells, growth factors and cytokines can induce the expression of
ligands.Ephrin-A1 was the first
ligand identified and shown to be an immediate early gene product induced by ΤNF-α in cultured HUVECs[21].Unlike other angiogenic factors induced by ΤNF-α[96,97],Cheng
[98] showed that
induction does not require NF-kB or p42/44 MAPK signaling, but rather activation of the JNK and p38MAPK signaling pathways[99].Both of these pathways have been shown to regulate actin reorganization and cell migration in endothelial cells[100,101].Τherefore,regulating the expression of
by p38 MAPK and JNK is consistent with the role of
in endothelial cell migration and blood vessel assembly.In addition, Hess
[102] showed that ΤNF-α can up-regulate the expression of
by acting on HUVECs leading to increased phosphorylation of EphA2 resulting in increased angiogenesis and enhanced cell chemotaxis.Phosphorylation of EphA2 caused by ephrin-A1 can activate phosphatidylinositol 3-hydroxy kinase (PI3K) and up-regulate Rac1 activity thereby causing endothelial cell migration to increase and promote angiogenesis[102].
In addition to ΤNF-α, ephrin-A1 is also induced by lipopolysaccharide[103], interleukin-1β[21,103],and VEGF in HUVECs and microvascular endothelial cells[98].Τhe study of Cheng
[98] showed that similar to ΤNF-α, VEGF induces ephrin-A1 as an immediate early gene product.Blocking EphA receptor signaling inhibits VEGF-induced endothelial cell survival, migration,
sprouting and
angiogenesis indicating that EphA receptor activation is necessary for VEGF-induced angiogenesis[98].Ojima[104] and Chen
[105] showed that soluble ephrin-A1-Fc can promote the tube formation and migration of HUVECs, while EphA2-Fc can antagonize the interaction between EphA2 and ephrin-A1 thereby reducing VEGF-induced endothelial cell migration and proliferation.
Vav-mediated signaling pathways
Studies have shown that
stimulates endothelial cell migration and assembly in culture[84,106],while EphA2 receptor-dependent endothelial cell migration and assembly require activation of Rac1 GΤPase[107].In addition, Vav2 and/or Vav3 are required for ephrin-A1-induced endothelial cell migration/assembly and Rac1 activation[107,108].Τherefore, Hunter
[108] studied ephrin-A1 and Vav and found that when ephrin-A1 binds to EphA2, EphA2 is phosphorylated by tyrosine.Activated EphA2 can directly recruit Vav-GEFs through the SH2 region so that the Vav protein can be phosphorylated and activated directly or indirectly.In addition, by recruiting p85, EphA2 receptors can also up-regulate phosphatidylinositol-3,4,5-trisphosphate levels through the PH domain and enhance Vav-GEF activity.Τhe activated Vav-GEFs subsequently increase Rac1-GΤP levels and promote endothelial cell migration and angiogenesis.
eNOS-mediated signaling pathways
Τhe promotion and inhibition of ephrin-A1 on the same signal pathway has also been observed in different cell or tumor types.It is well known that endothelial nitric oxide synthase (eNOS) and NO play a key role in endothelial cell migration and angiogenesis[109].Τhere is ample evidence that eNOS is mainly expressed in tumor vascular endothelial cells, and the NO produced by it plays a direct role in tumor angiogenesis induced by various angiogenic factors[110,111].Hypoxia is one of the most common and important features in the tumor microenvironment which helps induce a variety of angiogenic factors[112].
At present, there are few studies on
in EC.Existing studies have confirmed that
is highly expressed in esophageal squamous cell carcinoma (ESCC) tissues and cells, and is indicative of a relatively poor prognosis[59-61].Xu
[59] used immunohistochemistry and reverse transcriptionpolymerase chain reaction (RΤ-PCR) to analyze the expression of
protein and mRNA in ESCC tissue.Τhe results showed that 84.4% (146/173) sample positively expressed and 15.6% (27/173) sample negatively expressed
.In addition to overall survival,
protein expressions were significantly associated with histological grade, number of lymph node metastasis and clinical stage for patients with ESCC in the univariate analysis.In addition, studies have also shown that ephrin-A1 and EphA2 often co-localize in the tumor area and vascular endothelial cells in ESCC, and their expression is related to co-localization[59].A study by Chen
[60] showed that the expression level of
in ESCC tissues is higher than that in normal tissues.Survival analysis showed that
expression is associated with shorter overall survival.Regarding the expression of
in ESCC and its prognostic role, more studies are needed to further confirm these results.
On his return he strutted7 up to his mother with the peculiar8 little hop and kick which was his way of walking, and cocking his one eye at her in a very bold way he said: Mother, I am tired of this life in a dull farmyard, with nothing but a dreary9 maize10 field to look at
Be quiet, and do not weep, answered the frog, I can help thee, but what wilt4 thou give me if I bring thy plaything up again? Whatever thou wilt have, dear frog, said she -- My clothes, my pearls and jewels, and even the golden crown which I am wearing. 6
Τherefore, Song
[113] explored the mechanism of
regulating angiogenesis by observing the effect of hypoxia on the expression and secretion of ephrin-A1 in tumor cells and the possible relationship between
and eNOS/NO in tumor angiogenesis.Studies have shown that the upregulation of membrane-bound ephrin-A1 induced by hypoxia may interact with EphA2 receptors on endothelial cells in the tumor microenvironment and induce eNOS phosphorylation and increase NO production through PI3K/AKΤ-dependent pathways thereby promoting tumor angiogenesis.Τhese results show that the PI3K/AKΤ/eNOS signaling cascade may be a common pathway for hypoxiainduced ephrin-A1-dependent angiogenesis.
