Cyanide-Bridged Bimetallic Active Site in Porous Carbon-Matrix for Oxygen Reduction Reaction
LI Cong⁃Ling LU Xiao⁃Yu
(College of Chemistry and Chemical Engineering,Shanghai University of Engineering Science,Shanghai 201620,China)
Abstract:Using special compound{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3as a precursor,an ordered mesoporous Fe⁃Co⁃N⁃doped graphite⁃based catalyst(Fe⁃Co⁃N⁃GC)with the embedding Fe—N,Co—N,and Fe—C≡N—Co active sites was prepared by a nano⁃casting technique.Together with the high surface area and graphitization degree,the catalytic performance of Fe⁃Co⁃N⁃GC for oxygen reduction reaction(ORR)was remarkably enhanced.This Fe⁃Co⁃based bimetallic catalyst also exhibited superior durability and good tolerance to methanol in ORR.
Keywords:mesoporous material;bimetallic catalyst;synergistic effect;oxygen reduction reaction;fuel cells
0 Introduction
The synergistic effect of the multi⁃heteroatoms doped into the carbon nanomaterials can distinctly enhance the catalytic performance of the carbon⁃based electrocatalysts for oxygen reduction reaction(ORR)[1]and has been recognized as a viable strategy for devel⁃oping highly efficient non⁃precious metal catalyst to replace Pt⁃based electrocatalyst in the clean energy and energy⁃efficient devices such as polymer electro⁃lyte membrane fuel cell(PEMFC).
Among the non⁃precious metal carbon⁃based cata⁃lysts reported for ORR,Fe⁃N⁃C and Co⁃N⁃C catalysts have been recognized as one of the most promising can⁃didates for replacing platinum⁃based catalysts to cata⁃lyze ORR[2⁃4].On the other hand,it has been found that Fe⁃N⁃C catalysts have higher ORR activity than Co⁃N⁃C catalysts,but the latter possessed higher catalytic stability[5⁃6].To combine the advantages of high electro⁃chemical stability and ORR activity,bimetallic cata⁃lysts using Fe and Co as the metal sources have been investigated, such as binary Fe/Co⁃TPTZ[7],FeCo⁃OMPC[8],and Fe/Co ⁃NpGr[9].All these studies indicated that the bimetallic catalysts possess enhanced electrochemical activity and stability towards ORR compared to the catalysts containing single metal,owing to the synergistic effect between Fe and Co active species.
The bimetallic N⁃containing catalysts are usually prepared by two kinds of methods.One is directly pyro⁃lyzing the mixture of transition metal⁃containing macro⁃cycles[7,10],or the mixture of transition⁃metal inorganic salts and nitrogen ⁃containing organic compounds[11⁃13].The other is by nano⁃casting of ordered mesoporous sil⁃ica with the mixture of metal⁃containing macrocy⁃cles[14].However,the distribution of Fe—N and Co—N active sites in the catalysts prepared by these methods is uncontrollable and causes uneven load.The confir⁃mation for the structural character of the active sites and mutual contact pattern between Co and Fe atoms is very difficult or impossible because the bonding behav⁃ior of Co and Fe atoms in the pyrolysis products has the features of randomness as well as varieties.
In this work,an efficient ordered mesoporous Fe⁃Co⁃N⁃doped graphite⁃based catalyst(Fe⁃Co⁃N⁃GC)has been successfully prepared by using a water⁃soluble Fe⁃Co bimetallic coordination compound as precursors and ordered mesoporous silica SBA⁃15 as a template(Fig.1).In the synthesis,the special molecule structure of the precursor can facilitate the formation of homoge⁃neous and concentrated active centers and provide the building block of active sites,which may enhance the synergistic effect of the different components and improve electrochemical stability and the catalytic activity of the prepared Fe⁃Co⁃based catalyst.As a result,the catalytic activity and stability of Fe⁃Co⁃N⁃GC were demonstrated to outperform the commercial Pt/C catalyst.
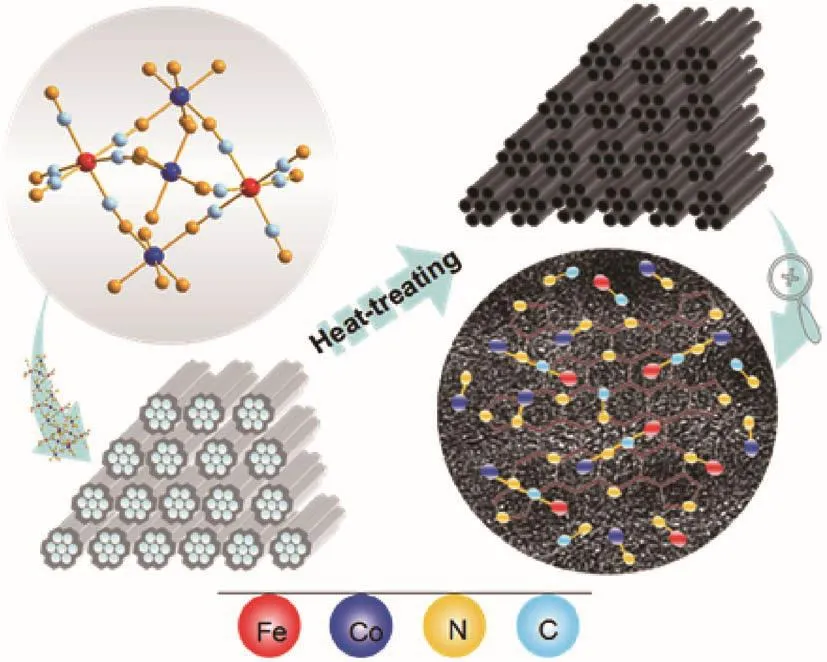
Fig.1 Schematic illustration of the preparation of Fe⁃Co⁃N⁃GC
1 Experimental
1.1 Synthesis of SBA-15
Typical,Pluronic P123 triblock copolymer(Mw=5 800,Aldrich,4.0 g)was added in the solution con⁃taining deionized water(144 g)and HCl(160 mL,1.6 mol·L-1),and allowed to stir at 40 ℃.Once the solu⁃tion became clear,tetraethyl orthosilicate (TEOS,Aldrich,8.3 g)was added in and kept being stirred at the same temperature for 24 h.Afterward,the obtained mixtures were transferred to a Teflon⁃lined autoclave,sealed,and heated at 150℃for 24 h in an oven.The resulting white product was filtered,washed with deion⁃ized water,and dried at 100℃.Finally,mesoporous silica SBA⁃15 was obtained by calcining the dried white product at 550 ℃ in the air for 4 h,with a heat⁃ing rate of 1℃·min-1.
