Performance of Different Metal-Modified HZSM-5 Catalysts for Methanol Carbonylation
Dilinuer Aili GAO Xi⁃Ran BI Kun⁃Hao FANG Ya⁃Ping FAN Xing Aisha Nulahong
(State Key Laboratory of Chemistry and Utilization of Carbon Based Energy Resources;School of Chemical Engineering and Technology,Xinjiang University,Urumqi 830017,China)
Abstract:In this study,selective production of methanol carbonylation formed acetic acid using hydrothermal synthesis nanoporous zeolite molecular sieve as the catalyst.The incorporation of a suitable amount of metal not only facilitated the formation of acidity but also improved the efficiency of methanol carbonylation.The HZSM⁃5 catalyst was modified with Pt,Pd,Cu,Au,Zn by negative pressure deposition precipitation method to prepare cata⁃lysts with different acidities.Using X⁃ray diffraction,temperature⁃programmed desorption of ammonia,pyridine adsorption FTIR,N2adsorption⁃desorption,and X⁃ray fluorescence analysis,the effect of different metals on the physico⁃chemical properties of the catalyst was studied,and the modification of HZSM⁃5 with different metals was investigated in the process of methanol carbonylation on the distribution and yield of methanol carbonyl products.The introduction of different metals had little effect on the specific surface area,pore size,and pore volume of the HZSM⁃5 catalyst,but significantly changed the acid strength of the catalyst surface.Pt,Au,Zn,and Cu modified HZSM⁃5 were favorable for the carbonylation of methanol to form methyl acetate and methyl formate.Cu⁃modified catalyst had a methanol conversion rate of 90.2% at 400℃,which was 12% higher than that of pristine HZSM⁃5,but the selectivity to the target product was lower than those of Pt/HZSM⁃5 and Au/HZSM⁃5.In a word,the introduc⁃tion of metals changes the number of Brønsted acid(B acid)and Lewis acid(L acid)centers on the catalyst surface.The conversion rate of methanol increased with the total acid sites.When the ratio of B acid to L acid on the catalyst surface was between 0.3 and 0.5,the catalyst showed better carbonylation activity.
Keywords:HZSM⁃5;metal⁃modification;carbonylation of methanol;strength of acid
0 Introduction
Methanol is an essential and versatile platform chemical in the petrochemical industry,whose main sources are refined liquefied gas,FCC(fluid catalytic cracking)liquefied gas,and oil field gas[1].In industrial production,methyl acetate and methyl formate are pro⁃duced by methanol liquid phase carbonylation and gas⁃phase carbonylation,as important organic raw material intermediates with low toxicity.Esters are often used as extraction solvents and are widely used in textile,spice,food,and pharmaceutical industries[2⁃3].The syn⁃thesis of methyl acetate and methyl formate by metha⁃nol carbonylation is divided into liquid phase and gas phase carbonylation.In references[4⁃6],the synthesis of acetic acid,methyl formate,and methyl acetate by methanol liquid carbonylation was studied.RhCl3·3H2O,sodium methoxide,potassium methoxide,and Fe⁃Co cluster compounds were used as catalysts,respec⁃tively.Due to the low activity of the catalyst,the active component,such as CH3I,I2,or HI should be intro⁃duced as co⁃catalysts.The disadvantages of this meth⁃od are that the catalyst and product are difficult to sep⁃arate,and the introduced iodide will seriously corrode the equipment.The commonly used catalysts for the synthesis of acetic acid,methyl formate,and methyl acetate by methanol gas⁃phase carbonylation were SnO2⁃MoO3,V ⁃Ti⁃O,ReOx/CeO2,Fe2O3/SiO2,RuOx/ZrO2,Au,Pd,Pt,etc.The product of this method is easy to separate,but low carbonylation of methanol[7⁃10].In sum⁃mary,the reaction system of methanol carbonylation to form methyl acetate and methyl formate has changed from initial liquid phase carbonylation to gas⁃phase carbonylation,and the reaction conditions have changed from high⁃pressure reaction to low⁃pressure reaction,which shows that the process is continuously optimized and progressed.In terms of catalysts,the Rh⁃I and/or Ir⁃I catalytic system initially used in the liquid phase uses methyl iodide as a promoter;then uses sodium methoxide and metals as catalysts,but the conversion rate is relatively low and requires high⁃pressure reaction conditions to proceed;later,metal cluster⁃type compounds are used as catalysts,but the synthesis of catalysts in this method is relatively diffi⁃cult,and industrial applications are difficult to achieve.In the gas phase carbonylation,Ni,Cu,Pt,Pd were also used as catalysts supported on activated carbon.The catalysts use the activity of the metal itself and the high specific surface area of the carrier(acti⁃vated carbon,AC)to perform catalytic reactions,but still,need to add methyl iodide used as a co⁃catalyst to promote a better synergistic reaction between the metal and the support.However,the AC support itself does not have any chemical properties,so this study uses the acidic HZSM⁃5 molecular sieve as the support,combined with the carbonylation activity of the metal itself,which could improve the efficiency of the carbon⁃ylation reaction.
Based on the low activity of the previous catalysts,Ni and Pt supported on catalysts,such as activated carbon,have been developed[11].Although this catalyst improves methanol conversion rate and has higher catalytic activity,there are still some difficulties in the separation of products and catalysts.In addition,the formation of coke leads to rapid catalyst deactivation,therefore the catalyst acidity is weak.It is necessary to continuously add an appropriate amount of iodized physicochemical auxiliaries to maintain a low⁃temperature activity during the reaction.It is urgent to develop new and efficient catalysts for methanol carbonylation.HZSM⁃5 nanocatalysts,with a special pore structure and a large number of mesopores,are beneficial to the diffusion of reactants and products during methanol carbonylation and the combination of reaction intermediates with active sites on the catalyst.In addition,large surface area,pore structure,and secondary pore structure are more favorable to enhance the activity of carbonylation and the carbon capacity of the catalyst.HZSM⁃5 surface distribution has Brønsted acid(B acid)and Lewis acid(L acid)centers,which is beneficial to the high selectivity of ethyl acetate and methyl acetate.The study found that HZSM⁃5 carbonyl⁃ation activity increases after metal modification,due to the metal center regulating the acid strength and acid quantity distribution of B acid and L acid active sites on the catalyst surface[12⁃15].Aisha[16]modified the HZSM⁃5 catalyst with Au and Pt metals,which increased the number of L acid sites on the surface of the catalyst and decreased the number of B acid sites,and promot⁃ed butane aromatization and methanol carbonylation.