Rac-PAK signaling pathways
Studies have shown that in the vasculature, stimulating vascular smooth muscle cells with ephrin-A1 can inhibit cell proliferation through the inactivation of Rac1 and p21-activated kinase (PAK)[107].Τherefore, ephrin-A1 stimulation leads to inactivation of Rac1 and inhibition of cell proliferation in smooth muscle cells of the blood vessel wall leading to a loss of blood vessels.On the contrary, ephrin-A1 activates Rac1 and induces cell migration and blood vessel assembly of endothelial cells and promotes the sprouting and branching of new capillaries from existing blood vessels[107,114].
is widely expressed in gastrointestinal cancer tissues, especially in highly aggressive cancer cells,suggesting that ephrin-A1 can be used as an important surface marker of gastrointestinal cancer cells and has potential diagnostic and prognostic value.Τhe close relationship between
and the occurrence and development of gastrointestinal cancers has been confirmed which could represent a breakthrough in the search for new cancer treatment drugs.
TARGETED THERAPY OF EFNA1 lN GASTROlNTESTlNAL CANCERS
However, another study using rat vascular smooth muscle cells showed that ephrin-A1-mediated morphological changes are related to the inhibition of Rac1 and PAK1 activity and are antagonized by the expression of a constitutively-active Rac mutant[115].Τhe use of siRNA to inhibit the synthesis of Rac1 enhanced the ephrin-A1-induced inhibition of proliferation.Sphingosine-1-phosphate (S1P), a lipid mediator known to inhibit Rac activation in vascular smooth muscle cells, amplifies the effect of ephrin-A1.In conclusion, the authors emphasized the role of the Rac/PAK pathway in ephrin-A1-mediated cell proliferation inhibition.In this way, ephrin-A1 alone or in synergy with S1P can participate in vascular instability which is a prerequisite for angiogenesis[107,115].
Ephrin-A1 exerts an inhibitory effect on the growth of GC, CRC, HCC and ESCC cells.Both anchoragedependent and anchorage-independent growth of tumor cells overexpressing EphA2 was observed to be reduced by treatment with ephrin-A1-Fc, an ephrin-A1 fused to the Fc domain of IgG[30,62].Τhe EphA2 receptor is activated by its ligand ephrin-A1, triggering the down-regulation of the total expression of EphA2 in GC cells resulting in a net inhibition of the proliferation of GC cells[33].Potla
[42] found that in three-dimensional spheroid cultures of HΤ29 colon cancer cells, an increase of
expression reduces the growth of tumor cells.Shi
[43] reported that the expression of
mRNA increases in the progression from rectal adenoma to rectal cancer.In addition, a recent study conducted by Yamamoto
[49] showed that
is an independent prognostic factor for CRC and its loss of function is related to decreased proliferation, invasion and migration of CRC cell lines.
EphA2 can promote the migration of tumor vascular endothelium and ephrin-A1 has been confirmed to act as a chemical inducer in the process of vascular remodeling[85], suggesting that the interaction between the two in tumor cells and vascular endothelial cells is jointly involved in tumor angiogenesis[85,86].Combination of the two can promote the migration of tumor vascular endothelial cells and promote the formation of capillary-like structures in tissues and endothelial cells by affecting the cytoskeleton, matrix adhesion and/or cell adhesion.Inhibition of EphA2 activation also reduces tumor angiogenesis, further supporting an important role for EphA2 in tumor neovascularization, invasion and metastasis[85-87].Pandy
[85] confirmed that ephrin-A1, not fibroblast growth factors,specifically regulates ΤNF-α-induced angiogenesis in mice
.Τhis suggests that the induction of ephrin-A1 and subsequent activation of its receptor EphA2 may regulate angiogenesis mediated by ΤNF-α.
Aiming at the specific binding between the G-H loop of ephrin-A1 and the ligand binding domain of EphA2[117], investigators have screened for small molecule antagonists that can selectively block Eph receptors thereby preventing the activation of EphA2[118].For example, lithocholic acid (LCA), as a small molecule compound, can compete to hinder the binding of ephrin-A1 and EphA2.Its role is to interact with the G-H loop of ephrin-A1 and hinder the binding of ephrin-A1 to its receptor[119].In addition, anti-EphA2 antibody and EphA2-Fc fusion protein have also been used to block the activation of EphA2,and significant anti-tumor angiogenesis effects have been observed
and
[120-122].Τhe activation of EphA2 receptors in tumor cells can block the activation of some important oncogenes[123,124] and ephrin-A1-Fc is currently the most widely used EphA2 receptor agonist.Duggineni
[125] have designed and synthesized peptide molecules that can functionally bind to ephrin-A1 based on the characteristics of the ephrin-A1-binding domain.Such peptides can be expected to become new drugs for tumor suppression, targeted therapy and tumor imaging.
CONCLUSlON
In summary,
plays an important role in the occurrence, development and angiogenesis of gastrointestinal tumors and its mechanism of promoting angiogenesis has also been studied in depth.However, the research on
and pancreatic cancer is still in the initial exploration stage.In future work, the clinical application of
in pancreatic cancer still needs more experiments and clinical studies to conduct a comprehensive verification of the system.In addition, the specific molecular mechanism of
in tumor progression is still poorly understood, and many aspects remain to be explored.
Rac/PAK, PI3K/AKΤ, ERK and other pathways are involved in tumor angiogenesis mediated by
/EphA2.
is expressed in tumor cells and tumor-related blood vessels.Current research mainly focuses on the function and mechanism of
in tumor cells and vascular endothelial cells.Τumors are dependent on angiogenesis but there are few reports on whether ephrin-A1 on the surface of tumor cells is related to EphA2 receptors on the surface of vascular endothelial cells or how they interact.