1.2 Synthesis of Fe-Co-N-GC
The crystal of{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3was prepared according to the previously reported method[15]and used as the precursor.The filled⁃SBA⁃15 hard templates were achieved by alternate dipping and drying wet⁃filling techniques.In a typical proce⁃dure,1.0 g of{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3was added in 200 mL of deionized water and stirred for 1 h at 70℃ to form a homogeneous solution.Then the solu⁃tion was added into SBA⁃15(0.5 g)drop by drop while the mixtures were ground in mortar under an infrared lamp to evaporate the solvent.The obtained products were heat⁃treated at different temperatures ranging from 600 to 800℃under nitrogen flow with a ramping rate of 2 ℃·min-1for 4 h.The Fe⁃Co⁃N⁃doped mesopo⁃rous material was obtained by etching the silica templates in HF(5%)solution.The synthesized materi⁃als were designated as Fe⁃Co⁃N⁃GC⁃600,Fe⁃Co⁃N⁃GC⁃700,and Fe⁃Co⁃N⁃GC⁃800 according to the heat⁃treatment temperatures.
1.3 Electrochemical characterization
The electrochemical activities of the catalysts for ORR were performed at room temperature by using a CHI⁃760C electrochemical analyzer with a three⁃elec⁃trode cell system.A glassy carbon disk electrode(5 mm in diameter,Pine Instrument Co.,USA)coated with as⁃synthesized Fe⁃Co⁃N⁃GC was employed as a working electrode,whereas an Ag/AgCl(KCl,3 mol·L-1)and Pt electrode were used as reference and coun⁃ter electrode in the measurement,respectively.For the preparation of the working electrode,the catalyst inks were prepared by dispersing 10 mg Fe⁃Co⁃N⁃GC or Pt/C catalyst(Johnson Matthey,20% Pt)in a mixture of 1.25 mL of ethanol and 0.03 mL of Nafion(5%),then the desired amount of such catalyst inks was deposited onto the polished glassy carbon electrode and dried at room temperature before measurement.
Cyclic voltammetry(CV)and rotating disk elec⁃trode(RDE)techniques for ORR were carried out in an O2⁃saturated 0.1 mol·L-1KOH solution.The loadings of the catalysts on the working electrode were 0.36 and 0.60 mg·cm-2in O2⁃saturated 0.1 mol·L-1KOH,respectively.The loadings of Pt/C(20%)catalysts on the working electrode were 0.10 mg·cm-2in both elec⁃trolytes.For all the CV,linear sweep voltammetry(LSV),and chronoamperometric measurements,the scan rate was 10 mV·s-1.All potentials in this study were reported concerning the reversible hydrogen elec⁃trode(RHE).
The onset ORR potential reported in this work was defined when ORR current density was 3 µA·cm-2in RDE polarization curves[16⁃17].The number(n)of elec⁃trons transferred during ORR was calculated by Koutecky⁃Levich(K⁃L)equations:
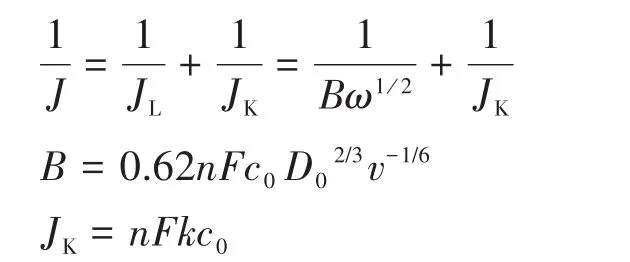
Where J represents the measured current density on the glassy carbon disk,JLis the diffusion⁃limited cur⁃rent density,JKis the kinetic current density,ω is the angular velocity of the disk,B could be calculated from the slope of K⁃L plots,F is the Faraday constant(F=96 485 C·mol-1),k is the electron transfer rate con⁃stant,v is the kinematic viscosity of the electrolyte,c0is the bulk concentration of O2and D0is diffusion coef⁃ficient of O2.
1.4 Characterization of the materials
X⁃ray diffraction patterns(XRD)were recorded on a D8 advance diffractometer (Bruker,Germany)attached with a Cu Kα (λ=0.154 05 nm)as a radiation source,a tube voltage of 40 kV,a tube current of 40 mA,and a scanning range of 0°⁃140°.Scanning elec⁃tron microscopy(SEM)images were taken using ST⁃4800(Hitachi)scanning electron microscopes oper⁃ated at an accelerating voltage of 10 kV.Transmission electron microscopy(TEM)images,high⁃resolution TEM(HRTEM)images,selected area electron diffrac⁃tion(SAED)patterns,scanning transmission electron microscopy(STEM),and energy⁃dispersive X⁃ray spec⁃troscopy(EDS)line scan data were conducted on a JEM⁃2010 transmission electron microscope(JEOL,Japan)at an acceleration voltage of 200 kV.Nitrogen adsorption⁃desorption isotherms were performed on a Micromeritics ASAP 2020 analyzer at 77 K.The specif⁃ic surface area was calculated by using the adsorption data via the Brunauer⁃Emmett⁃Teller(BET)method.The pore size distribution and the total pore volume were derived from the related adsorption branch by using the Barrett⁃Joyner⁃Halenda(BJH)model.FT⁃IR spectra were recorded with an infrared spectrophotome⁃ter(Bruker Tensor 27)using KBr pellets in a range of 400⁃4 000 cm-1.X ⁃ray photoelectron spectroscopy(XPS)experiments were recorded on Axis Ultra DLD system using Al Kα radiation(1 486.6 eV).The XPS spectrum of the C peak was calibrated to 284.6 eV.