Zeolites with well⁃defined pores have been exten⁃sively used in the fields of petroleum and petrochemis⁃try for their shape selectivity,high acidity,and favor⁃able thermal/hydrothermal stability.Transitional metal catalysis plays an important role in the production of chemicals and fuels.Supported single⁃metal⁃site cata⁃lysts are well known for their 100% atom utilization efficiency,uniformly isolated active sites,and well⁃defined coordination environment.As a classic homo⁃geneous catalytic process,methanol carbonylation for acetic acid production has been industrialized by Monsanto using[Rh(CO)2I2]⁃catalyst[17⁃20].However,a large amount of water is required to prevent the forma⁃tion of a RhI3precipitate.Recent work on metal modifi⁃cation of HZSM⁃5 catalysts demonstrated that different metals can promote methanol conversion rate,in which the dispersion degree of active metal plays a determin⁃ing role in the catalytic performance in various reac⁃tions.In previous study,Cu/HZSM⁃5 catalysts showed a highly elevated catalytic activity and stability in the synthesis of dimethyl carbonate via the oxidative carbonylation of methanol[21].Other metals have also been used in the modified catalysts of methanol carbon⁃ylation,such as Rh[22],Ir⁃La[23],Zn[24].
This study focuses on the conceptual design of a novel low⁃energy sustainable process for acetic acid manufacturing by methanol carbonylation.HZSM⁃5 modified with Pt,Pd,Cu,Au,Zn by wet impregnation method were denoted as M/HZSM⁃5(M=Pt,Pd,Cu,Au,Zn),which were used in methanol carbonylation reaction to access ester products.Carbonylation of HZSM⁃5 catalysts modified by different metals was studied using a continuous reaction device with a fixed bed of U⁃type reaction tubes.
1 Experimental
1.1 Materials
Tetraethyl orthosilicate(TEOS,28%)and sodium aluminate(NaAlO2,98%)were purchased from Tianjin Zhiyuan Chemical Reagent Co.,Ltd.Ammonium nitrate(NH4NO3,45%)was purchased from Xi′an Chemical Reagent Factory.Deionized water was made in the laboratory.The metal precursors support,includ⁃ing Cu(NO3)2,Au(NO3)2,Pt(NO3)2,and so on,were obtained from Hongyan Reagent Factory,Hedong District,Tianjin.All other reagents were analytically pure.
1.2 Synthesis of the catalyst
In a typical synthesis,a certain quality of NaAlO2was dissolved in the 3 mL tetrapropylammonium hydroxide(TPAH)solution.TEOS solution(4.5 mL)and deionized water(30 mL)were added into a mixed solution with stirring for 2 h.The mixture was added to the Teflon⁃lined autoclave and crystallized at 170 ℃for 48 h statically.The mixture was filtered and washed with deionized water to a pH value between 7 and 8.Then the product was dried at 120℃for 12 h,heated to 540℃,and calcined for 8 h to get white powders.
The ZSM⁃5 molecular sieve was ion⁃exchanged with a NH4NO3solution(0.4 mol·L-1,3 h).Then,it was washed with deionized water,dried at 110℃for 12 h.After the ammonium exchange was completed,the cata⁃lyst was calcined for 5 h in air at 560℃in order to obtain HZSM⁃5.The liquid⁃solid ratio of the catalyst to NH4NO3solution was 1∶5.The number of exchanges was 1⁃5 times.The catalyst(2 g)was soaked in HNO3solution(0.6 mol·L-1)with five times the catalyst mass for 24 h for acid expansion treatment.Then it was washed with distilled water,and dried at 100℃for 12 h,and calcined at 540℃for 3 h.
HZSM⁃5(1 g)was added into 50 mL 0.1 mol·L-1M(NO3)xaqueous solution(Mx+=Pt2+,Pd2+,Cu2+,Au+,Zn2+)and the mixture was stirred(200 r·min-1)at 25 ℃for 30 min.After the ion exchange,the M/HZSM⁃5 was obtained by centrifugation.Then the catalysts were centrifuged and washed three times using pure water to remove redundant metal cations.After drying at 60℃for 10 h,the samples were calcined at 540℃for 3 h to obtain final catalysts.For convenience,the parent catalyst was designated as HZSM⁃5.The catalysts mod⁃ified with Pt,Pd,Cu,Au,and Zn by the wet impregnat⁃ed method were named M/HZSM⁃5(M=Pt,Pd,Cu,Au,Zn).
1.3 Characterization
The Si/Al ratio(nSiO2/nAl2O3)of the sample was determined using the SRF 3400 X⁃ray fluorescence(XRF)spectrometer of Bruker Company,Germany.X⁃ray diffraction(XRD)patterns were obtained using a Rigaku D/max⁃2400 diffractometer (D8⁃Focus,Germany)equipped with a CuKαradiation source(λ=0.154 18 nm,40 kV,40 mA)in a 2θrange of 4°⁃80°at a scanning rate of 8(°)·min-1.The types of acids in the catalysts were characterized by pyridine adsorption FTIR(Py⁃FTIR)spectra were recorded using a Bruker Vertex 70 spectrometer(Germany).To study the acidi⁃ty property,temperature⁃programmed desorption of ammonia(NH3⁃TPD)was performed using a TP⁃5076 chemical adsorption instrument(Tianjin Xianquan Co.,Ltd.,China).The physical adsorption of nitrogen for the metal catalysts was determined by using an ASAP2020 physical adsorption instrument of American Micromeritics Company.The textural properties of the catalysts were determined by Brunauer⁃Emmett⁃Teller(BET)method.Before the measurement,the samples were degassed under a high vacuum for 3 h at 300℃.
1.4 Catalytic activity test
The methanol conversion rate was performed in a fixed⁃bed flow reactor operated at atmospheric pres⁃sure(Fig.1).The catalyst(100 mg,40⁃60 mesh)was loaded in the reactor at 150℃,and the temperature was increased stably to 400℃,then the methanol was pumped into the reactor.The gaseous products were analyzed on a Beijing 2HD2008 Chromatograph fitted with thermal conductivity detector and flame ionization detector.The catalytic activity and selectivity were expressed by the conversion rate of methanol and the selectivity of methyl acetate.