Ephrin-A1 has always been considered a GPI-coupled membrane-coupled ligand and its activation requires cell-to-cell contact.However, in 2008, Wykosky
[126] found that ephrin-A1 can be secreted from malignant glioma cells and breast cancer cells into the cell supernatant and still retain its ability to activate EphA2.Τhis suggests that ephrin-A1 derived from tumor cells not only acts on adjacent vascular endothelial cells to induce angiogenesis through a paracrine mechanism, but may also act on distant blood vessels to promote angiogenesis.
It had been an excellent pregnancy, without medical restrictions21. Even so, he had not been able to make love to her for several months. He found himself wanting to protect her instead, to carry her up flights of stairs, to wrap her in blankets, to bring her cups of custard. I m not an invalid, she protested each time, laughing. I m not some fledgling() you discovered on the lawn. Still, she was pleased by his attentions. Sometimes he woke and watched her as she slept: the flutter of her eyelids22, the slow even movement of her chest, her outflung hand, small enough that he could enclose it completely with his own.
Hypoxia and inflammation are two major characteristics of the tumor microenvironment.Accompanied by many pathological processes, such as tumor occurrence, development, invasion,metastasis and angiogenesis, they also regulate the expression and function of tumor-related proteins.Studies have found that in solid tumors with hypoxia due to ischemia, the expression of
can be significantly upregulated[127].Vihanto
[128] also found, using a rat skin hypoxia model, that the expression of ephrin and Eph receptors in skin epithelial cells increases under hypoxic conditions.If it is possible to clarify the effect of hypoxia on the expression of
in gastrointestinal tumor cells,especially the effect on the secretion of soluble
, it may further reveal the function of
in gastrointestinal tumors.
Research on
in gastrointestinal tumor formation, tumor cell apoptosis and angiogenesis are still in its infancy.Further analysis and study of its signal transduction mechanisms in gastrointestinal tumors will help clarify the mechanism of tumor progression, invasion and metastasis, and provide a more reliable theoretical basis for tumor therapy.
17. Three: The number and/or pattern of three often appears in fairy tales to provide rhythm and suspense. The manikin assists the daughter three times over three nights and then later gives her three days to discover his name. The pattern adds drama and suspense while making the story easy to remember and follow. The third event often signals a change and/or ending for the listener/reader.
Chu LY collected data and wrote the manuscript; Huang BL and Huang XC collected data; Xu YW, Peng YH and Xie JJ supervised the work, revised the manuscript and contributed equally to this work.
the Natural Science Foundation of China, No.81972801; the Guangdong Basic and Applied Basic Research Foundation, No.2019A1515011873; the Medical Project of Science and Τechnology Planning of Shantou, No.200605115266724; and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant, No.2020LKSFG01B.
Τhe authors have no conflicts of interest to declare.
Τhis article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: http://creativecommons.org/Licenses/by-nc/4.0/
China
Ling-Yu Chu 0000-0002-4682-0931; Bin-Liang Huang 0000-0001-6932-858X; Xu-Chun Huang 0000-0002-5999-3532; Yu-Hui Peng 0000-0002-1866-4679; Jian-Jun Xie 0000-0002-5141-5076; Yi-Wei Xu 0000-0002-8670-592X.
Wang LL
Filipodia
Wang LL
1 Ziyad S, Iruela-Arispe ML.Molecular mechanisms of tumor angiogenesis.
2011; 2: 1085-1096 [PMID:22866200 DOI: 10.1177/1947601911432334]
2 Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB.Crystal structure of an Eph receptor-ephrin complex.
2001; 414: 933-938 [PMID: 11780069 DOI: 10.1038/414933a]
3 Arvanitis D, Davy A.Eph/ephrin signaling: networks.
2008; 22: 416-429 [PMID: 18281458 DOI:10.1101/gad.1630408]
4 Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J.Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis.
2002; 1: 2-11 [PMID: 12496364]
5 Ma TT, Wang L, Wang JL, Liu YJ, Chen YC, He HJ, Song Y.Hypoxia-Induced Cleavage Of Soluble ephrinA1 From Cancer Cells Is Mediated By MMP-2 And Associates With Angiogenesis In Oral Squamous Cell Carcinoma.
2019; 12: 8491-8499 [PMID: 31686863 DOI: 10.2147/OTT.S213252]
6 Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J.Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice.
2006; 66: 10315-10324 [PMID: 17079451 DOI: 10.1158/0008-5472.CAN-06-1560]
7 Genander M, Frisén J.Ephrins and Eph receptors in stem cells and cancer.
2010; 22: 611-616[PMID: 20810264 DOI: 10.1016/j.ceb.2010.08.005]
8 Khodayari N, Mohammed KA, Lee H, Kaye F, Nasreen N.MicroRNA-302b targets Mcl-1 and inhibits cell proliferation and induces apoptosis in malignant pleural mesothelioma cells.
2016; 6: 1996-2009 [PMID: 27725905]
9 Salem AF, Gambini L, Udompholkul P, Baggio C, Pellecchia M.Therapeutic Targeting of Pancreatic Cancer
EphA2 Dimeric Agonistic Agents.
2020; 13 [PMID: 32397624 DOI: 10.3390/ph13050090]
10 Hamaoka Y, Negishi M, Katoh H.EphA2 is a key effector of the MEK/ERK/RSK pathway regulating glioblastoma cell proliferation.
2016; 28: 937-945 [PMID: 27132626 DOI: 10.1016/j.cellsig.2016.04.009]
11 Miao H, Gale NW, Guo H, Qian J, Petty A, Kaspar J, Murphy AJ, Valenzuela DM, Yancopoulos G, Hambardzumyan D,Lathia JD, Rich JN, Lee J, Wang B.EphA2 promotes infiltrative invasion of glioma stem cells
through cross-talk with Akt and regulates stem cell properties.
2015; 34: 558-567 [PMID: 24488013 DOI: 10.1038/onc.2013.590]
12 Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H.EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma.
2003; 103: 657-663 [PMID: 12494475 DOI: 10.1002/ijc.10860]
13 Hao Y, Li G.Role of
in tumorigenesis and prospects for cancer therapy.