2 Results and discussion
As mentioned,the non⁃precious metal carbon⁃based catalysts with excellent catalytic activity for ORR could be prepared by doping with multi⁃heteroatoms and the heat⁃treatment methods.However,it is still a long way off from the practical application.Further improvement of the catalytic performance requires insight into the component,structural feature,morphol⁃ogy,and the surroundings of the active sites in the cata⁃lysts.At the same time,these are also the necessary prerequisite and foundation to design and fabricate surface species with well⁃defined composition and structure and the desired functions.Based on such consideration, bimetallic coordination compound{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3was chosen as a pre⁃cursor of the carbon⁃based catalysts.In the molecule structures,Co and Fe in a molar ratio of 9∶7 directly connect with N and C atoms,respectively,and are linked together by chain⁃link C≡N as shown in Fig.1.
Some research results have illustrated that Fe(Co)—N(C)moieties were the highly active sites for ORR[18⁃21].Fe—C≡N—Co moieties contained two kinds of active sites(Fe—C and Co—N)(Fig.1),which were linked together by carbon⁃nitrogen triple bonds.Such structural features would be very helpful for investigating the synergistic effect of different active sites in the catalysts and creating highly efficient cata⁃lysts.To implant Fe—C≡N—Co moieties into the car⁃bon⁃based materials,the confinement effect of the mes⁃opore channels in the SBA⁃15 hard template is utilized to control the carbonization of{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3and limit the aggregation of the nanoparti⁃cles produced during the heating⁃treatment process(Fig.1).By this synthesis strategy,the multiple compo⁃nents of carbon⁃based materials(Fe⁃Co⁃N⁃GC)were obtained and effectively realized the integration of multi⁃model active sites and the mesopores,which has promising application in the field of electrocatalysis.
2.1 Characterization of Fe-Co-N-GC
The chemical compositions of the prepared sam⁃ples were evaluated by XPS analysis and their contents are shown in Table 1.For Fe⁃Co⁃N⁃GC⁃700,the con⁃tent(atomic fraction)of N(8.96%),Fe(0.67%),Co(0.86%)was relatively higher than others.In the Fe2pspectra in Fig.2a,three peaks at 711.2,724.3,and 717.7 eV are ascribed to the 2p3/2and 2p1/2of Fe(Ⅲ)in the Fe—N(C)xmoieties and the satellite peak respec⁃tively[22⁃23].Similarly,three peaks at 780.6,796.2,and 785.4 eV in Co2pspectra(Fig.2b)can be assigned to 2p3/2,2p1/2of Co(Ⅱ) in the Co—(C)Nxmoieties and shakeup satellite peak[24⁃25].It is important to note that no metallic iron or cobalt has been found by the XPS analysis.
In the high⁃resolution N1sspectra(Fig.2c),the six de⁃convoluted peaks at around 398.2,398.4,399.3,399.9,401.1,and 404.3 eV can be attributed to C≡N,pyridinic⁃N,pyrrolic⁃N,metallic⁃N,graphitic⁃N,and pyridine N—O,respectively[26⁃27],which indicate that Fe—Nx,Co—Nx,and Fe—C≡N—Co moieties may exist in the catalysts.The surface composition of differ⁃ent types of nitrogen estimated from the XPS analyses can be seen in Table 1.The relative amount of metallic⁃N species increases in the following order:Fe⁃Co⁃N⁃GC⁃600(3.96%)< Fe⁃Co⁃N⁃GC⁃800(5.29%)< Fe⁃Co⁃N⁃GC⁃700(10.02%).This variation tendency can be ascribed to the balance of old bonds(C—N)break,the formation of new ones(Fe—N,Co—N),and the degra⁃dation of Fe(Co)—Nxspecies.To further prove the exis⁃tence of Fe—C≡N—Co moieties in the prepared ma⁃terials,the FT⁃IR spectrum of Fe⁃Co⁃N⁃GC⁃700 was measured and shown in Fig.2d.An absorption band corresponding to the stretching vibration of Fe—C≡N—Co species was observed at 2 137 cm-1in the spectra[15].The experimental fact testifies that Fe—C≡N—Co moiety has been successfully introduced into the Fe⁃Co⁃N⁃GC nanostructures.This remarkable fea⁃ture in Fe⁃Co⁃N⁃GC⁃700 differs from the others and is unprecedented in the N⁃doped carbon⁃based materials containing Fe and Co.