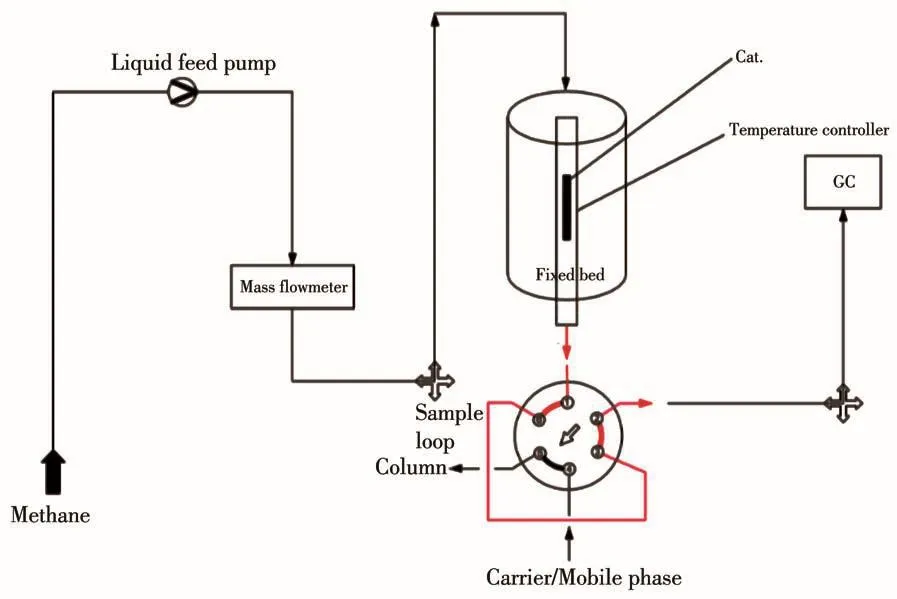
Fig.1 Diagram of mini⁃scale pulse reactor
2 Results and discussion
2.1 Characterization of metal modified catalysts
2.1.1 Chemical composition of different metal modified HZSM⁃5 catalysts
Table 1 shows the chemical composition of oxides of as⁃synthesized zeolite and as⁃synthesized M/HZSM⁃5.The synthesized HZSM⁃5 zeolite showed a high mass fraction of SiO2and a high Si/Al ratio.The loading rate of different metals for M/HZSM⁃5 was about 90%,and the Si/Al ratio was about 30.The ratio of measured value of Au,Pt,Pd,Zn,Cu content to the theoretical value was more than 90%,and the metal loading rate of the active component was higher.Moreover,the error of metal loading was less than 10%,which has little effect on the shape selectivity of the catalyst pore.
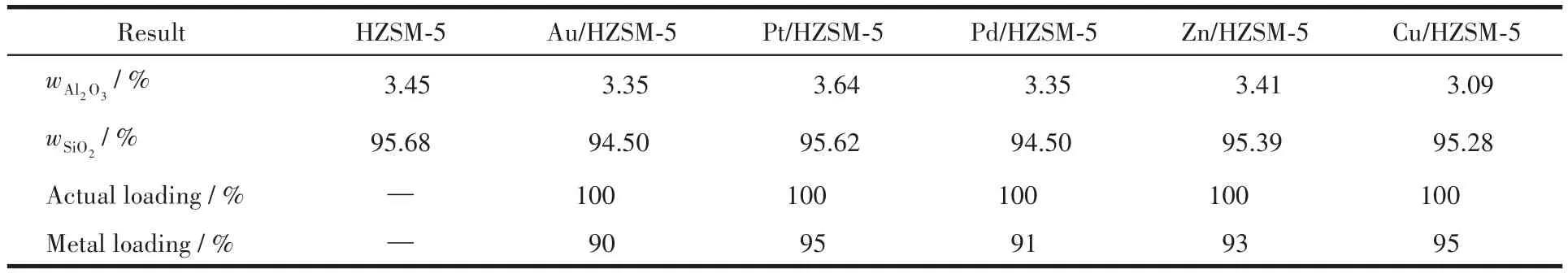
Table 1 XRF measurement results of different metal modified HZSM-5 catalysts*
2.1.2 XRD analysis
The XRD patterns of all the samples are shown in Fig.2a.A set of typical diffraction peaks(2θ=7°⁃9°and 23°⁃25°)corresponding to an MFI framework were clearly identified on all the samples,indicating that the MFI structure are wellmaintained after the Au,Pt,Zn,Cu,Pd modification.Metal phases were not detected,which indicates that the metal Au,Pt,Zn,Cu,Pd are dispersed in the HZSM⁃5 catalyst.After metal modifi⁃cation,the intensity of peaks at low angles(2θ=7.8°,8.7°)slightly increased,and there was no significantchange at high angles,indicating the decrease of crys⁃tallinity of the catalyst after metal modification[24].
2.1.3 Textural characteristic analysis by nitrogen physical absorption
The N2adsorption⁃desorption isotherms and the textural properties of all the samples are presented in Fig.2b and Table 2,respectively.As shown in Fig.2b,the typical type Ⅳ adsorption isotherms and H3 type hysteresis loops[14]have been found for M/HZSM⁃5,where the N2adsorption and desorption increased rapidly atp/p0<0.05 andp/p0>0.8.At the same time,whenp/p0>0.8,the parent catalyst was microporous mesoporous composite multistage zeolite whether before and after modification.The micropores are the inner channels of the catalyst,and the mesopores are produced by the accumulation of crystals between the catalysts.