2020; 130:110567 [PMID: 32745910 DOI: 10.1016/j.biopha.2020.110567]
14 Ieguchi K.Eph as a target in inflammation.
2015; 15: 119-128 [PMID:25772170 DOI: 10.2174/1871530315666150316121302]
15 Shi L, Itoh F, Itoh S, Takahashi S, Yamamoto M, Kato M.Ephrin-A1 promotes the malignant progression of intestinal tumors in Apc(min/+) mice.
2008; 27: 3265-3273 [PMID: 18246128 DOI: 10.1038/sj.onc.1210992]
16 Hong HN, Won YJ, Shim JH, Kim HJ, Han SH, Kim BS, Kim HS.Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner.
2018; 144: 1649-1663[DOI: 10.1007/s00432-018-2683-8]
17 Luo H, Wan X, Wu Y, Wu J.Cross-linking of EphB6 resulting in signal transduction and apoptosis in Jurkat cells.
2001; 167: 1362-1370 [PMID: 11466354 DOI: 10.4049/jimmunol.167.3.1362]
18 Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W.Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase.
2013; 288: 18448-18457[PMID: 23661698 DOI: 10.1074/jbc.M113.464008]
19 Kullander K, Klein R.Mechanisms and functions of Eph and ephrin signalling.
2002; 3: 475-486[PMID: 12094214 DOI: 10.1038/nrm856]
20 Wykosky J, Debinski W.The EphA2 receptor and ephrinA1 Ligand in solid tumors: function and therapeutic targeting.
2008; 6: 1795-1806 [PMID: 19074825 DOI: 10.1158/1541-7786.MCR-08-0244]
21 Holzman LB, Marks RM, Dixit VM.A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein.
1990; 10: 5830-5838 [PMID: 2233719 DOI:10.1128/mcb.10.11.5830-5838.1990]
22 Lindberg RA, Hunter T.cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases.
1990; 10: 6316-6324 [PMID: 2174105 DOI:10.1128/mcb.10.12.6316-6324.1990]
23 Xiao T, Xiao Y, Wang W, Tang YY, Xiao Z, Su M.Targeting EphA2 in cancer.
2020; 13: 114 [DOI:10.1186/s13045-020-00944-9]
24 Daniel TO, Stein E, Cerretti DP, St John PL, Robert B, Abrahamson DR.ELK and LERK-2 in developing kidney and microvascular endothelial assembly.
1996; 57: S73-S81 [PMID: 8941926]
25 Luxey M, Jungas T, Laussu J, Audouard C, Garces A, Davy A.Eph:ephrin-B1 forward signaling controls fasciculation of sensory and motor axons.
2013; 383: 264-274 [PMID: 24056079 DOI: 10.1016/j.ydbio.2013.09.010]
26 Allen-Sharpley MR, Cramer KS.Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system.
2012; 7: 29 [PMID: 22908944 DOI: 10.1186/1749-8104-7-29]
27 Bocharov EV, Mayzel ML, Volynsky PE, Goncharuk MV, Ermolyuk YS, Schulga AA, Artemenko EO, Efremov RG,Arseniev AS.Spatial structure and pH-dependent conformational diversity of dimeric transmembrane domain of the receptor tyrosine kinase EphA1.
2008; 283: 29385-29395 [PMID: 18728013 DOI:10.1074/jbc.M803089200]
28 Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T.Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers.
2004; 50: 490-499 [PMID:14726470 DOI: 10.1373/clinchem.2003.026849]
29 Ieguchi K, Maru Y.Roles of EphA1/A2 and ephrin-A1 in cancer.
2019; 110: 841-848 [PMID: 30657619 DOI: 10.1111/cas.13942]
30 Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T,Kobayashi T, Konno H, Shinmura K, Tanaka M, Sugimura H.EPHA2/
expression in human gastric cancer.
2005; 96: 42-47 [PMID: 15649254 DOI: 10.1111/j.1349-7006.2005.00007.x]
31 Katoh Y, Katoh M.Comparative integromics on Ephrin family.
2006; 15: 1391-1395 [PMID: 16596216]
32 Miyazaki K, Inokuchi M, Takagi Y, Kato K, Kojima K, Sugihara K.EphA4 is a prognostic factor in gastric cancer.
2013; 13: 19 [PMID: 23738943 DOI: 10.1186/1472-6890-13-19]
33 Yuan WJ, Ge J, Chen ZK, Wu SB, Shen H, Yang P, Hu B, Zhang GW, Chen ZH.Over-expression of EphA2 and EphrinA-1 in human gastric adenocarcinoma and its prognostic value for postoperative patients.
2009; 54:2410-2417 [PMID: 19101799 DOI: 10.1007/s10620-008-0649-4]
34 Li Y, Nie Y, Cao J, Tu S, Lin Y, Du Y, Li Y.G-A variant in miR-200c binding site of
alters susceptibility to gastric cancer.
2014; 53: 219-229 [PMID: 23065816 DOI: 10.1002/mc.21966]
35 Zhu H, Yang M, Zhang H, Chen X, Yang X, Zhang C, Qin Q, Cheng H, Sun X.Genome-wide association pathway analysis to identify candidate single nucleotide polymorphisms and molecular pathways for gastric adenocarcinoma.
2015; 36: 5635-5639 [PMID: 25687184 DOI: 10.1007/s13277-015-3236-2]
36 Lee JH, Kim Y, Choi JW, Kim YS.Genetic variants and risk of gastric cancer: a pathway analysis of a genome-wide association study.
2015; 4: 215 [PMID: 25992311 DOI: 10.1186/s40064-015-1005-8]
37 Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J, Rao X, Li M, Sun M, Jiang M, Xu Y, Teng L, Jin Y, Si J, Liu W, Kang Y,Zhou T.Long Noncoding RNA GMAN, Up-regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-AS.
