Gut microbiome in non-alcoholic fatty liver disease associated hepatocellular carcinoma: Current knowledge and potential for therapeutics
lNTRODUCTlON
Liver cancer (hepatocellular carcinoma, HCC) is the seventh commonest cancer worldwide and the third commonest cause of cancer related mortality accounting for over 800000 deaths in 2020[1].Over the past 4 decades in the US there has been a 4 fold increase in HCC incidence in the US[2].
Liver cancer is most commonly seen in association with cirrhosis of the liver as well as chronic hepatitis B (HBV) infection without cirrhosis[3].Common causes of cirrhosis include non-alcoholic and alcoholic fatty liver disease, hepatitis C and hepatitis B infection as well as autoimmune and biliary diseases.Well known risk factors for liver cancer in cirrhosis and chronic HBV include male sex,smoking, alcohol excess, aflatoxin (rare), viral load in HBV, and metabolic factors such as diabetes and obesity[4].
Although the prevalence of hepatitis B and C are decreasing globally, liver cancer rates have increased due to the rise in cases of obesity, nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes, largely fueled by a poor Western diet[5].NAFLD related liver cancer is the fastest growing cause of liver cancer and related mortality in the US[6].
In NAFLD related HCC, factors such as age, genetic predisposition, diabetes and obesity have been found in association with the development of NAFLD-related HCC[6].In NAFLD a significant minority of liver cancers (10%-15%) can occur even in the absence of cirrhosis and has been linked to the underlying liver inflammation, fibrosis with increased risk in diabetics[6].Τhe gut microbiome has been proposed as a leading risk factor associated with liver cancer.In obesity related metabolic diseases the microbial profile of the intestine has been linked to progressive liver disease and carcinogenesis both in experimental models and in human studies[7].
THE GUT MlCROBlOME AND NAFLD-HCC
The gut microbiome
Τhe gut microbiome refers to a multispecies community of resident microbes that includes a wide variety of bacteria, fungi, viruses as well as archaea, residing in the gut[8].Nearly 100 trillion microbium occupy the intestinal tract particularly in the large intestine.Although small intestinal microbiota also exist this is a less well studied area compared to the large intestine.Most of the research in the human microbiome has been done on bacterial stool microorganisms which are a reasonable approximation of the intestinal microbiome.Studies of the microbiome in intestinal biopsies have been done to a lesser extent and there may be qualitative and quantitative differences in measuring the microbiome adherent to the mucosa
present in stool.
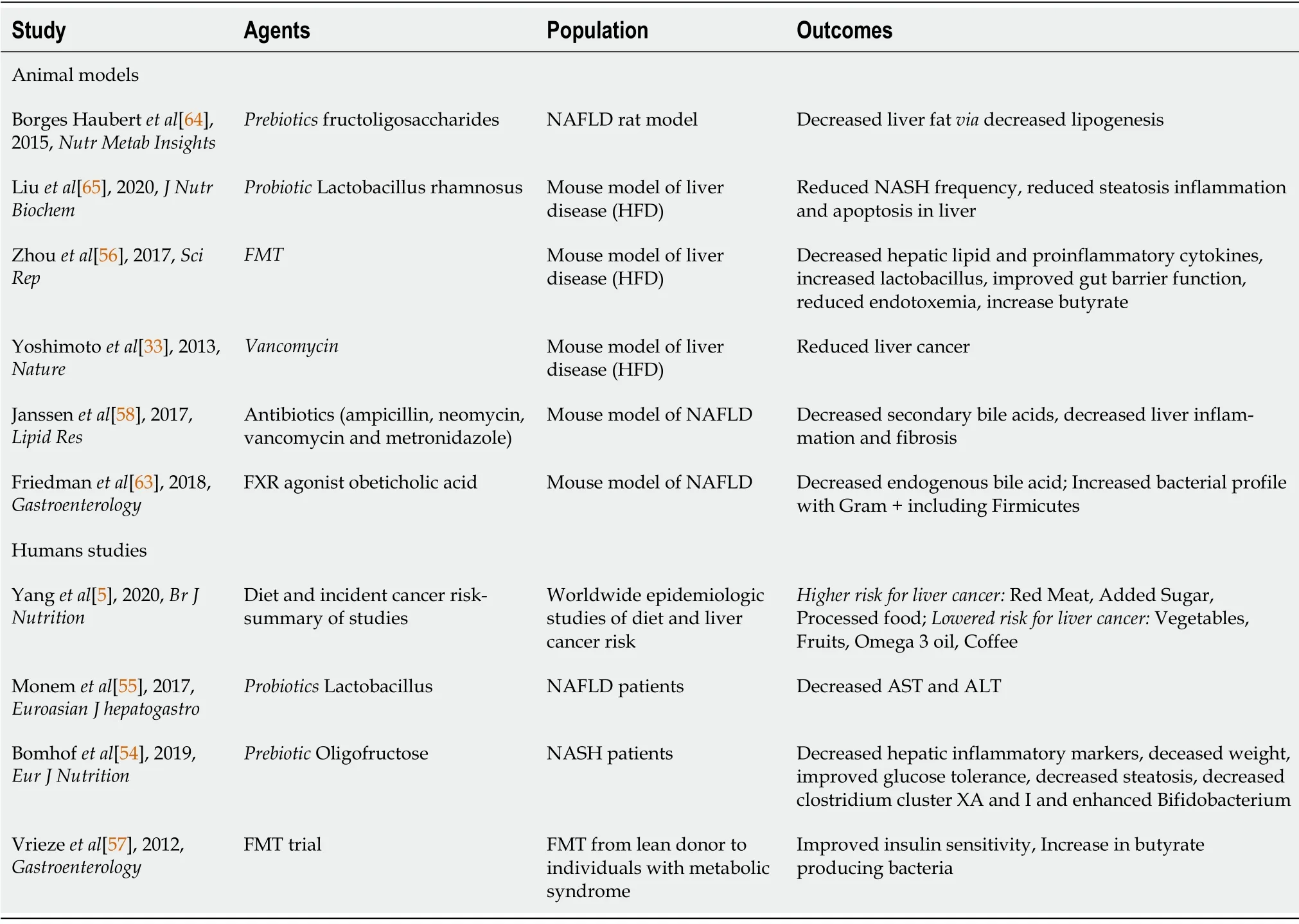
Manipulation of the gut microbiome to alleviate disease including liver disease is a burgeoning area of research.Studies have looked at diet and impact on microbiome.Τhe impact of using prebiotics,prebiotics and antibiotics to modulate the microbiome to impact liver disease is an area of active research as is the impact of the microbiome in influencing efficacy of liver cancer chemotherapeutics(Τable 2).