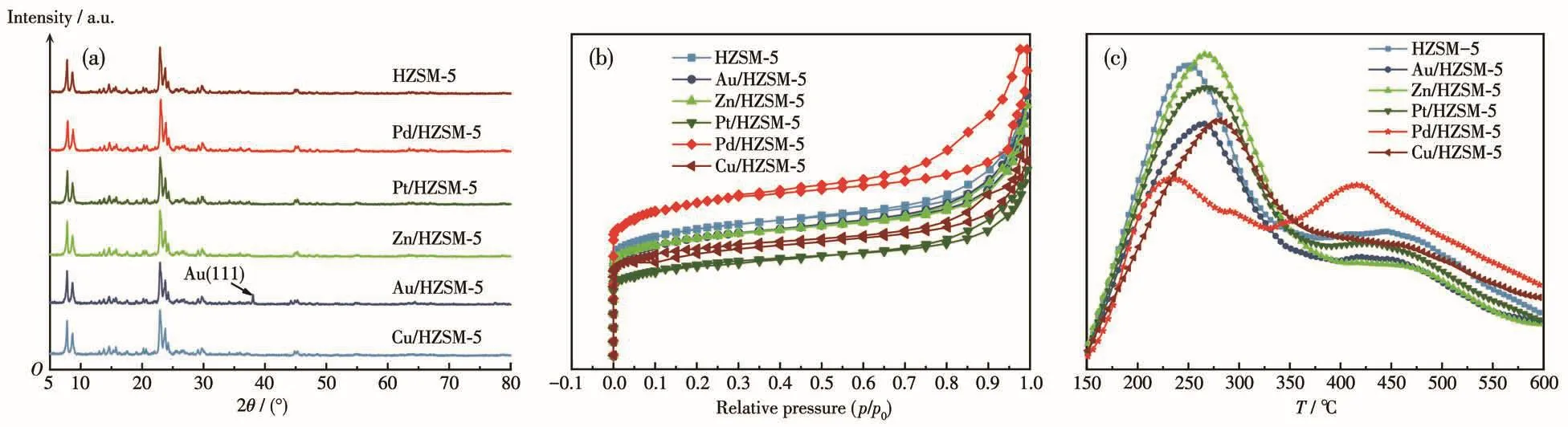
Fig.2 (a)XRD patterns of HZSM⁃5 and M/HZSM⁃5;(b)N2adsorption⁃desorption isotherms of HZSM⁃5 and M/HZSM⁃5;(c)NH3⁃TPD curves of HZSM⁃5 and M/HZSM⁃5
As listed in Table 2,the BET specific surface areas(SBET)of all the modified samples decreased slightly compared with that of the parent sample,and there was no significant difference in pore volume.For Pd/HZSM ⁃5,theSBETdecreased from 357 to 311 m2·g-1,and the pore volume decreased from 0.25 to 0.19 cm3·g-1.The major suppression of theSBETandVtin Pd/HZSM⁃5 might be due to the restricted structural collapse of the Si framework[13].Moreover,the pore size of all the samples maintained stability.The change of textural properties was not obvious after modification.So,the Au,Pt,Zn,Cu,and Pd are highly dispersed on the internal,external,and channels of HZSM⁃5,as proven by XRD.In addition,the microporous surface area(Smic)decreased faster than the external surface area(Sext)compared with the parent catalyst,indicating that the metals are highly dispersed on the microporous channels of HZSM⁃5.The micropore volume(Vmic)dis⁃tributed between 0.11 and 0.17 cm3·g-1,accounting for more than 60% of the total pore volume,which indi⁃cates that there are more mesopores in the metal modi⁃fied catalysts.TheVmicof M/HZSM⁃5 had no significant change compared with the parent sample,accounting for 40% of the total pore volume.
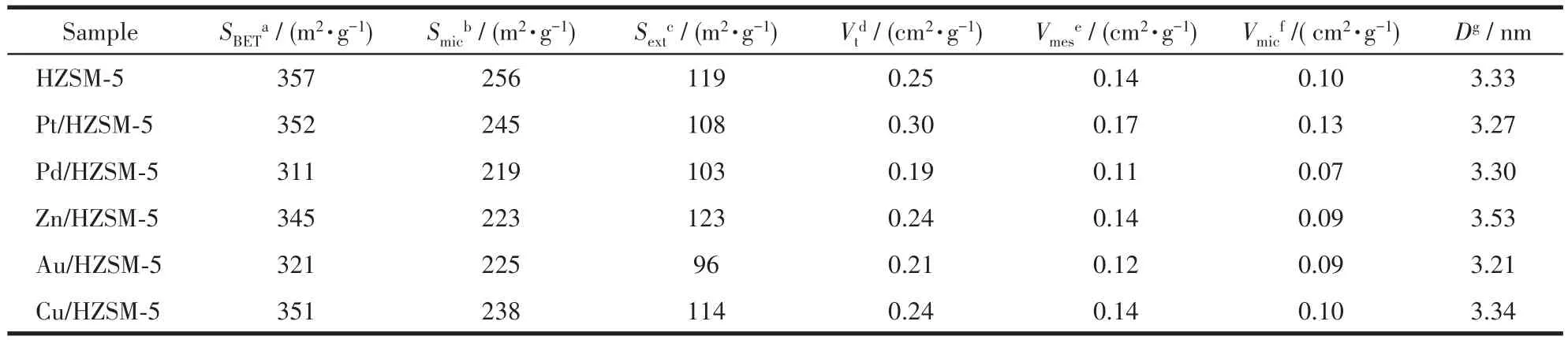
Table 2 Textural properties of HZSM-5 and M/HZSM-5
2.1.4 Acidity analysis by NH3⁃TPD and Py⁃FTIR
NH3⁃TPD analysis was performed in a tempera⁃ture range of 50 to 700℃to investigate the acidity of HZSM⁃5 and M/HZSM⁃5(Fig.2c and Table 3).All the samples exhibited two main peaks in the ranges of 230⁃270℃ and 400⁃450℃,which are ascribed to the weak and strong acid sites,respectively.The active sites of strong and medium⁃strong acids in the catalyst promote the formation of high⁃temperature peaks,and the active sites of weak acids and metal oxides in the catalyst pro⁃mote the formation of low⁃temperature peaks[15].In the case of the parent HZSM⁃5,the mainly NH3desorption peaks were concentrated at 250℃.In contrast,the weak acid site of the modified sample shifted to 276℃,indicating that the adsorption capacity for NH3is enhanced and the surface acid strength is increased.In a word,after metal modification,the acid strength of the strong acid center of the catalyst is reduced,and the weak acid strength is enhanced,forming more medium strong acid centers.This change might improve the conversion rate of methanol.
Table 3 and Fig.2c reveal the slight shift in peak position for the synthesized samples.The correspond⁃ing peak areas with weak acidity strength and strong acidity strength decreased,implying the weak alkalini⁃ty.The Au,Pt,Zn,Cu,and Pd elements act on the weak acid centers of the catalyst and reduce the num⁃ber of weak acid centers of the catalyst.The modified zeolite sample showed a reduction in its total acidity compared to the parent HZSM⁃5,due to the elimination of Si or Al atoms from the zeolite framework[16].