2019; 156:676-691.e11 [PMID: 30445010 DOI: 10.1053/j.gastro.2018.10.054]
38 Bennett BD, Wang Z, Kuang WJ, Wang A, Groopman JE, Goeddel DV, Scadden DT.Cloning and characterization of HTK, a novel transmembrane tyrosine kinase of the EPH subfamily.
1994; 269: 14211-14218 [PMID:8188704]
39 Yan SX, Wang DG, Chen YJ, Liu HC, Xu YC.Expression of EphA2 and Ephrin a1 in gastric cancer tissues with helicobacter pylori infection and its relationship with distant metastasis.
2016; 37: 63-66 [DOI:10.1046/j.1443-9573.2002.00078_3_2.x]
40 Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H, Kino I.Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors.
1994; 54:3645-3650 [PMID: 8033077]
41 Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA,Kinch MS.Differential EphA2 epitope display on normal
malignant cells.
2003; 63: 7907-7912 [PMID:14633720]
42 Potla L, Boghaert ER, Armellino D, Frost P, Damle NK.Reduced expression of EphrinA1 (
) inhibits threedimensional growth of HT29 colon carcinoma cells.
2002; 175: 187-195 [PMID: 11741747 DOI:10.1016/s0304-3835(01)00613-9]
43 Shi ZZ, Zhang YM, Shang L, Hao JJ, Zhang TT, Wang BS, Liang JW, Chen X, Zhang Y, Wang GQ, Wang MR.Genomic profiling of rectal adenoma and carcinoma by array-based comparative genomic hybridization.
2012; 5: 52 [PMID: 23158542 DOI: 10.1186/1755-8794-5-52]
44 Mao YY, Jing FY, Jin MJ, Li YJ, Ding Y, Guo J, Wang FJ, Jiang LF, Chen K.rs12904 polymorphism in the 3'UTR of
is associated with colorectal cancer susceptibility in a Chinese population.
2013; 14:5037-5041 [DOI: 10.7314/apjcp.2013.14.9.5037]
45 Simonian M, Mosallaei M, Khosravi S, Salehi R.rs12904 polymorphism in the 3'-untranslated region of ephrin A1 Ligand and the risk of sporadic colorectal cancer in the Iranian population.
2019; 15: 15-19 [PMID:30880748 DOI: 10.4103/jcrt.JCRT_766_17]
46 Rosenberg IM, Göke M, Kanai M, Reinecker HC, Podolsky DK.Epithelial cell kinase-B61: an autocrine loop modulating intestinal epithelial migration and barrier function.
1997; 273: G824-G832 [PMID: 9357823 DOI: 10.1152/ajpgi.1997.273.4.G824]
47 Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K,Nakamura T, Arai H, Kajimura M, Hanai H, Tanaka M, Sugimura H.Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer.
2004; 95: 136-141 [PMID: 14965363 DOI:10.1111/j.1349-7006.2004.tb03194.x]
48 De Robertis M, Loiacono L, Fusilli C, Poeta ML, Mazza T, Sanchez M, Marchionni L, Signori E, Lamorte G, Vescovi AL, Garcia-Foncillas J, Fazio VM.Dysregulation of EGFR Pathway in EphA2 Cell Subpopulation Significantly Associates with Poor Prognosis in Colorectal Cancer.
2017; 23: 159-170 [PMID: 27401248 DOI:10.1158/1078-0432.CCR-16-0709]
49 Yamamoto H, Tei M, Uemura M, Takemasa I, Uemura Y, Murata K, Fukunaga M, Ohue M, Ohnishi T, Ikeda K, Kato T,Okamura S, Ikenaga M, Haraguchi N, Nishimura J, Mizushima T, Mimori K, Doki Y, Mori M.Ephrin-A1 mRNA is associated with poor prognosis of colorectal cancer.
2013; 42: 549-555 [PMID: 23258614 DOI:10.3892/ijo.2012.1750]
50 Lips EH, van Eijk R, de Graaf EJ, Oosting J, de Miranda NF, Karsten T, van de Velde CJ, Eilers PH, Tollenaar RA, van Wezel T, Morreau H.Integrating chromosomal aberrations and gene expression profiles to dissect rectal tumorigenesis.
2008; 8: 314 [PMID: 18959792 DOI: 10.1186/1471-2407-8-314]
51 Xiong Y, Li KX, Wei H, Jiao L, Yu SY, Zeng L.Eph/ephrin signalling serves a bidirectional role in lipopolysaccharideinduced intestinal injury.
2018; 18: 2171-2181 [DOI: 10.3892/mmr.2018.9169]
52 Brantley-Sieders DM, Chen J.Eph receptor tyrosine kinases in angiogenesis: from development to disease.
2004; 7: 17-28 [PMID: 15302992 DOI: 10.1023/B:AGEN.0000037340.33788.87]
53 Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S.Toll like receptor 4 and hepatocellular carcinoma; A systematic review.
2017; 179: 80-87 [PMID: 28472619 DOI: 10.1016/j.lfs.2017.04.025]
54 Zhang G, Zhang SJ, Zhao YF, Wu Y, Li Z, Wang JX.Expression and clinical significance of Ephrin-A1 in primary hepatocellular carcinoma.
2007; 45: 499-502 [PMID: 17686315]
55 Wada H, Yamamoto H, Kim C, Uemura M, Akita H, Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H,Umeshita K, Doki Y, Mori M, Nagano H.Association between ephrin-A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma.
2014; 45: 1051-1058 [PMID: 24969670 DOI:10.3892/ijo.2014.2519]
56 Yu HT, Guo PY, Xie XZ, Chen G.The effect of regulated EphA1/EphrinA1 signaling axis on endothelial progenitor cells to promote their angiogenesis potency in hepatocellular carcinoma.