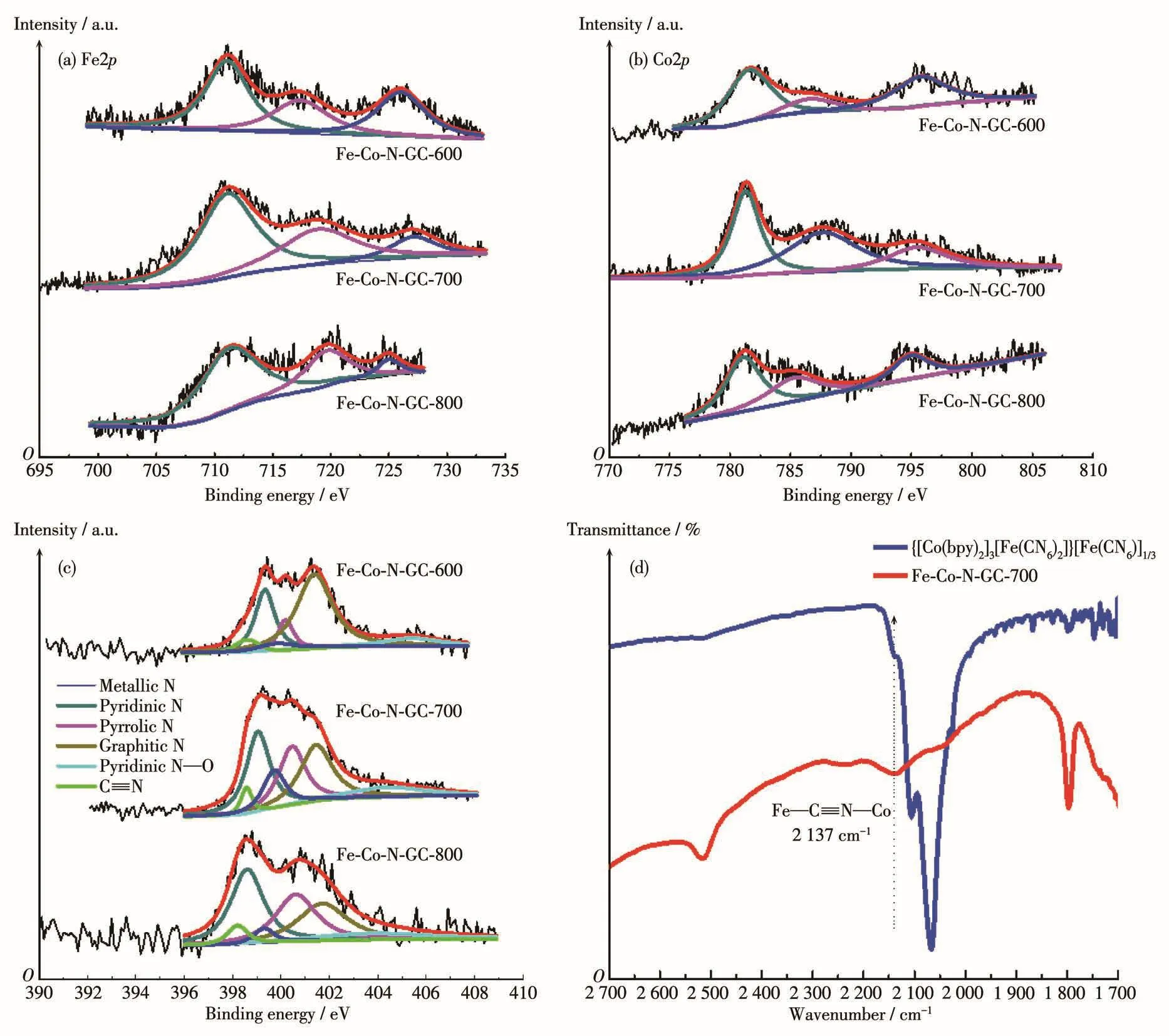
Fig.2 XPS spectra of(a)Fe2p,(b)Co2p,and(c)N1s for Fe⁃Co⁃N⁃GC⁃600,Fe⁃Co⁃N⁃GC⁃700,and Fe⁃Co⁃N⁃GC⁃800;(d)Enlarged FT⁃IR spectra for{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3and Fe⁃Co⁃N⁃GC⁃700
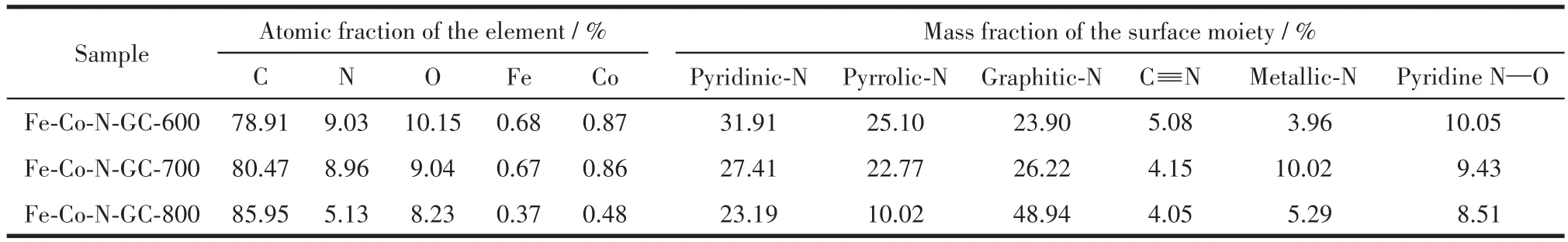
Table 1 Surface composition and relative content of the component elements in different catalysts evaluated from XPS analysis
The morphology and crystal structure of the cata⁃lysts were characterized by XRD,TEM,and N2adsorp⁃tion⁃desorption analyses.The peaks with the higher intensity at 2θ=25°and 43°in Fig.3a indicate that thecarbons in pore⁃walls have higher graphitization degrees[28].The intensity of these two peaks increases at higher heat⁃treatment temperatures,suggesting a high⁃er graphitization degree of the catalyst frameworks.The low⁃angle X⁃ray diffraction of the catalysts in Fig.3b shows a remarkable diffraction peak at about 0.9°(2θ),which can be indexed to the(100)plane of hexagonal structures.
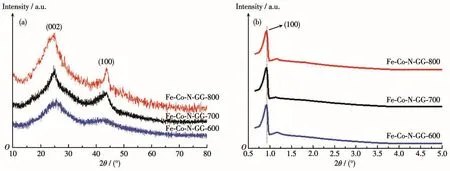
Fig.3 (a)Large⁃angle and(b)small⁃angle XRD patterns of Fe⁃Co⁃N⁃GC⁃600,Fe⁃Co⁃N⁃GC⁃700,and Fe⁃Co⁃N⁃GC⁃800
From the low⁃magnification TEM images in Fig.4a and 4b,ordered 2D hexagonal mesostructures with rod⁃like morphology were observed.These results certify that the prepared materials are well⁃reverse replicas of the SBA⁃15 hard template[29].