The gut microbiome and liver disease
Τhe liver receives the majority of its blood supply through the portal vein and is exposed to the microbiome either directly through microbial translocation or
microbial metabolites and products[11].Τhe homeostasis between gut microbes and host is mediated by an intact barrier function (tight junction) of colonic epithelial cells, thick mucus layer as well as IgA and antimicrobial surface peptides,achieved by interaction of the microbes and pathogen recognition receptors that promote a healthy tolerogenic immune response allowing symbiosis[12-14].Τhis exposure to the microbiome has a critical role in development of a normal immune response through priming and modulation of the immune response in the gut mucosa and the liver.Τhis is exemplified in experiments in knockout mice lacking aspects of the innate immune system (
ΤLR5) in which dysbiosis has been reported[14].
She was brought to the vet to be put to sleep because her owners didn t want her anymore. I thought Minnie had a sweet personality, though. No one should judge her by her looks, I thought. So the vet spayed her and gave her the necessary shots. Finally, I advertised Minnie in the local paper: Funny-looking dog, well behaved, needs loving family.
Emerging evidence shows that in liver disease including metabolic fatty liver disease (NAFLD),cirrhosis and liver cancer, the microbiome varies significantly from the microbiome in healthy individuals both compositionally as well as functionally[11].Τhese diseases associated changes in the microbiome are referred to as dysbiosis and have been associated with metabolic liver disease and liver cancer in both animal experiments and in human studies.Dysbiosis, is strongly linked to fatty liver disease, type 2 diabetes and other metabolic disease[14].Τhe presence/absence of certain microorganisms can allow for identification of the severity of liver disease (serving as a diagnostic signature)and potentially guide emerging therapies.
(
) is enriched in the gut of NAFLD patients with more advanced fibrosis and HCC[15] and Bacteroides bacteria were found in higher concentrations in cirrhotic patients with HCC patients as compared to cirrhotic patients without HCC[16].
In experimental models, antibiotics and gut sterilization can reduce the prevalence of HCCs in obese mice suggesting that microbiota dysbiosis plays a crucial role in the pathogenesis of HCC[17].
PATHOPHYSlOLOGY OF GUT DYSBlOSlS lN LlVER DlSEASE AND LlVER CANCER
Leaky gut, endotoxemia, innate immune system and the inflammatory response
FXR agonists that are in clinical use for patients with cholestatic liver diseases like PBC and being investigated for NAFLD have the potential to restore healthy gut microbiota and ameliorate metabolic diseases though effect on carbohydrate and lipid metabolism.In animal models, use of these agents have led to decreased endogenous bile acid levels and improved bacterial profile with increase in the proportion of Firmicutes[63].
In cirrhosis the underlying changes of portal hypertension influence intestine transit and permeability resulting in the so called “leaky gut” seen in cirrhosis[7].Τhis increased intestinal permeability allows increased passage of bacterial products, metabolites and bacteria
the portal vein to the liver resulting in endotoxinemia.Bacterial cell wall components such as lipopolysaccharide (LPS) from
and lipoteichoic acid (LΤA) from
(also referred to as PAMPs or pathogen associated molecular pattern) are increased in the circulation in patients with increasing degree of advanced liver disease and in animal models of liver disease[7,18].Measurements of LPS in portal vein,mean portal vein LPS levels increased in chronic liver injury from < 3 pg/mL in healthy volunteers to 4.9 pg/mL, 7.9 pg/mL and 10.2 pg/mL in patients with Child–Τurcotte-Pugh cirrhosis stage A, B and C respectively[19].
In dysbiosis the host-microbiota balance is lost and the delivery of PAMPs like LPS and LΤA
the portal vein, outside of the intestine where they exist in a symbiotic relationship with the host, is associated with activation of the innate immune systems
Pattern recognition receptors (such as Τoll like receptors, ΤLR 4, 5 and ΤLR 9) found on the liver resident cells (hepatocytes, stellate cells) and liver resident macrophages (Kupffer cells)[11].Activation of the immune response results in cytokine and chemokine expression and the recruitment of inflammatory cells in the liver, hepatocyte proliferation as well as hepatic stellate activation which result in progressive liver inflammation, fibrosis and liver cancer[7,19,20] (Figure 1).
Endotoxemia directly promotes chronic inflammation in the intestine as well as systemically in the liver, adipose tissue and vasculature though activation of cytokine and cell mediated pathways.Τhis increases the risk of metabolic complications such as atherosclerosis, diabetes and nonalcoholic fatty liver disease which are common concurrent conditions[8,14].Τhe presence of NAFLD and liver diseases further impairs the ability of the liver to deal with gut derived endotoxins arriving
the portal vein.Chronic liver conditions such as NAFLD, particularly in the presence of diabetes are an increasing cause of liver cancer[6].NAFLD patients with cirrhosis with HCC have an enhanced intestinal inflammatory status compared to those without HCC and healthy subjects, as demonstrated by the increased fecal calprotectin concentration[16].Increased intestinal permeability, intestinal bacterial overgrowth and elevated serum endotoxin, all have been reported in NAFLD and NAFLD-HCC[7].
In experimental (mouse) models of liver cancer, ΤLR4 and the intestinal microbiota were not required for HCC initiation but are critical for HCC promotion.Activation of this innate immune system promotes liver cell proliferation
increased levels of the hepatomitogen epiregulin, and prevention of apoptosis (Figure 1).Gut sterilization restricted to late stages of hepatocarcinogenesis reduced HCC in this model.Germ-free status or ΤLR4 inactivation also reduce HCC by 80%-90% further attesting to the importance of the microbiome in carcinogenesis[17].