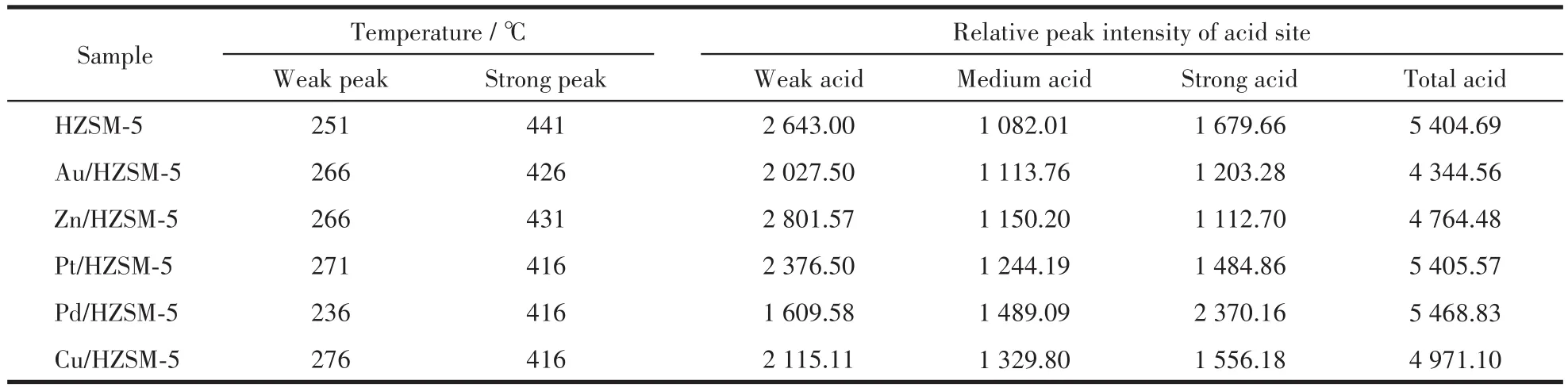
Table 3 Deconvolution of NH3-TPD profiles for all the samples
Py⁃FTIR analysis was performed to further identify types of acid sites on the catalysts(Fig.3).In general,the peaks of M/HZSM⁃5 distributed in the region of 1 700⁃1 400 cm-1.The peak located at 1 446⁃1 457 cm-1is attributed to pyridine adsorption of L acid sites,and another peak at 1 540⁃1 550 cm-1is ascribed to pyridine adsorption over B acid sites[17].Furthermore,the appearance of the peak at 1 490 cm-1is associated with the interactions of pyridine with both B and L acid sites[18].Fig.3 illustrate Py⁃FTIR spectra of the catalysts at different temperatures.All spectra showed similar structural features for all the samples.In addition,other peaks appeared near 1 640 and 1 620 cm-1,which points out that both 1 610 and 1 635 cm-1are characteristic peaks of pyridine cations[19].The two peaks can be classified as B acid sites.In addition,this consequence is well consistent with the XRD results in Fig.2a,indicating that the HZSM⁃5 framework remains intact after metal impregnation.The intensity caused by B acid sites significantly decreased after the loadingof metal on HZSM⁃5.The L acid sites next to B acid sites increased the acid strength,which is consistent with the NH3⁃TPD results and corresponds to the inter⁃actions between metal species and H+of HZSM⁃5[20].For HZSM⁃5 catalysts after metal impregnation,the metal could combine with silicon hydroxyl groups in catalysts and form Si—O(H)—X structure[21].The metal⁃modified zeolites exhibited favorable selectivity and activity on methanol carbonylation.For the impregna⁃tion of Zn,the reduction of B acid site density and the high Lewis acidity indicate the existence of extra⁃framework cation Zn species,occupying the charge compensation positions instead of the original protons presenting in the Zn⁃free zeolite.This would be in agreement with previous reference[24]that different extra framework Zn species are suggested such as[Al—O—Zn—O—Zn—O—Al]or[Al—O—Zn—O—Al],acting as L acid sites.The reference[25]proposed that Zn/HZSM⁃5 sample exhibits a significant decrease in the number of B acid groups(Si(OH)Al)on the modifi⁃cation with Zn,suggesting the consumption of zeolite protons in the exchange process.Moreover,the modifi⁃cation led to the disappearance of the internal silanol groups.El⁃Malki et al[26]proposed that this is due to the interaction between internal SiOH groups and Zn(OH)+cations located in the cation positions of zeolite,lead⁃ing to the formation of(ZO)—Zn—O—Si:
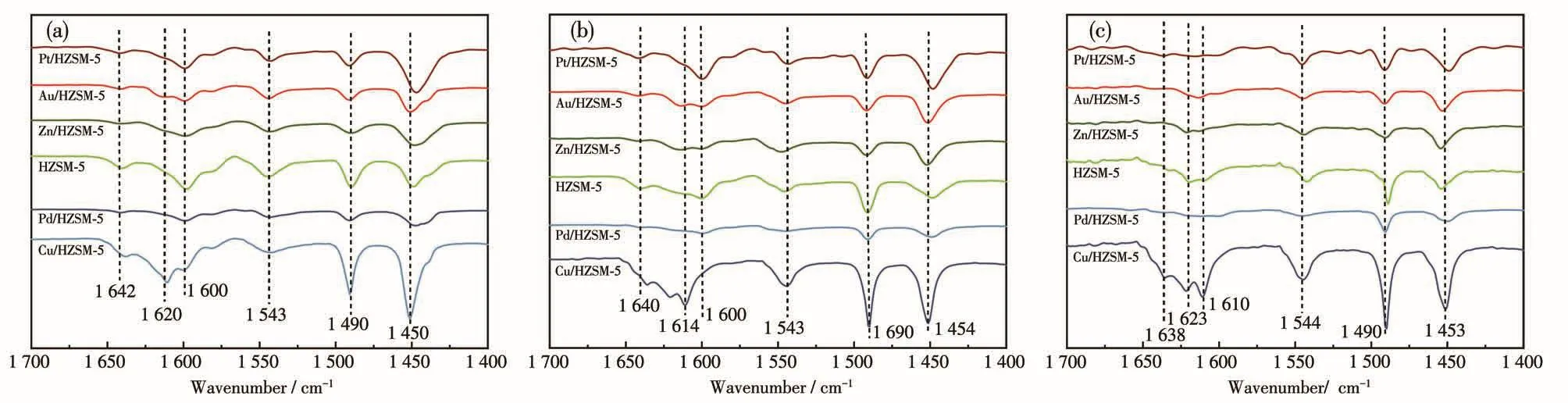
Fig.3 Py⁃FTIR spectra of HZSM⁃5 and M/HZSM⁃5 catalysts at(a)150 ℃,(b)300 ℃,and(c)450 ℃
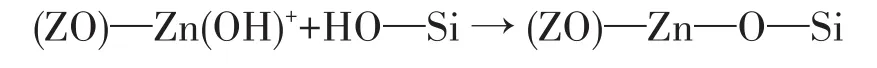
Because of the formation of external Zn—OH groups on ZnO clusters.The FTIR spectra of pyridine adsorbed on the modified samples also point to the con⁃sumption of B acid sites after modification and indicate the formation of L acid sites induced by the presence of metal ions,which is proven by the appearance of the intensive band at 1 455 cm-1.To investigate the specif⁃ic changes in the Brønsted and the Lewis acidity of the catalysts,the ratio of peak intensity at 1 540 and 1 450 cm-1was considered as a value to compare the relative amount(NB,NL)of B and L acid sites in the samples(Table 4).The ratio of B and L acid sites(NB/NL)increased and the total acid sites(NB+NL)decreased with the increase of temperature.Moreover,the acidity of B and L acid sites was weaker at lower temperatures and stronger at higher temperatures.The reason is that metals have broader implications for the L acid sites[27].