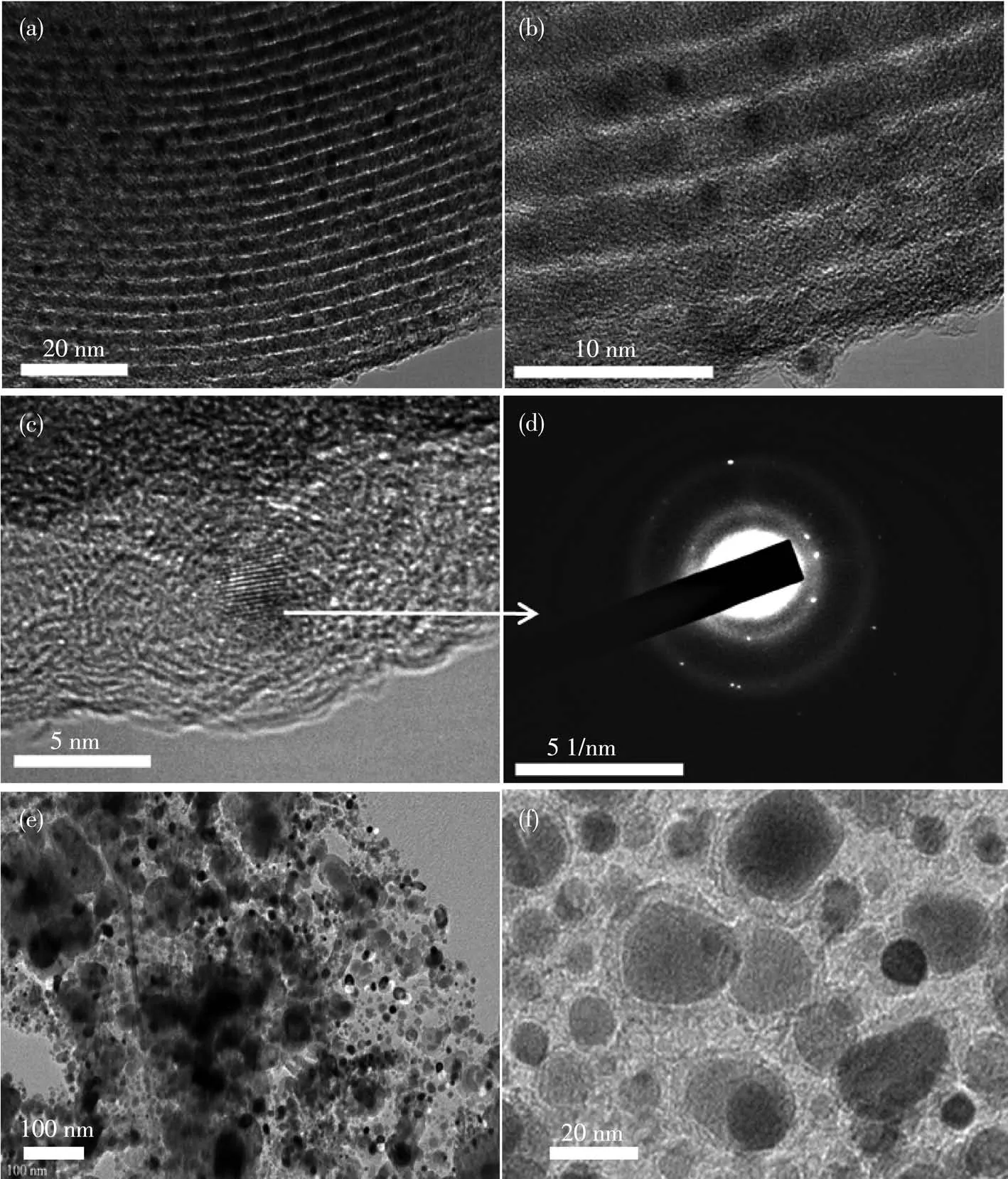
Fig.4 (a,b)TEM and(c)HRTEM images of Fe⁃Co⁃N⁃GC⁃700 and(d)the corresponding SAED images;(e,f)TEM images of the catalyst prepared without using SBA⁃15 template
N2⁃adsorption and desorption isotherms of Fe⁃Co⁃N⁃GC showed type Ⅳ isotherm and an H2⁃type hyster⁃esis loop,indicating the uniform mesoporous structure(Fig.5a)[30].A very narrow pore⁃size distribution was obtained by calculation from the adsorption branch based on the BJH model(Fig.5b).The detailed surface parameters are summarized in Table 2.Compared to the prepared materials at different temperatures,Fe⁃Co⁃N⁃GC⁃700 had a higher BET surface area(1 017 m2·g-1)than the others,which may be ascribed to the opti⁃mized structure of carbon framework and micropores at this heat⁃treating temperature.Mesopore sizes were found to increase(from 4.1 to 4.7 nm)due to the shrinkage of the pore walls while the heat⁃treatment temperature was from 600 to 800℃[31].An optimization between BET surface and pore structure will be a bene⁃fit for more active sites exposed to the reactants and rapid transportation of O2for ORR.