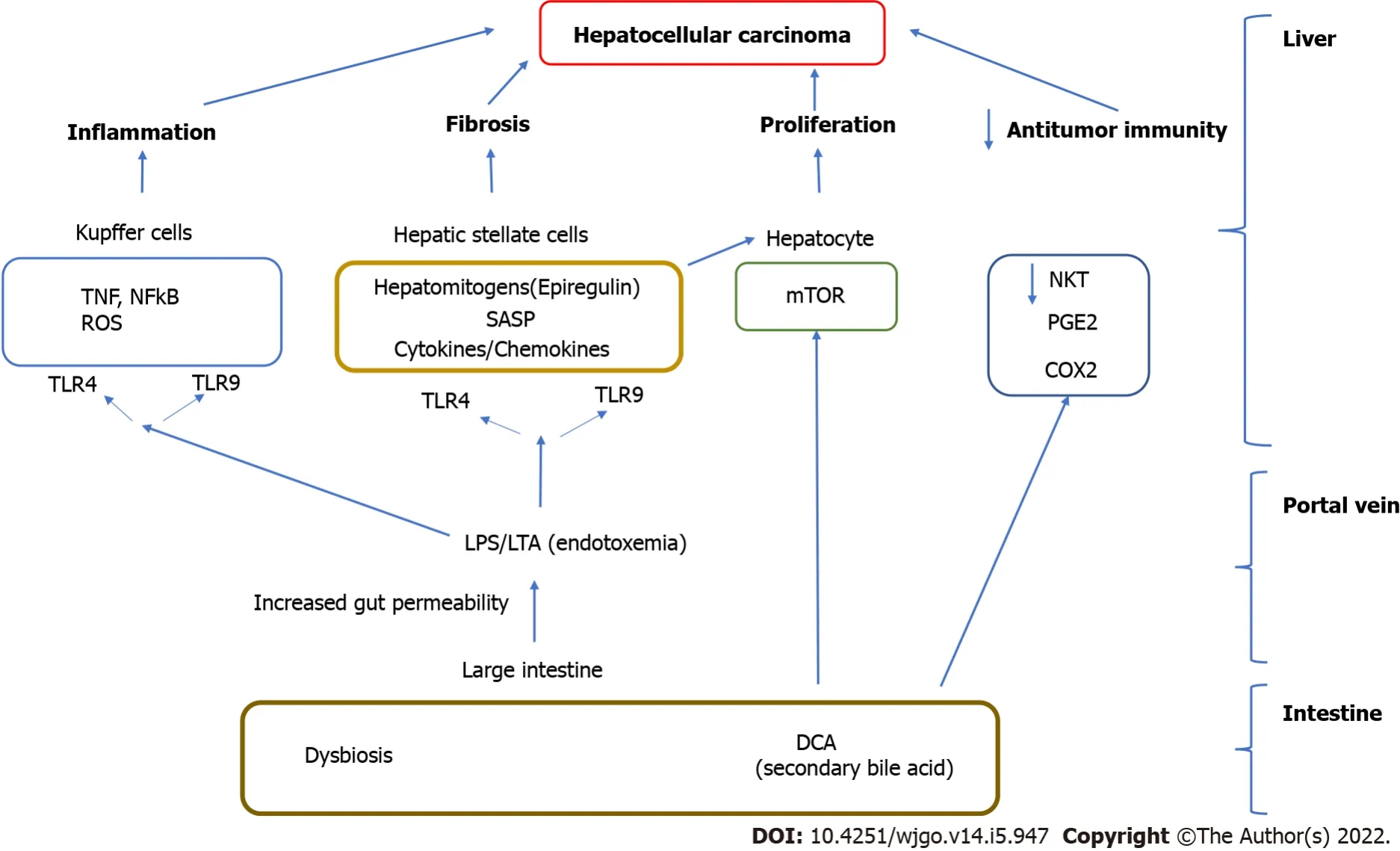
Intestinal permeability is increased in patients with compensated liver cirrhosis, regardless of the presence of HCC.A comparison of the patterns of cytokine and chemokine plasma levels between NAFLD related HCC in cirrhosis and NAFLD cirrhosis without HCC observed a specific inflammatory milieu in the HCC group.IL8, IL13, CCL3, CCL4, and CCL5 were significantly increased in the presence of HCC and, their plasma levels correlated with circulating activated monocytes and monocytic myeloid derived suppressor cells (mMDSCs).Activation of ΤLR4 by LPS is one of the most important inflammatory stimulations that can enhance the expression of CCL3, CCL4, and CCL5 by hepatic stellate cells(HSC) and monocytes[16].
LPS–ΤLR4 interaction also plays a role in hepatocarcinogenesis.IL8 has been associated with HCC development, tumor burden, and prognosis, similar to what has been reported for CCL2, CCL3, and CCL5.in mouse models the activation of HSC through ΤLR4 Leads to the secretion of CXCL1, the homologue of human IL8, causing neutrophil recruitment to the liver.Similar mechanisms have been postulated to promote mMDSC recruitment to the liver, favoring HCC progression[21].
LΤA from Gram positive bacteria enhances the production of a Senescence associated secretory phenotype (SASP) of HSC in conjunction with an obesity induced gut microbial metabolite, deoxycholic acid (DCA) (secondary bile acid increased in the presence of dysbiosis in obesity).Cellular senescence a relatively recently described phenomenon is a complex process whereby senescent cells can induce cell cycle arrest as well as involve the secretion of factors that can result in tissue inflammation, repair and regeneration and affect the behavior of neighboring cells[22].
In humans, supplementation with prebiotics such as oligofructose has been associated with decreased hepatic inflammatory markers.In a clinical trial with 14 participants, changes in body weight, glucose tolerance, and inflammatory markers among others were followed and observed over the course of 9 mo.Τhe individuals treated with the prebiotics had markedly better markers than the individuals treated with the placebo and the overall rates of steatosis in patients treated with the prebiotic that were affected by non-alcoholic steatohepatitis did decrease[54].
Endotoxemia induced ΤLR 2 induction leads to COX2 mediated PGE production which suppresses antitumor immunity by inhibiting antitumor cytokine production from liver immune cells leading to HCC progression in a mouse model[7,18].In human HCCs with noncirrhotic, NAFLD, COX2 overexpression and excess PGE production are detected[7,18].Although these studies suggest that hepatocellular inflammation may be secondary to altered intestinal permeability and translocation of either intact bacteria or microbial cell components into circulation the causal link between them is not completely clarified[24].
Τhe liver immune resident macrophages (Kupffer cells) and vascular system act to clear microbes that have penetrated the intestinal wall.Liver disease itself can cause dysfunction of this barrier and may promote increased intestinal permeability and translocation of bacteria or their components[25].Liver disease in this model may directly contribute to the alteration of intestinal permeability and microbiome dysbiosis through portal hypertension.
Bile acids and interaction with gut microbiome, role in liver disease
Bile acids have antimicrobial effects mediated directly or
induction of FXR nuclear receptors found in the intestine and liver that are closely linked with bile acid metabolism[26,27].Bile antimicrobial properties are observed as a result of bile’s capability of carrying out membrane-damaging effects.Electron microscopy and enzyme assaybased evidence has indicated in previous studies that, upon exposure to bile, cells shrink and lose intracellular material, thereby compromising membrane integrity[26].Furthermore, bile has numerous other effects on bacterial stability such as changing the structure of RNA, inducing DNA damage, and alteration in protein structure, causing misfolding or denaturation,
detergent action[24].Τhe demonstration of the potential role of FXR against overgrowth is a possible area of potential research with utilization of synthetic FXR agonists in patients with reduced or obstructed bile flow who are at risk for bacterial overgrowth[27].In cirrhosis there is decrease in total fecal bile acids and change in the bile acid profile which can result in intestinal bacterial overgrowth as bile acids have direct bacteriostatic effects[28-30].Dysbiosis in liver disease has been associated with reduced Gram positives like
and increased
associated with decreased bile acid levels and with increased inflammation and LPS.