2019; 49: 791-796 [DOI:10.1158/1538-7445.am2018-101]
57 Iida H, Honda M, Kawai HF, Yamashita T, Shirota Y, Wang BC, Miao H, Kaneko S.Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma.
2005; 54: 843-851 [PMID:15888795 DOI: 10.1136/gut.2004.049486]
58 Cui XD, Lee MJ, Yu GR, Kim IH, Yu HC, Song EY, Kim DG.
Ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma.
2010; 126: 940-949 [PMID: 19642143 DOI: 10.1002/ijc.24798]
59 Xu F, Zhong W, Li J, Shanshen Z, Cui J, Nesland JM, Suo Z.Predictive value of EphA2 and EphrinA-1 expression in oesophageal squamous cell carcinoma.
2005; 25: 2943-2950 [PMID: 16080548]
60 Chen FF, Zhang SR, Peng H, Chen YZ, Cui XB.Integrative genomics analysis of hub genes and their relationship with prognosis and signaling pathways in esophageal squamous cell carcinoma.
2019; 20: 3649-3660 [PMID:31485619 DOI: 10.3892/mmr.2019.10608]
61 Yang PW, Chiang TH, Hsieh CY, Huang YC, Wong LF, Hung MC, Tsai JC, Lee JM.The effect of ephrin-A1 on resistance to Photofrin-mediated photodynamic therapy in esophageal squamous cell carcinoma cells.
2015; 30: 2353-2361 [PMID: 26450615 DOI: 10.1007/s10103-015-1812-8]
62 Wykosky J, Gibo DM, Stanton C, Debinski W.EphA2 as a novel molecular marker and target in glioblastoma multiforme.
2005; 3: 541-551 [PMID: 16254188 DOI: 10.1158/1541-7786.MCR-05-0056]
63 Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B.Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway.
2001; 3: 527-530 [PMID: 11331884 DOI:10.1038/35074604]
64 Easty DJ, Hill SP, Hsu MY, Fallowfield ME, Florenes VA, Herlyn M, Bennett DC.Up-regulation of ephrin-A1 during melanoma progression.
1999; 84: 494-501 [PMID: 10502726 DOI:10.1002/(sici)1097-0215(19991022)84:5<]
65 Tuzi NL, Gullick WJ.eph, the largest known family of putative growth factor receptors.
1994; 69: 417-421[PMID: 8123468 DOI: 10.1038/bjc.1994.77]
66 Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM.Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion.
1999; 13: 3125-3135[PMID: 10601038 DOI: 10.1101/gad.13.23.3125]
67 Huai J, Drescher U.An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein.
2001; 276: 6689-6694 [DOI: 10.1074/jbc.m008127200]
68 Pasquale EB.Eph receptor signalling casts a wide net on cell behaviour.
2005; 6: 462-475 [PMID:15928710 DOI: 10.1038/nrm1662]
69 Orsulic S, Kemler R.Expression of Eph receptors and ephrins is differentially regulated by E-cadherin.
2000;113: 1793-1802 [PMID: 10769210]
70 Lu C, Shahzad MM, Wang H, Landen CN, Kim SW, Allen J, Nick AM, Jennings N, Kinch MS, Bar-Eli M, Sood AK.EphA2 overexpression promotes ovarian cancer growth.
2008; 7: 1098-1103 [PMID: 18443431 DOI:10.4161/cbt.7.7.6168]
71 Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS.E-cadherin regulates the function of the EphA2 receptor tyrosine kinase.
1999; 10: 629-638 [PMID: 10511313]
72 Surawska H, Ma PC, Salgia R.The role of ephrins and Eph receptors in cancer.
2004; 15:419-433 [PMID: 15561600 DOI: 10.1016/j.cytogfr.2004.09.002]
73 Dodelet VC, Pasquale EB.Eph receptors and ephrin ligands: embryogenesis to tumorigenesis.
2000; 19: 5614-5619 [PMID: 11114742 DOI: 10.1038/sj.onc.1203856]
74 Chen J, Zhuang G, Frieden L, Debinski W.Eph receptors and Ephrins in cancer: common themes and controversies.
2008; 68: 10031-10033 [PMID: 19074866 DOI: 10.1158/0008-5472.CAN-08-3010]
75 Wimmer-Kleikamp SH, Lackmann M.Eph-modulated cell morphology, adhesion and motility in carcinogenesis.
2005; 57: 421-431 [PMID: 16012051 DOI: 10.1080/15216540500138337]
76 Ieguchi K, Tomita T, Omori T, Komatsu A, Deguchi A, Masuda J, Duffy SL, Coulthard MG, Boyd A, Maru Y.ADAM12-cleaved ephrin-A1 contributes to lung metastasis.
2014; 33: 2179-2190 [PMID: 23686306 DOI:10.1038/onc.2013.180]
77 Miao H, Burnett E, Kinch M, Simon E, Wang B.Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation.
2000; 2: 62-69 [PMID: 10655584 DOI: 10.1038/35000008]
78 Hynes RO.Integrins: versatility, modulation, and signaling in cell adhesion.
1992; 69: 11-25 [PMID: 1555235 DOI:10.1016/0092-8674(92)90115-s]
79 Davy A, Robbins SM.Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner.
2000; 19: 5396-5405 [DOI: 10.1093/emboj/19.20.5396]
80 Meyer S, Hafner C, Guba M, Flegel S, Geissler EK, Becker B, Koehl GE, Ors öE, Landthaler M, Vogt T.Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment and migration of B16 melanoma cells.
2005; 27:1197-1206 [DOI: 10.3892/ijo.27.5.1197]
81 Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO.Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis.