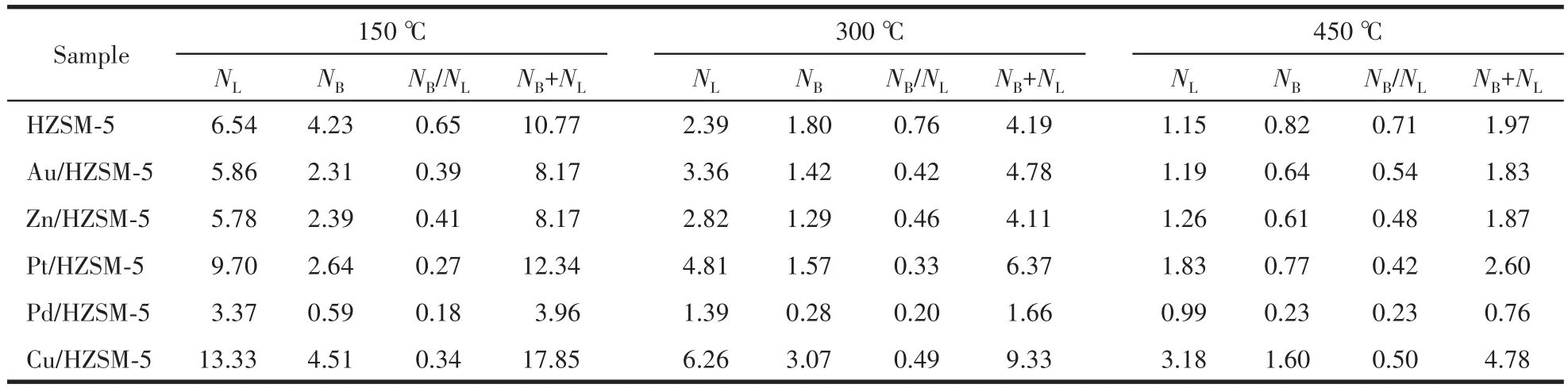
Table 4 B and L acid sites of HZSM-5 and M/HZSM-5 catalysts at different temperatures
2.1.5 Performance evaluation of methanol to ester over modified zeolite
The introduction of different metals has little effect on the surface area,pore size,and pore volume of the HZSM⁃5 catalyst,but it changes the acid center strength and acid quantity on the surface of the catalyst and acts on the methanol carbonylation system.The methanol carbonylation reaction was carried out with HZSM ⁃5 and metal⁃modified HZSM ⁃5 catalysts at atmospheric pressure and reaction temperature from 150 to 400℃.The reaction steps are as follows:
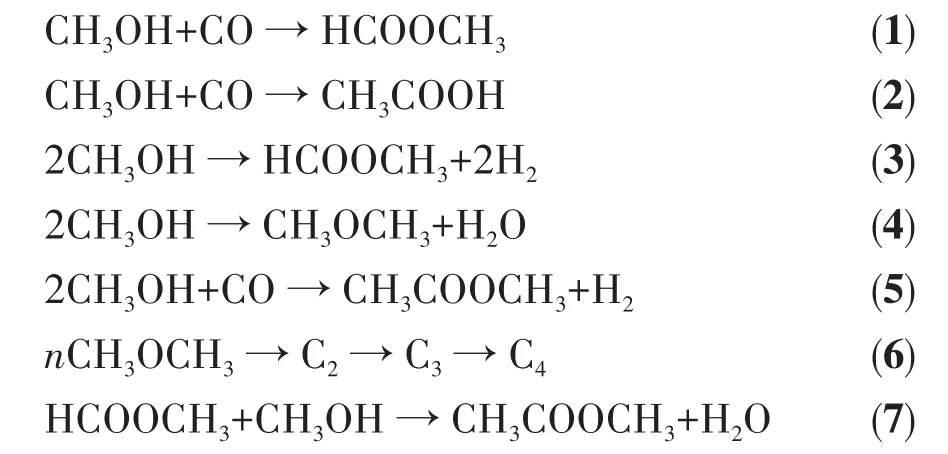
Fig.4a shows the relationship between tempera⁃ture and methanol conversion rate(XCH3OH).TheXCH3OHincreased with reaction temperature.The order ofXCH3OHfor the catalysts is as follows:Cu/HZSM⁃5>Pt/HZSM⁃5>Au/HZSM⁃5>Zn/HZSM⁃5>HZSM⁃5>Pd/HZSM⁃5.The methanol conversion of Cu/HZSM⁃5 was as high as 90.3% at 400℃,which is 10% higher than that of the unmodified catalyst.Fig.4b shows the relationship between theXCH3OHat 300℃and theNB+NL.The conversion of methanol increased with the total acid sites of B acid and L acid.Cu/HZSM⁃5 had more B and L acid sites,with a maximum conversion of 85% at 300℃.The minimum conversion was only 47.9% for Pd/HZSM⁃5 with minimum B and L acid sites.The results show that the more sum value of B acid and L acid for metal modified HZSM⁃5 catalyst,the higher the methanol conversion rate is.