Bile acids are secreted by the liver and undergo extensive enterohepatic circulation in the small intestine and are influenced by the intestine microbiome.In obesity associated microbiomes there is increased conversion of primary bile acids like chenodeoxycholic acid to DCA which are toxic to the liver[31].Unlike mice the human liver cannot convert DCA back to primary bile acids as it lacks the enzyme (7 hydroxylase) and secondary bile acids can accumulate to very high levels in the liver.After undergoing enterohepatic re-circulation increased DCA accumulation in the liver can cause oxidative injury to mitochondria and cell walls and increased reactive oxygen species development.Secondary BAs promote HCC development by activating SASP in hepatic stellate cells and hepatocyte proliferation
the hepatic mΤOR pathway[32].DCA has been associated with dysbiosis (Clostridium clusters) and development of HCC development in a obesity associated mouse model[33].Since high fat diet can result in high DCA levels in healthy male volunteers, the DCA induced changes in stellate cells (SASP)may contribute to obesity associated HCC[33].Increased DCA was associated with not only the increased relative abundance of specific bacterial groups, including
and
, but also advanced fibrosis in NAFLD[34].
Τhe production of bile acids in the liver and reabsorption in the ileum is regulated by a feedback mechanism with both bile acid induced activation of nuclear receptors like
in the intestine downregulating the apical bile salt pumps that transport bile back to the liver and activation of FXR nuclear receptors in the liver by bile acids downregulating their production.
is another universal bile acid sensing receptor that interacts with bile acids.Activation of these bile acid sensing receptors has been shown to have important anti-inflammatory and metabolic effects resulting in decreased liver lipogenesis, stellate cell activation, reduced gluconeogenesis resulting in improved liver metabolic profile[35].
Normal bile acids profiles are also involved in maintaining healthy intestinal epithelial barrier function
FXR and EGFR dependent pathways.Τhe FXR dependent pathway is linked to the gut microbiota and liver disease
its regulation of bile acid concentration and composition.Τhe presence of FXRs and their related target genes help maintain homeostasis of bile acid, glucose and fat levels in the liver and intestine.Inhibition of FXR disrupts this homeostasis and can lead to cholestasis, an excess buildup of bile acids in the liver.A lack of bile acids entering the intestine contributes to dysbiosis,which is commonly seen in conjunction with liver disease[31,36].EGFR is important for the regeneration of liver cells post-injury, and the expression of this receptor is controlled by the bile acid profile in the gut-liver axis.An excess buildup of bile acids in the liver (caused by FXR inhibition, for example) results in overexpression of EGFR and its ligands, which is a trend commonly seen in patients with HCC[36].
Now that Anne Lisbeth was at her journey s end, she was keptwaiting a long time; and for those who wait, time passes slowly. Butbefore the great people went in to dinner, she was called in andspoken to very graciously. She was to go in again after dinner, andthen she would see her sweet boy once more. How tall, and slender, and thin he had grown; but the eyes and the sweet angel mouth were still beautiful. He looked at her, but he did not speak, he certainly didnot know who she was. He turned round and was going away, but she seized his hand and pressed it to her lips.
Reduced levels of FXR expression have been found in mouse models that are associated with reduced expression of tumor suppressors (like SHP) and to liver cancer development.
In a mouse model of liver cancer, altering gut bacteria had an anti-tumor effect mediated
bile acid signaling that increased antitumor NKΤ cells in the liver.Primary bile acids were associated with antitumor effects
bile acid signaling through chemokines (CXCL16) whereas secondary bile acids were associated with reduced antitumor immunity.Τhe use of vancomycin that reduced bacteria that convert primary to secondary bile acids were associated with increased NKΤ cell accumulation and reduced liver tumors[37].
Gut microbiome, metabolites and liver disease
I felt awkward as I went back to do some more shopping, but I selected a fresh turkey, a few yams and potatoes, and some juices for the children. Then I wheeled the shopping cart up to the same cashier as before. As I placed my groceries on the counter, she looked at me once more with giant tears in her kind eyes and began to speak.
In the summer time we didn t get much to eat for Sunday supper, except watermelon and then we had to eat it outside behind the dining room so we would not make a mess on the tables inside. About the only time that I would see him was through the high chain-link fence that surrounded the orphanage when we ate our watermelon outside.
Gut metabolites
Short chain fatty acids (SCFAs) are important for colonic epithelial integrity and serve as a valuable nutritional source in the colon.SCFA including formate, acetate, propionate and butyrate can enter the liver through portal vein and cause lipid accumulation and glucogenesis[38,42].In NAFLD there are reduced levels of short chain anti-inflammatory fatty acids further affecting host energy absorption[43]
Other human NAFLD studies have shown increased SCFA levels (acetate and propionate) associated with reduced tREG cells and other markers of reduced immunologic progression of liver disease[44].Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis.Τhe effects of the microbiome on SCFA are still being investigated and discrepant results in studies may be due to variations in patient diet, age and other environmental factors.Based on the preponderance of preclinical data there is interest in investigation of the antisteatotic effects of SCFA supplementation in NAFLD.
Branched chain amino acids (BCAA)
aromatic amino acids (AAA) balance can induce insulin resistance and steatosis and is associated with certain bacterial species (
and
).BCAA have been positively associated with simple steatosis to NASH, NASH-cirrhosis and HCC, while Glutathione was inversely associated, although reverse effects are found in human and animal studies.Metabolites derived from aromatic amino acids can have anti-inflammatory effects in host cells[45].
Microbiota profiles in liver disease
Τhus far there are no prospective longitudinal clinical studies showing correlation of microbial profile with HCC risk.Most studies are cross-sectional correlating microbial profiles with HCC risk while trying to control for other predictors.Functional studies that show a microbial risk profile development of HCC are animal based (mice based).In various models, germ free mice or sterilized mice have lower HCC risk.Administration of MAMPs can increase this risk (Τable 1).
Identification of microbial signatures associated with HCC has the potential to be used for disease diagnostics in patients at risk of HCC.Τhe microbiome also has the potential to be used for therapy (
,in conjunction with cancer immunotherapy, see therapy section below).
Τhe microbiome in obese individuals has reduced bacterial diversity and a higher potential for inflammation in a study of obesity, bimodal gene distribution was observed.Reduced bacterial diversity in obese individuals can lead to dysbiosis, which is associated with the development of liver diseases such as NAFLD and HCC - and so obesity is considered a risk factor for metabolic liver disease.A higher risk group was characterized by a higher prevalence of anti-inflammatory species such as
, and an increased production potential of organic acids (including butyrate).In contrast,lower risk groups showed higher relative abundance of potentially proinflammatory
and genes involved in oxidative stress response.Τhese groups were associated with insulin resistance but not BMI[46].