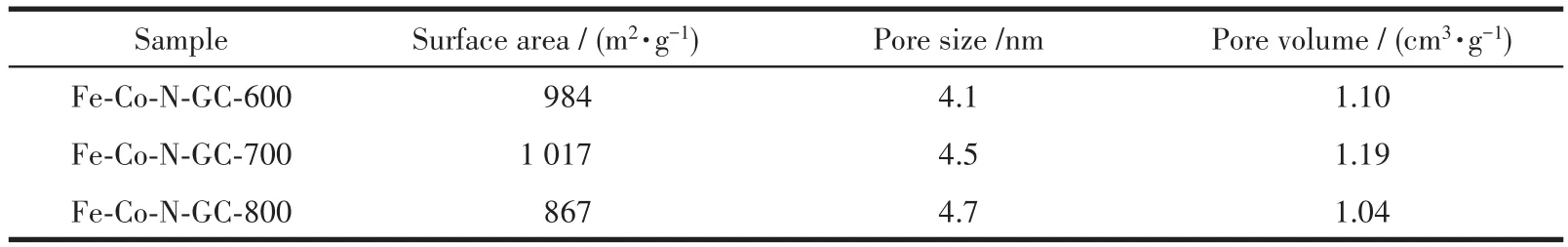
Table 2 Texture parameters of Fe-Co-N-GC-600,Fe-Co-N-GC-700,and Fe-Co-N-GC-800
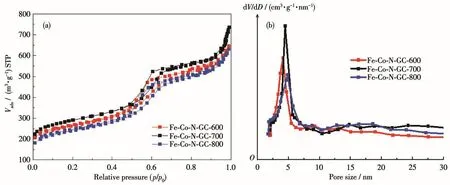
Fig.5 (a)N2adsorption⁃desorption isotherms and(b)pore size distribution curves of Fe⁃Co⁃N⁃GC⁃600,Fe⁃Co⁃N⁃GC⁃700,and Fe⁃Co⁃N⁃GC⁃800
Moreover,an interesting phenomenon is shown in Fig.4a and 4b was that some small nanoparticles were embedded in the surface of mesoporous frameworks in the prepared materials.For characterizing these nanoparticles,their microstructure,and chemical com⁃ponent and appearance had been investigated by HRTEM images(Fig.4c),TEM⁃EDS mapping(Fig.6)for Fe,Co and N elements,line⁃scanning EDS analysis(Fig.7)by using scanning⁃mode TEM,and the SAED analysis(Fig.4d).The EDS mapping images revealed that Fe,Co,and N atoms were well dispersed both on the surface of the carbon frame and carbon nanoparti⁃cles.Fig.7 further attested that the distribution of Fe,Co,and N atoms in the nanoparticles was the same as that in the mesoporous framework.The graphitic lattice fringes could be distinctly observed in the HRTEM images of the nanoparticles in Fig.4c.The correspond⁃ing SAED patterns in Fig.4d for Fe⁃Co⁃N⁃GC⁃700 showed clear diffraction rings and dots,which exhibit⁃ed a higher degree of graphitization in the nanoparti⁃cles.These facts above sufficiently verified that the nanoparticles and mesoporous frameworks of Fe⁃Co⁃N⁃GC⁃700 possessed the same component but a different degree of graphitization.To compare the superiority of the application of the hard template,a catalyst pre⁃pared without using the SBA⁃15 template was also pre⁃sented.TEM images(Fig.4e and 4f)showed agglomera⁃tion of carbon⁃coated nanoparticles,which mainly formed intra⁃particle pores.
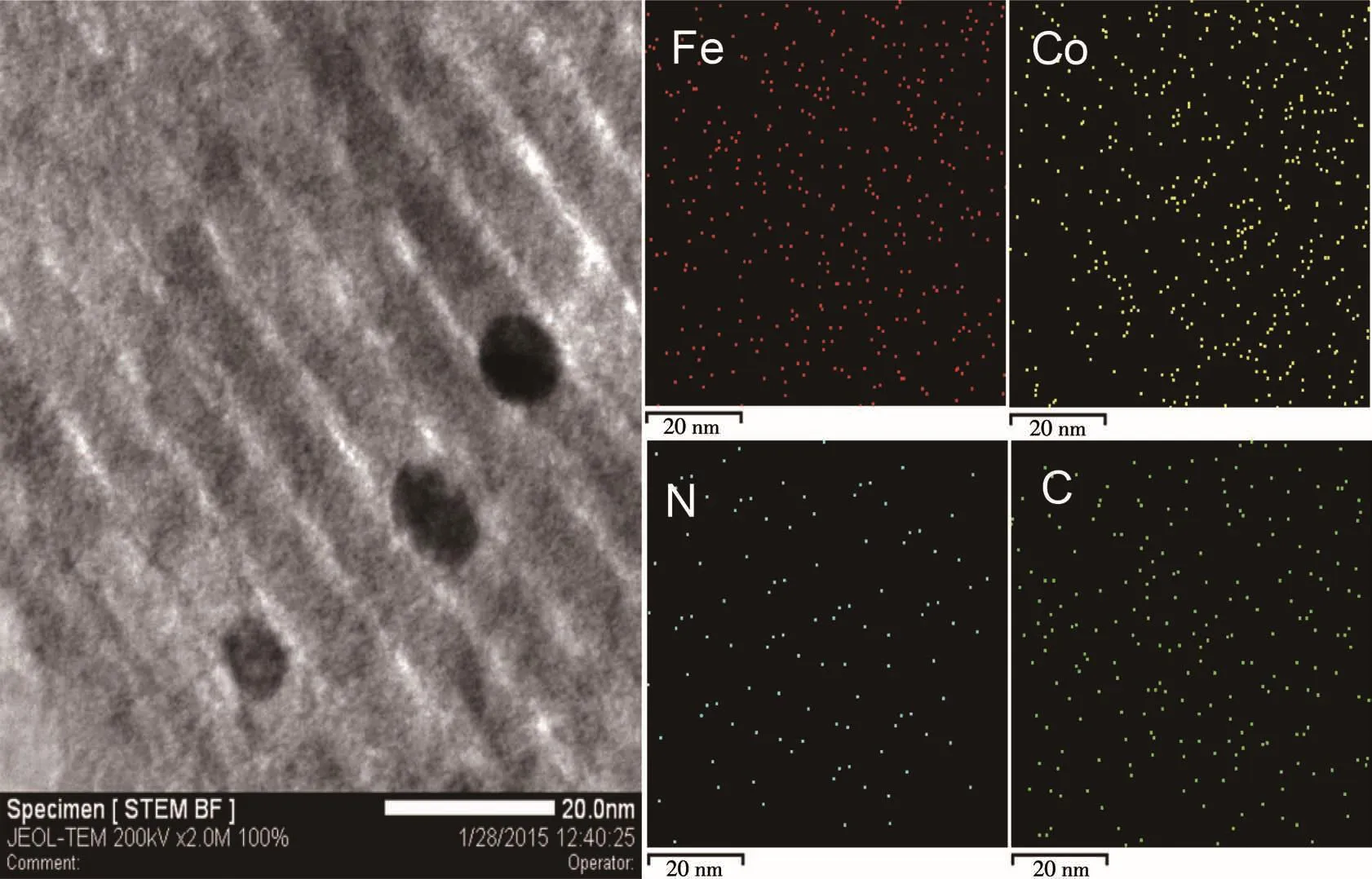
Fig.6 STEM image of Fe⁃Co⁃N⁃GC⁃700 and the corresponding EDS elemental mapping images
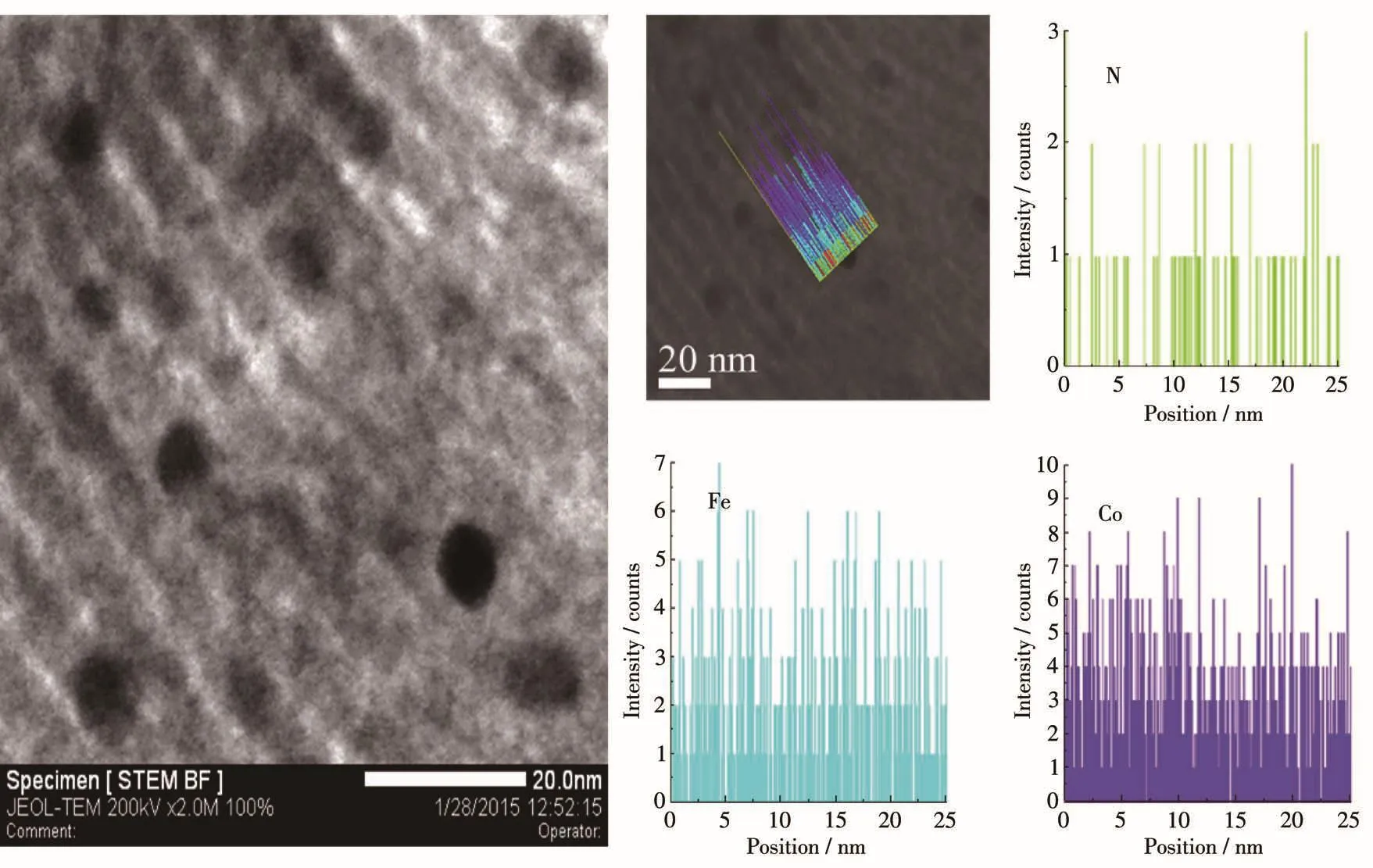
Fig.7 STEM image of Fe⁃Co⁃N⁃GC⁃700 and the corresponding EDS line scan images
2.2 Electrocatalytic activity for ORR