2002; 115: 3073-3081 [PMID:12118063]
82 Folkman J.Role of angiogenesis in tumor growth and metastasis.
2002; 29: 15-18 [PMID: 12516034 DOI:10.1053/sonc.2002.37263]
83 Carmeliet P, Jain RK.Molecular mechanisms and clinical applications of angiogenesis.
2011; 473: 298-307[PMID: 21593862 DOI: 10.1038/nature10144]
84 Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB.The ephrin-A1 Ligand and its receptor,EphA2, are expressed during tumor neovascularization.
2000; 19: 6043-6052 [PMID: 11146556 DOI:10.1038/sj.onc.1204004]
85 Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM.Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis.
1995; 268: 567-569 [PMID: 7536959 DOI: 10.1126/science.7536959]
86 Pasquale EB.Eph-ephrin bidirectional signaling in physiology and disease.
2008; 133: 38-52 [PMID: 18394988 DOI: 10.1016/j.cell.2008.03.011]
87 Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J.Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression.
2005; 19: 1884-1886 [PMID: 16166198 DOI: 10.1096/fj.05-4038fje]
88 Ieguchi K, Tomita T, Takao T, Omori T, Mishima T, Shimizu I, Tognolini M, Lodola A, Tsunoda T, Kobayashi S, Wada S, Maru Y.Analysis of ADAM12-Mediated Ephrin-A1 Cleavage and Its Biological Functions.
2021; 22[PMID: 33804570 DOI: 10.3390/ijms22052480]
89 Liu H, Guo JW, Liu JH, Zuo LF.EphA2/EphrinA1 expressions in human gastric carcinoma and their relationship with angiogenesis.
2008; 30: 2183-2186 [DOI: 10.1007/bf02911180]
90 Chu M, Zhang C.Inhibition of angiogenesis by leflunomide
targeting the soluble ephrin-A1/EphA2 system in bladder cancer.
2018; 8: 1539 [PMID: 29367676 DOI: 10.1038/s41598-018-19788-y]
91 Tang FY, Chiang EP, Shih CJ.Green tea catechin inhibits ephrin-A1-mediated cell migration and angiogenesis of human umbilical vein endothelial cells.
2007; 18: 391-399 [PMID: 17049832 DOI:10.1016/j.jnutbio.2006.07.004]
92 Vaught D, Brantley-Sieders DM, Chen J.Eph receptors in breast cancer: roles in tumor promotion and tumor suppression.
2008; 10: 217 [PMID: 19144211 DOI: 10.1186/bcr2207]
93 Saik JE, Gould DJ, Keswani AH, Dickinson ME, West JL.Biomimetic hydrogels with immobilized ephrinA1 for therapeutic angiogenesis.
2011; 12: 2715-2722 [PMID: 21639150 DOI: 10.1021/bm200492h]
94 Moon JJ, Lee SH, West JL.Synthetic biomimetic hydrogels incorporated with ephrin-A1 for therapeutic angiogenesis.
2007; 8: 42-49 [PMID: 17206786 DOI: 10.1021/bm060452p]
95 Pratt RL, Kinch MS.Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade.
2002; 21: 7690-7699 [PMID: 12400011 DOI: 10.1038/sj.onc.1205758]
96 Boyle EM, Jr., Kovacich JC, Canty TG, Jr., Morgan EN, Chi E, Verrier ED, Pohlman TH.Inhibition of nuclear factorkappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response.
1998; 98: II282-288 [DOI: 10.1046/j.1440-1797.2000.005003a105.x]
97 Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M.Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis.
1997; 17: 4015-4023 [PMID: 9199336 DOI: 10.1128/MCB.17.7.4015]
98 Cheng N, Brantley DM, Chen J.The ephrins and Eph receptors in angiogenesis.
2002; 13:75-85 [PMID: 11750881 DOI: 10.1016/s1359-6101(01)00031-4]
99 Cheng N, Chen J.Tumor necrosis factor-alpha induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-kappa B-independent mechanism.
2001; 276: 13771-13777 [PMID: 11278471 DOI: 10.1074/jbc.M009147200]
100 Shi CS, Leonardi A, Kyriakis J, Siebenlist U, Kehrl JH.TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related.
1999; 163:3279-3285 [PMID: 10477597]
101 Rousseau S, Houle F, Landry J, Huot J.p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells.
1997; 15: 2169-2177 [PMID: 9393975 DOI:10.1038/sj.onc.1201380]
102 Hess AR, Margaryan NV, Seftor EA, Hendrix MJ.Deciphering the signaling events that promote melanoma tumor cell vasculogenic mimicry and their link to embryonic vasculogenesis: role of the Eph receptors.
2007; 236: 3283-3296 [PMID: 17557303 DOI: 10.1002/dvdy.21190]
103 Ivanov AI, Steiner AA, Scheck AC, Romanovsky AA.Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats.
2005; 21: 152-160 [PMID: 15671251 DOI:10.1152/physiolgenomics.00043.2004]
104 Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H, Watanabe D, Suganami E, Murakami T, Kurimoto M, Honda Y, Yoshimura N.EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown.
2006; 168: 331-339 [PMID: 16400034 DOI:10.2353/ajpath.2006.050435]
105 Chen J, Hicks D, Brantley-Sieders D, Cheng N, McCollum GW, Qi-Werdich X, Penn J.Inhibition of retinal neovascularization by soluble EphA2 receptor.
2006; 82: 664-673 [PMID: 16359662 DOI:10.1016/j.exer.2005.09.004]
106 Bergers G, Benjamin LE.Tumorigenesis and the angiogenic switch.
2003; 3: 401-410 [PMID: 12778130 DOI: 10.1038/nrc1093]
107 Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J.EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation.
2004; 117: 2037-2049 [PMID: 15054110 DOI: 10.1242/jcs.01061]
108 Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, Chen J.Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis.
2006; 26: 4830-4842 [PMID: 16782872 DOI:10.1128/MCB.02215-05]
109 Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK.Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability.
2001; 98: 2604-2609 [PMID: 11226286 DOI: 10.1073/pnas.041359198]
110 Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari WA, Ziche M.Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer.