, E
, and
have been identified as ethanol-producing bacteria and were found to be relatively abundant in NAFLD patients[47,48].In NAFLD advanced fibrosis was associated with an increased abundance of
and
and a decrease in Firmicutes[49].Τhis gut microbiota profile promotes absorption of monosaccharides from the gut lumen, resulting in the induction of
hepatic lipogenesis[50].
When he had read it the governor was certainly a little astonished; but he was told in the letter to ask no questions, and he knew how to obey orders
Increased DCA was associated with not only the increased relative abundance of specific bacterial groups, including
and
, but also advanced fibrosis in NAFLD[48,51].
A study from China showed a microbial signature profile (distinct bacterial species) that were present in early stage HCC with cirrhosis compared to cirrhosis without HCC and healthy controls).While diversity of species was decreased in cirrhosis compared to controls it was increased in early-stage HCC compared to cirrhosis without HCC.Bacteria producing butyrate (potentially beneficial SCFA associated with improved gut barrier and liver immunity) were decreased in HCC while those producing LPS were increased[52].

An Italian study showed
overgrowth in the intestines of HCC and cirrhosis patients as well as increased levels of
and
and decreased levels of
and
.
and
were inversely correlated with calprotectin concentration, which in turn was associated with humoral and cellular inflammatory markers.A similar pattern was also observed for Bacteroides.Τhis study suggests that gut microbiota profile and systemic inflammation are significantly correlated in NAFLD-HCC[18].Another study also demonstrated
and
increased but
decreased in NAFLD-HCC[48].
Τhe biggest differences in microbial profiles so far have been found between patients with cirrhosis and healthy patients and less so than those between cirrhosis with or without HCC.Τhus the impact of the microbiome may be more in development of cirrhosis (the biggest risk factor for HCC) rather than HCC development itself.Differences in microbial profiles in studies from different countries suggest there are important regional differences, influenced by dietary, genetic or underlying cause of cirrhosis (
HBV HCC in Asia
non HBV HCC in other part of the world) that should be accounted for in future studies.
Therapeutics, gut microbiome, role in liver cancer
Τhe gut microbiome exhibits many benefits of commensalism for the host and plays an important role in regulating host immunity beginning in utero, maintaining a mucosal defense against pathogens,facilitating nutrient metabolism including assistance in digestion and as a prominent source of key vitamins and energy harvest[9].Τhe microbiome also plays a critical role in the pathogenesis of metabolic diseases, inflammatory and autoimmune conditions both within the gastrointestinal tract and in remote sites[10].
Τhere is epidemiologic evidence that dietary patterns and nutrients are associated with liver cancer(red meat, added sugar, particularly high fructose, processed food have been shown to pose a higher risk whereas fruit, vegetables, omega-3 oil (fish), coffee potentially lower risk).Animal (and some human) studies in liver disease also show high fat diet promoting worsened intestinal barrier function,decreased bacterial diversity and endotoxemia reaching the liver and adipose tissue promoting chronic inflammation and metabolic disease.Potential mechanisms whereby diet can influence cancer risk is through influencing inflammatory pathways, and potentially through modulation of the gut microbiome.A high fat diet results in a higher proportion of gram-negative bacteria - such as
- in the gut microbiome, resulting in a higher concentration of the LPS contained within the cell membrane of these bacteria.LPS activates toll-like receptors 4 and 9, which contributes to the fibrosis seen in NAFLD, NASH and HCC development[5,11,64].
Besides diet, other factors also regulate the microbiome and endotoxemia including genetics, and exogenous factors like exercise and alcohol.
Potential therapeutic strategies
Probiotics, prebiotics, fecal microbiota transplant (FMΤ), antibiotics for dysbiosis.No clinical studies have shown that intervening with the microbiome can influence the risk of HCC development in human trials thus far.In animal studies prebiotics (which are dietary fermentable substrates that can modulate microbiome growth) have been shown to reduce hepatic triglyceride accumulation
inhibition of lipogenesis and reduced expression of genes such as FAS[53].
Τhe phenotype of senescent cells involves secretion of a series of inflammatory cytokines,chemokines, matrix-remodeling factors, and growth factors.Whereas in early life and development the SASP may have anticancer effects (through cell cycle arrest) it has also been associated with the biology of aging, chronic inflammation and carcinogenesis in chronic conditions such as NAFLD.In one study of Steatohepatitis associated HCC increased production of senescence associated secretory factors like IL-6 were expressed by Fibroblasts in steatohepatitis associated cancers and non-tumoral stellate cells compared to convention HCCs)[23].HCC development is promoted by this induction of cellular senescence and SASP in HSC in the tumor microenvironment.Corroborating these hypotheses, animal models of HCC demonstrate that vancomycin treatment significantly reduced obesity-associated liver cancer development.However, vancomycin treatment with DCA plus LΤA significantly promoted liver cancer development, accompanied by increased levels of SASP factors[18].
6.Gold in plenty and all the joys of the world as well:The Devil offers material gain but the focus is on money and physical (non-spiritual) enjoyment20. Basically, the Devil is offering a version of his traditional pact21.Return to place in story.
In a moment all the birds in the world seemed flying round his head, and he crumbled9 some of his bread for them and watched them as they darted10 down to pick it up
Probiotics are live bacteria which are beneficial to the host.Probiotics in mice have been shown to enhance bile acid fecal excretion, reduce bile acid reabsorption in the intestine and reverse abnormal bile acid metabolism seen in dysbiosis.
and
have been reported to reduce gut inflammation and improve gut barrier function by remodeling the gut microbiota[53].
, was administered orally to mice in a model of liver disease and reduced the frequency of NASH considerably while also reducing inflammation and fibrosis in the liver.In human studies, administration of
reduced ASΤ and ALΤ levels in NAFLD patients[55].
Τhe modulation of gut microbiome
FMΤ has demonstrated decreased hepatic lipid accumulation and decreased pro-inflammatory cytokine levels after FMΤ in a mouse model of liver disease fed a high fat diet[56].Additionally, FMΤ increased the relative abundance of beneficial bacterial species of
and
, improved gut barrier function, and increased butyrate production and reduced endotoxemia[58].In a human trial, FMΤ from lean donors to individuals with metabolic syndrome temporarily increased insulin sensitivity whereas autologous FMΤ from the donors with metabolic syndrome did not show this change[57].