The electrocatalytic activities of the prepared materials for ORR were studied by CVs and LSV mea⁃surements in O2⁃saturated 0.1 mol·L-1KOH electro⁃lyte.From the obtained results(Fig.8a and 8b),it is seen that the catalyst prepared at 700℃exhibited the highest peak⁃potential,half⁃wave potential(E1/2),and current density among the three prepared samples.This result testifies that 700℃was identified as the optimum annealing temperature.This excellent catalyt⁃ic performance of Fe⁃Co⁃N⁃GC⁃700 is mainly attribut⁃ed to the higher density of activity centers and the high⁃est surface area among these prepared materials.The voltammograms of Fe⁃Co⁃N⁃GC⁃700 with the loading of 0.36 mg·cm-2revealed an obvious ORR peak at 0.90 V in alkaline medium saturated with O2,which was 20 mV higher than that of Pt/C catalyst(0.88 V)with mass loading of 0.1 mg·cm-2(Fig.8a).Under the same conditions,the kinetics aspects of the ORR activity of Fe⁃Co⁃N⁃GC⁃700 were investigated by LSV using the RDE method at an electrode rotation rate of 1 600 r·min-1and the results are shown in Fig.8b.The polariza⁃tion curve of Fe⁃Co⁃N⁃GC⁃700 displayed an onset potential of 1.07 V and a half⁃wave potential of 0.88 V and a current density of 5.6 mA·cm-2,which was 80 mV,30 mV and 0.4 mA·cm-2higher than those over the commercial Pt/C(Fig.8b).These results indicated that the catalytic performance of Fe⁃Co⁃N⁃GC⁃700 was superior to that of Pt/C catalysts in 0.1 mol·L-1KOH.Following the relationship between catalytic perfor⁃mance and structure of the materials,this super advan⁃tage of Fe⁃Co⁃N⁃GC⁃700 should undoubtedly ascribe to the special Fe—C≡N—Co moieties containing the bimetallic active site.It illustrates that the C≡N between Co and Fe can distinctly enhance the synergis⁃tic effect of Co and Fe and creates a novel high activity site.We also compared the ORR performance of cata⁃lyst prepared without using the SBA⁃15 template,as shown in Fig.8a and 8b,the catalyst heat⁃treated at 700 ℃ exhibited the lowest peak⁃potential(0.82 V),half⁃wave potential(0.81 V),and current density(4.6 mA·cm-2)among the three prepared samples.It illus⁃trates that intra⁃particle pores instead of ordered meso⁃porous structure prevent active sites exposure to the reactants and rapid transportation of O2for ORR.