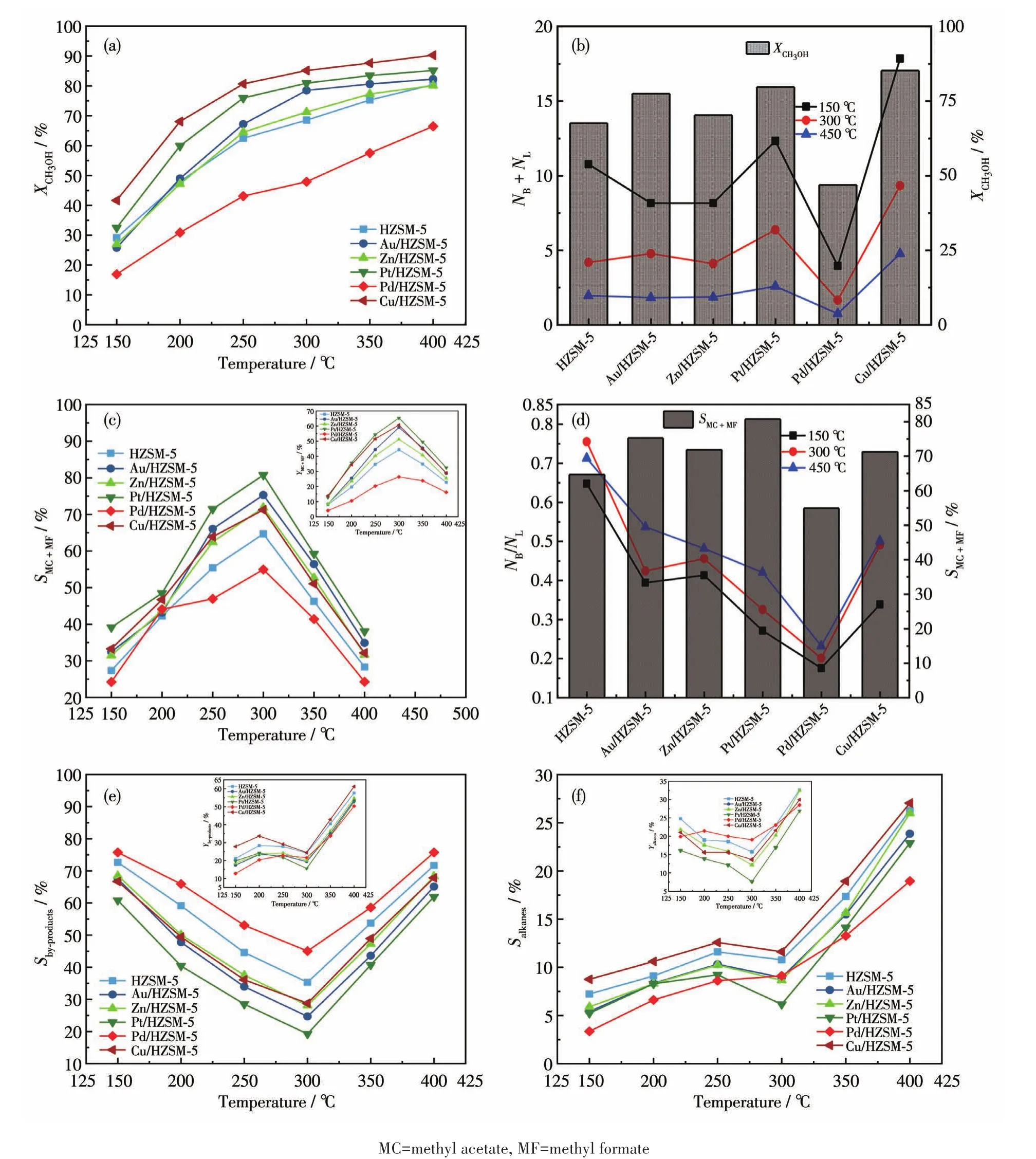
Fig.4 Performance of methanol to ester over HZSM⁃5 and M/HZSM⁃5:(a)XCH3OHat different temperatures;(b)relationship between NB+NLandXCH3OHat 300℃;(c)YMC+MF(Inset)and SMC+MFat different temperatures;(d)relationship between NB/NLand SMC+MFat 300 ℃;(e)Yby⁃products(Inset)and Sby⁃productsat different temperatures;(f)Yalkanes(Inset)and Salkanes at different temperatures
The carbonylation selectivity(the selectivity of methyl acetate and methyl formate,SMC+MF)for HZSM⁃5 and M/HZSM⁃5 catalysts is shown in Fig.4b.The yield(Inset)and selectivity of carbonylation products gradu⁃ally increased with the increase of temperature,reach⁃ing a maximum at 300℃.In particular,Pt/HZSM⁃5 had better selectivity(80.8%)than other samples,which increased by 25% compared with the unmodi⁃fied catalyst.The yield and selectivity of methyl for⁃mate and methyl acetate decreased when the tempera⁃ture rised to 350℃,because methyl acetate and meth⁃yl formate decompose to generate CO and H2at higher temperatures[29].Therefore,the carbonylation activity of M/HZSM⁃5 catalysts decreases as follows:Pt>Au>Cu>Zn>Pd.Combined with Fig.4d,it could be concluded that the SMC+MFdecreased with the increase of NB/NLat a higher temperature,indicating that the carbonylation activity of methanol decreased with the increase of NB/NL.However,it doesn′t mean that a lower NB/NLis ben⁃eficial to the reaction.For the impregnation of Pd,the minimum NB/NLof Pd/HZSM⁃5 was 0.18,and the selec⁃tivity of Pd/HZSM⁃5 was as low as 55%.In a system where methanol is converted to methyl acetate and for⁃mate,methanol is dehydrogenated at the B acid center to generate CH3O,CH3+,and CH2O groups,then CH3O and CH3+polymerize at the metal⁃L acid center to form methyl formate and methyl acetate,indicating that B acid and L acid have a synergistic effect on acid meth⁃yl formate and methyl acetate[28].In a word,a low NB/NLvalue is not conducive to the carbonylation reaction.Among all the samples,the NB/NLaround 0.3⁃0.5 can obtain a higher catalyst activity for carbonylation.