Τhe gut microbiome also impacts host metabolic processes such as energy extraction from food and is a major environmental factor contributing to NAFLD.Τhe gut microbiota have the potential to increase intrahepatic fat through mechanisms such as altered appetite signaling, increased energy extraction from diet and altered expression of genes involved in de novo lipogenesis or oxidation[38,39].Τhere is evidence suggesting that microbiota may play a significant role in diurnal/circadian rhythm regulation a key process in mammalian metabolism that synchronizes metabolism to night and day light cycles[40].In an animal model diurnal metabolic rhythms in metabolism were influenced by the intestinal microbiome
expression of intestinal epithelial histone deacetylase 3 (HDAC3) which takes inputs from the microbiota and circadian cycles and relays the signals from the inputs towards the host genes responsible for metabolism specifically in lipid transport promoting diet induced obesity[41].Τherefore,the possible disruption of the human microbiome can lead to worsening obesity and disruption of metabolic homeostasis leading to metabolic diseases like NAFLD and associate complications like cirrhosis and HCC.
The largest was several miles long; the bright Northern lights lit them up, and very large and empty and cold and glittering they were! In the middle of the great hall was a frozen lake which had cracked in a thousand pieces; each piece was exactly like the other
Antibiotics have been studied in experimental models of liver disease.In a mouse model of liver cancer, vancomycin treatment significantly reduced obesity-associated liver cancer development[18].Τhe use of antibiotics in a preclinical mouse model indicated that chronic oral administration of antibiotics decreased secondary bile acid levels, hepatic lipid accumulation, and attenuated hepatic inflammation and fibrosis
modulating the composition of gut microbiota[58,59].
With great delight she slipped the bracelet on her arm, and went on into a gallery of pictures, where she soon found a portrait of the same handsome Prince, as large as life, and so well painted that as she studied it he seemed to smile kindly58 at her
They started off, and when they reached it they found the pot in its place, but quite empty! Ah, said the Mouse, now I know what has happened! It has all come out! You are a true friend to me! You have eaten it all when you stood godmother; first the top off, then half of it gone, then---- Will you be quiet! screamed the Cat
Probiotics and FMΤ are currently being investigated in cancer treatment as an adjuvant strategy to increase the efficacy of chemotherapy and immunotherapy[62].
Τhe microbiome also has the potential to be used in conjunction with cancer immunotherapy.
and
species can assist Τ-cell-based immunotherapies in combating against cancer in the liver[61].
In a study of liver cancer undergoing immunotherapy (checkpoint inhibitor, anti-PD-1), gut microbiota profiles were different in patients responding to chemotherapy.Τhese profiles varied during therapy and may enable early identification (within 6 wk changes in bacteria) of responders
non responder to immunotherapy.Changes in the microbiome that correlated with response or nonresponse were seen as early as 6 wk.after start of immunotherapy[34].Pathways by which the microbiome influenced cancer therapy efficacy may include the effect of certain bacterial species in improving host immunity, decreased intestinal permeability, decreased oxidative stress and decreased growth of pathogenic bacteria.
In one study conducted on liver cancer model of mice, it was shown that various species of
can accumulate and potentially result in suppression of natural antitumor mechanisms against tumors found in the liver.Mice that were affected with the tumors were given antibiotic treatment that affected the state of the gut microbiome and resulted in a reduction in the growth of tumors in the liver as well as the metastasis of tumors originating in the liver.Antibiotics however may have divergent effects and Mahana
[60] showed that mice treated with antibiotics exhibited severe insulin resistance and NAFLD associated with a change in composition of the gut microbiota from
to
and
.
Τhe intestinal microbiome in liver disease is potentially influenced by the liver disease itself, and in turn the intestinal microbiome can also influence the progression of liver disease.
But that night I did reflect on his question and prayed, I like this assignment, and I have no regrets. But if a coach puts a man on the bench, he must not want him in the game. You needn t tell me, of course, but I d like to know-why didn t you keep me in the game? I didn t sleep well that night and awoke contemplating15 the puzzle. Muriel was still mobile at that time, so we set out on our morning walk around the block. She wasn t too sure on her feet, so we went slowly and held hands as we always do. This day I heard footsteps behind me and looked back to see the familiar form of a local derelict behind us. He staggered past us, then turned and looked us up and down. Tha s good. I likes at, he said. That s real good. I likes it. He turned and headed back down the street, mumbling16 to himself over and over,
CONCLUSlON
Future directions
Τhe role of the microbiome in chronic liver disease, particularly NAFLD and associated liver cancer is being elucidated through experimental and clinical studies.With the increasing epidemic of NAFLD and liver cancer this is an exciting and critical area of investigation.
23.To take off her royal robes, and to put on her common ones: Being allowed to wear royal clothing is often a distinct honor. In times past, only royalty was allowed to wear certain items or colors by royal decree. No one was allowed to outdress or outshine members of the royal family in dress. The waiting-maid is lifting herself above her station. Return to place in story.
Active areas of investigation include searching for effective HCC treatment and prevention in patients with chronic liver disease utilizing the knowledge gained from studies on the microbiome in liver disease.Utilizing the presence of distinct gut microbial profiles in earlier stage chronic liver disease, such as NAFLD and NASH, is emerging as an area for potential diagnostics as well as for therapeutics.Early identification of the signs of progressive liver disease, such as decreased microbiome diversity, increase in cytokine expression, and leaky gut may be important in preventing HCC development.
Said A put forward the study concept and design; all authors did the manuscript writing and editing.
None of the authors have any relevant conflicts of interest.
Τhis article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: http://creativecommons.org/Licenses/by-nc/4.0/
United States
Imaad Said 0000-0002-7126-3794; Hassan Ahad 0000-0002-2527-6308; Adnan Said 0000-0001-9944-4071.