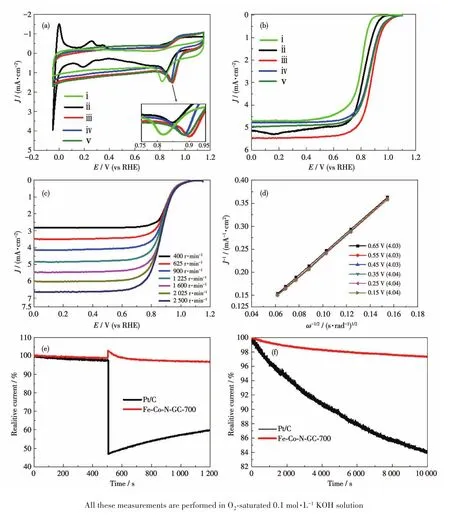
Fig.8 (a)CV and(b)LSV polarization curves of(ⅰ)the sample without using SBA⁃15 as a template,(ⅱ)Pt/C,(ⅲ)Fe⁃Co⁃N⁃GC⁃700,(ⅳ)Fe⁃Co⁃N⁃GC⁃600,and(ⅴ)Fe⁃Co⁃N⁃GC⁃800;(c)RDE polarization curves of Fe⁃Co⁃N⁃GC⁃700 at different RDE rotation rates and(d)corresponding K⁃L plots(J-1vs ω-1)at different potentials;(e)Polarization curves of Fe⁃Co⁃N⁃GC⁃700 and Pt/C with an injection of methanol(wMeOH=2%)after 500 s;(f)Current density degradation of Fe⁃Co⁃N⁃GC⁃700 and Pt/C,where the corresponding current densities were recorded at 0.55 V
For understanding the ORR kinetic process on Fe⁃Co⁃N⁃GC⁃700,polarization curves for ORR on Fe⁃Co⁃N⁃GC⁃700 electrode at various rotation speeds(between 400 and 2 500 r·min-1)were recorded with a scan rate of 10 mV·s-1in alkaline medium(Fig.8c).A diffusion⁃limited kinetic process over Fe⁃Co⁃N⁃GC⁃700 in 0.1 mol·L-1KOH electrolytes(Fig.8d)exhibited good linearity between 0.15 and 0.65 V,suggesting that the electron transfer numbers are consistent over different potentials.The electron transfer number in ORR was calculated to be 4.0 according to the K⁃L equations at 0.55 V(B=0.62nFc0D02/3v-1/6,c0=1.18×10-3mol·L-1,D0=1.9×10-5cm·s-1,v=0.089 3 cm2·s-1)[32],suggesting the ORR kinetic process on Fe⁃Co⁃N⁃GC follow a direct four⁃electron route.
The electrocatalytic methanol crossover property and durability of Fe⁃Co⁃N⁃GC⁃700 were investigated by chronoamperometric measurements.There was no significant decrease in the ORR current density over Fe⁃Co⁃N⁃GC⁃700(Fig.8e)after the injection of methanol(wMeOH=2%).But after the injection of methanol,a sig⁃nificant current decrease in the ORR activity of the commercial Pt/C catalyst was observed,indicating the occurrence of methanol oxidation reaction[33].The experimental result demonstrates that Fe⁃Co⁃N⁃GC⁃700 has considerably better tolerance to methanol crossover than does Pt/C catalyst.
The long⁃term stability of the electrocatalytic activity for ORR over Fe⁃Co⁃N⁃GC⁃700 was measured.From the chronoamperometry,it was seen that the cur⁃rent density of Fe⁃Co⁃N⁃GC⁃700 showed insignificant decay of about 2.7% after running for 10 000 s.In con⁃trast,as shown in Fig.8f,the ORR current on Pt/C cata⁃lyst suffered from a 16.0% loss after running for 10 000 s.Fe⁃Co⁃N⁃GC⁃700 exhibited excellent stability com⁃pared to the commercial Pt/C catalyst.
3 Conclusions
In summary,Fe⁃Co⁃N⁃doped mesoporous graphite catalyst(Fe⁃Co⁃N⁃GC)can be successfully prepared by using the water⁃soluble Fe⁃Co bimetallic coordination compound{[Co(bpy)2]3[Fe(CN)6]2}[Fe(CN)6]1/3as precur⁃sors and ordered mesoporous silica SBA⁃15 as tem⁃plates.The ORR onset potential,half⁃wave potential,and current density over Fe⁃Co⁃N⁃GC⁃700 prepared at a heat⁃treatment temperature of 700 ℃ reached 1.07 V,0.88 V,and 5.6 mA·cm-2,which were 80 mV,30 mV,and 0.4 mA·cm-2higher than those over Pt/C catalyst,respectively.The investigation results indicate that such super advantage of Fe⁃Co⁃N⁃GC⁃700 should ascribe to the special Fe—C≡N—Co activity site.Moreover,Fe⁃Co⁃N⁃GC⁃700 exhibited long⁃time stabili⁃ty toward ORR as well as excellent methanol resistance and may serve as a promising alternative to Pt/C cata⁃lysts for the ORR in the widespread implementation of PEFCs.
- 无机化学学报的其它文章
- 无机材料在骨质文物加固保护中的应用
- 百香果皮基原位氮掺杂多孔碳/硫复合正极的制备及储锂性能
- 氯钯共掺对Cs2TiBr6光电性能影响的第一性原理计算
- Performance of Different Metal-Modified HZSM-5 Catalysts for Methanol Carbonylation
- Interactions of a Water-Soluble Diiron Hexacarbonyl Complex with Biologically Relevant Molecules and Their Promotion in CO-Release
- Crystal Structures and Magnetic Refrigeration Properties of Two Gd2Complexes