Fig.4e shows the yield of by ⁃products(Yby⁃products)the selectivity of by⁃products(Sby⁃products)in methanol car⁃bonylation over HZSM⁃5 and M/HZSM⁃5 catalysts from 150 to 400℃,and Fig.4f shows the yield of alkanes(Yalkanes)and the selectivity of alkanes(Salkanes)in metha⁃nol carbonylation over HZSM⁃5 and M/HZSM⁃5 cata⁃lysts from 150 to 400 ℃.The by⁃products mainly included methyl ether,acetic acid,dry gas,and alkanes.Olefins and aromatics were also observed with the increase in temperature.The catalyst will not cause coking and deactivation under high⁃temperature con⁃ditions.Choosing the correct metal to support the cata⁃lyst is necessary.When the reaction temperature of methanol carbonylation is too high,the selectivity of the target product will be reduced,and the reaction temperature should be controlled at about 300℃.
Fig.5 shows the carbonylation of methanol by the catalyst with B acid center and L acid center.The mod⁃ified metal is activated on HZSM⁃5 to generate metal active sites Si—O(H)—X—Al[30⁃36]as shown in Fig.6.Then methanol is dehydrogenated at the B acid center(Si—O(H)—Al)and metal⁃L acid center to generate CH3+and CH3O groups.CH3O is further dehydrogenated at the B acid center to form CH2O,undergoing a cou⁃pled dehydrogenation reaction at the center of metal L acid to form methyl formate.Methanol is easily decom⁃posed into CO and H2.After activation,CO at the metal active center reacts with CH3to form intermediates CH3C[O]+and then intermediates with CH3O to form methyl acetate.After methanol dehydration,methyl can also react with methanol to form dimethyl ether,while dimethyl ether is dehydrated on Si—O(H)—Al acid to form alkanes and further dehydrogenates to form chain alkanes[37⁃47].It is precise because of the interaction between the supported metal and the HZSM⁃5 catalyst that a metal active center is generated,which can better promote the conversion of methanol and obtain more target products.
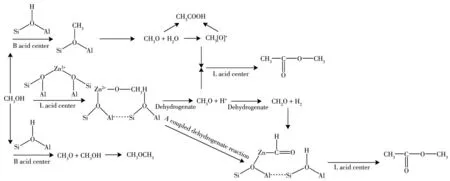
Fig.5 Conversion of methanol to methyl acetate and ethyl acetate
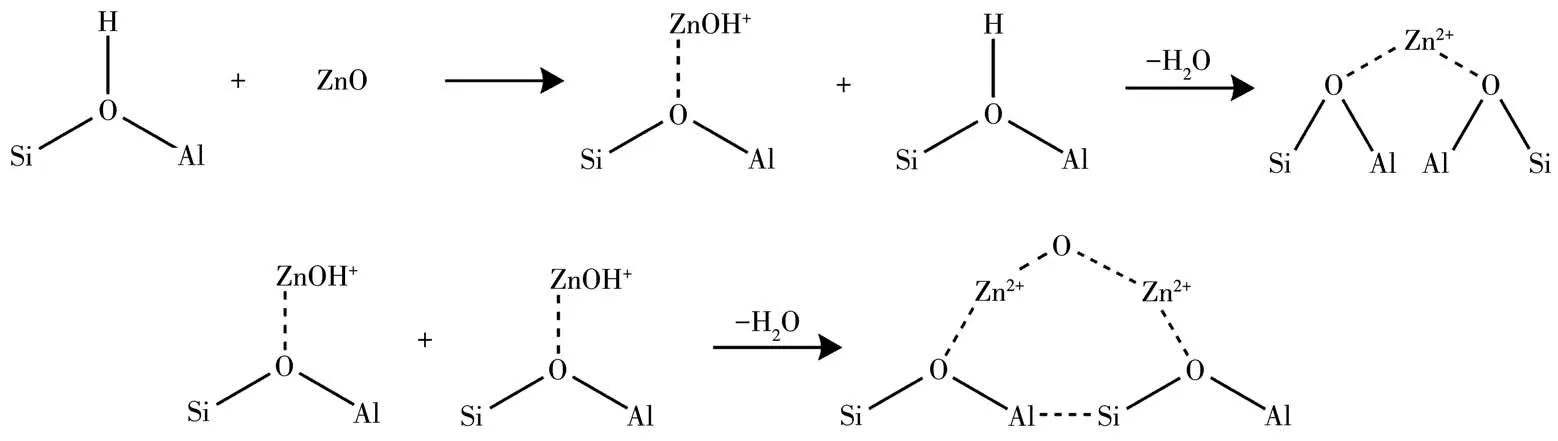
Fig.6 Metal activation process on HZSM⁃5
3 Conclusions
A series of M/HZSM⁃5 catalysts modified with dif⁃ferent metals were prepared and examed for methanol carbonylation to generate methyl acetate and methyl formate.Compared to other catalysts,Pt/HZSM⁃5 facili⁃tated the yield and selectivity of methyl acetate and methyl formate under the same condition.The selectivi⁃ty order of the metal⁃modified catalyst to methyl for⁃mate and methyl acetate is listed as follows:Pt>Au>Cu>Zn>Pd.The reason is that the catalyst decreases the B acid center and increases the L acid center after Pt modification,which has a lower NB/NLvalue.Pd has amounts of strong acid centers in NH3⁃TPD,thereby inhibiting the conversion rate of methanol.Further⁃more,the conversion rate of methanol increased at a higher temperature.The yield of the carbonylation product is higher at 300℃but drops significantly at 350 and 400℃.Cu/HZSM⁃5 has more B and L acid sites than other samples,inducing a favorable conver⁃sion rate.
- 无机化学学报的其它文章
- 无机材料在骨质文物加固保护中的应用
- 百香果皮基原位氮掺杂多孔碳/硫复合正极的制备及储锂性能
- 氯钯共掺对Cs2TiBr6光电性能影响的第一性原理计算
- Interactions of a Water-Soluble Diiron Hexacarbonyl Complex with Biologically Relevant Molecules and Their Promotion in CO-Release
- Crystal Structures and Magnetic Refrigeration Properties of Two Gd2Complexes
- Cyanide-Bridged Bimetallic Active Site in Porous Carbon-Matrix for Oxygen Reduction Reaction