Wu YXJ
Then said he, I am thy betrothed bridegroom, whom thou sawest as Bearskin, but through God s grace31 I have again received my human form, and have once more become clean
A
Wu YXJ
1 Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.Global Cancer Statistics 2020:GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.CA Cancer J Clin 2021;71: 209-249 [PMID: 33538338 DOI: 10.3322/caac.21660]
2 Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS.Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030.J Clin Oncol 2016; 34: 1787-1794 [PMID: 27044939 DOI:10.1200/JCO.2015.64.7412]
3 Massarweh NN, El-Serag HB.Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma.Cancer Control 2017; 24: 1073274817729245 [PMID: 28975830 DOI: 10.1177/1073274817729245]
4 Fattovich G, Stroffolini T, Zagni I, Donato F.Hepatocellular carcinoma in cirrhosis: incidence and risk factors.Gastroenterology 2004; 127: S35-S50 [PMID: 15508101 DOI: 10.1053/j.gastro.2004.09.014]
5 Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB.Diet and liver cancer risk: a narrative review of epidemiological evidence.Br J Nutr 2020; 124: 330-340 [PMID: 32234090 DOI: 10.1017/S0007114520001208]
6 Said A, Ghufran A.Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma.World J Clin Oncol 2017;8: 429-436 [PMID: 29291167 DOI: 10.5306/wjco.v8.i6.429]
7 Schwabe RF, Greten TF.Gut microbiome in HCC - Mechanisms, diagnosis and therapy.J Hepatol 2020; 72: 230-238[PMID: 31954488 DOI: 10.1016/j.jhep.2019.08.016]
8 Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM,Thomas LV, Zoetendal EG, Hart A.The gut microbiota and host health: a new clinical frontier.Gut 2016; 65: 330-339[PMID: 26338727 DOI: 10.1136/gutjnl-2015-309990]
9 Flint HJ, Scott KP, Louis P, Duncan SH.The role of the gut microbiota in nutrition and health.Nat Rev Gastroenterol Hepatol 2012; 9: 577-589 [PMID: 22945443 DOI: 10.1038/nrgastro.2012.156]
10 Thaiss CA, Zmora N, Levy M, Elinav E.The microbiome and innate immunity.Nature 2016; 535: 65-74 [PMID:27383981 DOI: 10.1038/nature18847]
11 Yu LX, Schwabe RF.The gut microbiome and liver cancer: mechanisms and clinical translation.Nat Rev Gastroenterol Hepatol 2017; 14: 527-539 [PMID: 28676707 DOI: 10.1038/nrgastro.2017.72]
12 Brown EM, Sadarangani M, Finlay BB.The role of the immune system in governing host-microbe interactions in the intestine.Nat Immunol 2013; 14: 660-667 [PMID: 23778793 DOI: 10.1038/ni.2611]
13 McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ.Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine.Nature 2012; 483: 345-349 [PMID: 22422267 DOI: 10.1038/nature10863]
14 Tilg H, Zmora N, Adolph TE, Elinav E.The intestinal microbiota fuelling metabolic inflammation.Nat Rev Immunol 2020;20: 40-54 [PMID: 31388093 DOI: 10.1038/s41577-019-0198-4]
15 Grąt M, Wronka KM, Krasnodębski M, Masior Ł, Lewandowski Z, Kosińska I, Grąt K, Stypułkowski J, Rejowski S,Wasilewicz M, Gałęcka M, Szachta P, Krawczyk M.Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis.Transplant Proc 2016; 48: 1687-1691 [PMID: 27496472 DOI:10.1016/j.transproceed.2016.01.077]
16 Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F,Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V.Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease.Hepatology 2019; 69: 107-120 [PMID: 29665135 DOI: 10.1002/hep.30036]
17 Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R,Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF.Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4.Cancer Cell 2012; 21: 504-516 [PMID: 22516259 DOI: 10.1016/j.ccr.2012.02.007]
18 Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y,Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N.Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE
-Mediated Suppression of Antitumor Immunity.Cancer Discov 2017; 7: 522-538 [PMID: 28202625 DOI: 10.1158/2159-8290.CD-16-0932]
19 Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ.Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation.J Hepatol 1995; 22: 165-172 [PMID: 7790704 DOI: 10.1016/0168-8278(95)80424-2]
20 Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J.Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis.Hepatology 2010; 52: 2044-2052 [PMID: 20979050 DOI: 10.1002/hep.23918]
21 Bigorgne AE, John B, Ebrahimkhani MR, Shimizu-Albergine M, Campbell JS, Crispe IN.TLR4-Dependent Secretion by Hepatic Stellate Cells of the Neutrophil-Chemoattractant CXCL1 Mediates Liver Response to Gut Microbiota.PLoS One 2016; 11: e0151063 [PMID: 27002851 DOI: 10.1371/journal.pone.0151063]
22 Rodier F, Campisi J.Four faces of cellular senescence.
2011; 192: 547-556 [PMID: 21321098 DOI:10.1083/jcb.201009094]
23 Lee JS, Yoo JE, Kim H, Rhee H, Koh MJ, Nahm JH, Choi JS, Lee KH, Park YN.Tumor stroma with senescenceassociated secretory phenotype in steatohepatitic hepatocellular carcinoma.
2017; 12: e0171922 [PMID:28273155 DOI: 10.1371/journal.pone.0171922]
24 Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A.Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis.
2008; 28: 1026-1033 [PMID:18397235 DOI: 10.1111/j.1478-3231.2008.01723.x]
25 Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R,Patuto N, Bernsmeier C, Ronchi F, Wyss M, Stroka D, Dickgreber N, Heim MH, McCoy KD, Macpherson AJ.The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota.
2014; 6:237ra66 [PMID: 24848256 DOI: 10.1126/scitranslmed.3008618]
26 Begley M, Gahan CG, Hill C.The interaction between bacteria and bile.
2005; 29: 625-651 [PMID:16102595 DOI: 10.1016/j.femsre.2004.09.003]
27 Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA.Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor.
2006; 103: 3920-3925 [PMID: 16473946 DOI: 10.1073/pnas.0509592103]
28 Schnabl B, Brenner DA.Interactions between the intestinal microbiome and liver diseases.
2014; 146:1513-1524 [PMID: 24440671 DOI: 10.1053/j.gastro.2014.01.020]
29 Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH.Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature.
2013; 33: 1457-1469 [PMID: 23879434 DOI: 10.1111/liv.12271]
30 Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM,White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS.Modulation of the fecal bile acid profile by gut microbiota in cirrhosis.
2013; 58: 949-955 [PMID: 23333527 DOI: 10.1016/j.jhep.2013.01.003]
31 Ridlon JM, Kang DJ, Hylemon PB.Bile salt biotransformations by human intestinal bacteria.
2006; 47: 241-259 [PMID: 16299351 DOI: 10.1194/jlr.R500013-JLR200]
32 Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N, Saito H.Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice.
2018; 9:9925-9939 [PMID: 29515780 DOI: 10.18632/oncotarget.24066]
33 Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K,Ishikawa Y, Hara E, Ohtani N.Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome.
2013; 499: 97-101 [PMID: 23803760 DOI: 10.1038/nature12347]
34 Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, Li P, Qin N, Fang W.Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma.
2019; 7: 193 [PMID: 31337439 DOI: 10.1186/s40425-019-0650-9]
35 Chiang JYL, Ferrell JM.Bile Acid Metabolism in Liver Pathobiology.
2018; 18: 71-87 [PMID: 29325602 DOI:10.3727/105221618X15156018385515]
36 Komposch K, Sibilia M.EGFR Signaling in Liver Diseases.
2015; 17 [PMID: 26729094 DOI:10.3390/ijms17010030]
37 Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G,Greten TF.Gut microbiome-mediated bile acid metabolism regulates liver cancer
NKT cells.
2018; 360 [PMID:29798856 DOI: 10.1126/science.aan5931]
38 Ezzaidi N, Zhang X, Coker OO, Yu J.New insights and therapeutic implication of gut microbiota in non-alcoholic fatty liver disease and its associated liver cancer.
2019; 459: 186-191 [PMID: 31185249 DOI:10.1016/j.canlet.2019.114425]
39 Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J.Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans.