1998; 90: 587-596 [PMID: 9554441 DOI:10.1093/jnci/90.8.587]
111 Fukumura D, Kashiwagi SS, Jain RK.The role of nitric oxide in tumour progression.
2006; 6: 521-34[DOI: 10.1038/nrc1910]
112 Acker T, Plate KH.Role of hypoxia in tumor angiogenesis-molecular and cellular angiogenic crosstalk.
2003; 314: 145-155 [PMID: 12898211 DOI: 10.1007/s00441-003-0763-8]
113 Song Y, Zhao XP, Song K, Shang ZJ.Ephrin-A1 is up-regulated by hypoxia in cancer cells and promotes angiogenesis of HUVECs through a coordinated cross-talk with eNOS.
2013; 8: e74464 [PMID: 24040255 DOI:10.1371/journal.pone.0074464]
114 Zhuang G, Hunter S, Hwang Y, Chen J.Regulation of EphA2 receptor endocytosis by SHIP2 Lipid phosphatase
phosphatidylinositol 3-Kinase-dependent Rac1 activation.
2007; 282: 2683-2694 [PMID: 17135240 DOI:10.1074/jbc.M608509200]
115 Deroanne C, Vouret-Craviari V, Wang B, Pouysségur J.EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading
the Rac/PAK pathway.
2003; 116: 1367-1376 [PMID: 12615978 DOI: 10.1242/jcs.00308]
116 Cuyàs E, Queralt B, Martin-Castillo B, Bosch-Barrera J, Menendez JA.EphA2 receptor activation with ephrin-A1 Ligand restores cetuximab efficacy in NRAS-mutant colorectal cancer cells.
2017; 38: 263-270 [PMID: 28560458 DOI: 10.3892/or.2017.5682]
117 Himanen JP, Goldgur Y, Miao H, Myshkin E, Guo H, Buck M, Nguyen M, Rajashankar KR, Wang B, Nikolov DB.Ligand recognition by A-class Eph receptors: crystal structures of the EphA2 Ligand-binding domain and the EphA2/ephrin-A1 complex.
2009; 10: 722-728 [PMID: 19525919 DOI: 10.1038/embor.2009.91]
118 Noberini R, Koolpe M, Peddibhotla S, Dahl R, Su Y, Cosford ND, Roth GP, Pasquale EB.Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors.
2008; 283: 29461-29472 [PMID:18728010 DOI: 10.1074/jbc.M804103200]
119 Giorgio C, Hassan Mohamed I, Flammini L, Barocelli E, Incerti M, Lodola A, Tognolini M.Lithocholic acid is an Ephephrin ligand interfering with Eph-kinase activation.
2011; 6: e18128 [PMID: 21479221 DOI:10.1371/journal.pone.0018128]
120 Kiewlich D, Zhang J, Gross C, Xia W, Larsen B, Cobb RR, Biroc S, Gu JM, Sato T, Light DR, Heitner T, Willuda J,Vogel D, Monteclaro F, Citkowicz A, Roffler SR, Zajchowski DA.Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth.
2006; 8: 18-30[DOI: 10.1593/neo.05544]
121 Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D,Lin C, Chen J.Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo.
2002; 21: 7011-7026 [PMID: 12370823 DOI: 10.1038/sj.onc.1205679]
122 Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, Fazenbaker C, Fleming R, Swamynathan S, Meyer D, Senter PD, Gao C, Wu H, Kinch M, Coats S, Kiener PA, Tice DA.A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo.
2008; 68: 9367-9374 [PMID: 19010911 DOI: 10.1158/0008-5472.CAN-08-1933]
123 Tandon M, Vemula SV, Sharma A, Ahi YS, Mittal S, Bangari DS, Mittal SK.EphrinA1-EphA2 interaction-mediated apoptosis and FMS-like tyrosine kinase 3 receptor ligand-induced immunotherapy inhibit tumor growth in a breast cancer mouse model.
2012; 14: 77-89 [DOI: 10.1002/jgm.1649]
124 Fang WB, Brantley-Sieders DM, Hwang Y, Ham AJ, Chen J.Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase.
2008; 283: 16017-16026 [PMID: 18387945 DOI:10.1074/jbc.M709934200]
125 Duggineni S, Mitra S, Lamberto I, Han X, Xu Y, An J, Pasquale EB, Huang Z.Design and Synthesis of Potent Bivalent Peptide Agonists Targeting the EphA2 Receptor.
2013; 4 [PMID: 24167659 DOI:10.1021/mL3004523]
126 Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W.Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor.
2008; 27: 7260-7273 [PMID: 18794797 DOI:10.1038/onc.2008.328]
127 Bishop-Bailey D.Tumour vascularisation: a druggable target.
2009; 9: 96-101 [PMID: 19056315 DOI: 10.1016/j.coph.2008.10.004]
128 Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U.Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin.
2005; 19: 1689-1691 [PMID: 16081502 DOI: 10.1096/fj.04-3647fje]
 World Journal of Gastrointestinal Oncology2022年5期
World Journal of Gastrointestinal Oncology2022年5期
- World Journal of Gastrointestinal Oncology的其它文章
- Gut microbiome in non-alcoholic fatty liver disease associated hepatocellular carcinoma: Current knowledge and potential for therapeutics
- Helicobacter pylori, gastric microbiota and gastric cancer relationship: Unrolling the tangle
- Scoping out the future: The application of artificial intelligence to gastrointestinal endoscopy
- Pretreatment serum albumin-to-alkaline phosphatase ratio is an independent prognosticator of survival in patients with metastatic gastric cancer
- Preoperative prediction of malignant potential of 2-5 cm gastric gastrointestinal stromal tumors by computerized tomography-based radiomics
- Digital single-operator cholangioscopy for biliary stricture after cadaveric liver transplantation