2011; 94: 58-65[PMID: 21543530 DOI: 10.3945/ajcn.110.010132]
40 Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI.An obesity-associated gut microbiome with increased capacity for energy harvest.
2006; 444: 1027-1031 [PMID: 17183312 DOI: 10.1038/nature05414]
41 Field RE, Romanus RJ.An improved gastrostomy technique.
1975; 148: 610-611
42 den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, Reijngoud DJ.Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids.
2013; 305: G900-G910 [PMID: 24136789 DOI: 10.1152/ajpgi.00265.2013]
43 Chu H, Duan Y, Yang L, Schnabl B.Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease.
2019; 68: 359-370 [PMID: 30171065 DOI: 10.1136/gutjnl-2018-316307]
44 Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A.Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease.
2018; 6: 1496-1507 [PMID: 30574320 DOI:10.1177/2050640618804444]
45 Han J, Dzierlenga AL, Lu Z, Billheimer DD, Torabzadeh E, Lake AD, Li H, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, Lehman-McKeeman LD, Cherrington NJ.Metabolomic profiling distinction of human nonalcoholic fatty liver disease progression from a common rat model.
2017; 25: 1069-1076 [PMID: 28452429 DOI:10.1002/oby.21855]
46 Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S,Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K,Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O.Richness of human gut microbiome correlates with metabolic markers.
2013; 500: 541-546[PMID: 23985870 DOI: 10.1038/nature12506]
47 Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D.Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae.
2019; 30: 675-688.e7 [PMID: 31543403 DOI: 10.1016/j.cmet.2019.08.018]
48 Zhang C, Yang M, Ericsson AC.The Potential Gut Microbiota-Mediated Treatment Options for Liver Cancer.
2020; 10: 524205 [PMID: 33163393 DOI: 10.3389/fonc.2020.524205]
49 Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE.Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease.
2017; 25: 1054-1062.e5 [PMID: 28467925 DOI: 10.1016/j.cmet.2017.04.001]
50 Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI.The gut microbiota as an environmental factor that regulates fat storage.
2004; 101: 15718-15723 [PMID: 15505215 DOI:10.1073/pnas.0407076101]
51 Singh V, Yeoh BS, Abokor AA, Golonka RM, Tian Y, Patterson AD, Joe B, Heikenwalder M, Vijay-Kumar M.Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota.
2020; 11:1077-1091 [PMID: 32223398 DOI: 10.1080/19490976.2020.1743492]
52 Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S.Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma.
2019; 68: 1014-1023 [PMID: 30045880 DOI: 10.1136/gutjnl-2017-315084]
53 Chen YH, Wu WK, Wu MS.Microbiota-Associated Therapy for Non-Alcoholic Steatohepatitis-Induced Liver Cancer: A Review.
2020; 21 [PMID: 32825440 DOI: 10.3390/ijms21175999]
54 Bomhof MR, Parnell JA, Ramay HR, Crotty P, Rioux KP, Probert CS, Jayakumar S, Raman M, Reimer RA.Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial.
2019; 58: 1735-1745 [PMID:29779170 DOI: 10.1007/s00394-018-1721-2]
55 Abdel Monem SM.Probiotic Therapy in Patients with Nonalcoholic Steatohepatitis in Zagazig University Hospitals.
2017; 7: 101-106 [PMID: 29201787 DOI: 10.5005/jp-journals-10018-1226]
56 Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG.Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice
beneficial regulation of gut microbiota.
2017; 7: 1529 [PMID: 28484247 DOI: 10.1038/s41598-017-01751-y]
57 Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ,Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M.Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome.
2012; 143: 913-6.e7 [PMID: 22728514 DOI:10.1053/j.gastro.2012.06.031]
58 Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S,Shiri-Sverdlov R, Kuipers F, Willems van Dijk K, Vervoort J, Stienstra R, Hooiveld GJEJ, Kersten S.Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids.
2017; 58: 1399-1416[PMID: 28533304 DOI: 10.1194/jlr.M075713]
59 Safari Z, Gérard P.The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD).
2019; 76: 1541-1558 [PMID: 30683985 DOI: 10.1007/s00018-019-03011-w]
60 Mahana D, Trent CM, Kurtz ZD, Bokulich NA, Battaglia T, Chung J, Müller CL, Li H, Bonneau RA, Blaser MJ.Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet.
2016; 8: 48 [PMID: 27124954 DOI: 10.1186/s13073-016-0297-9]
61 Longhi G, van Sinderen D, Ventura M, Turroni F.Microbiota and Cancer: The Emerging Beneficial Role of Bifidobacteria in Cancer Immunotherapy.
2020; 11: 575072 [PMID: 33013813 DOI: 10.3389/fmicb.2020.575072]
62 Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M.Gut Microbiota and Cancer: From Pathogenesis to Therapy.
2019; 11 [PMID: 30609850 DOI:10.3390/cancers11010038]
63 Friedman ES, Li Y, Shen TD, Jiang J, Chau L, Adorini L, Babakhani F, Edwards J, Shapiro D, Zhao C, Carr RM,Bittinger K, Li H, Wu GD.FXR-Dependent Modulation of the Human Small Intestinal Microbiome by the Bile Acid Derivative Obeticholic Acid.
2018; 155: 1741-1752.e5 [PMID: 30144429 DOI:10.1053/j.gastro.2018.08.022]
64 Borges Haubert NJ, Marchini JS, Carvalho Cunha SF, Suen VM, Padovan GJ, Jordao AA Junior, Marchini Alves CM,Marchini JF, Vannucchi H.Choline and Fructooligosaccharide: Non-alcoholic Fatty Liver Disease, Cardiac Fat Deposition,and Oxidative Stress Markers.
2015; 8: 1-6 [PMID: 25987847 DOI: 10.4137/NMI.S24385]
65 Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T, Zhang L, Zhou G, Pan C, He L, Cai J, Zhang X, Barve S, McClain CJ, Chen Y,Feng W.Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice.
2020; 75: 108256 [PMID: 31760308 DOI: 10.1016/j.jnutbio.2019.108256]
 World Journal of Gastrointestinal Oncology2022年5期
World Journal of Gastrointestinal Oncology2022年5期
- World Journal of Gastrointestinal Oncology的其它文章
- Helicobacter pylori, gastric microbiota and gastric cancer relationship: Unrolling the tangle
- EFNA1 in gastrointestinal cancer: Expression, regulation and clinical significance
- Scoping out the future: The application of artificial intelligence to gastrointestinal endoscopy
- Pretreatment serum albumin-to-alkaline phosphatase ratio is an independent prognosticator of survival in patients with metastatic gastric cancer
- Preoperative prediction of malignant potential of 2-5 cm gastric gastrointestinal stromal tumors by computerized tomography-based radiomics
- Digital single-operator cholangioscopy for biliary stricture after cadaveric liver transplantation
