Radiological imaging and non-surgical local treatments for cholangiocarcinoma
Angelo Della Corte, Ettore Di Gaeta, Stephanie Steidler, Francesco De Cobelli,3
1Department of Radiology, IRCCS San Raffaele Scientific Institute, 20132 Milan, Italy.
2Vita-Salute University, 20132 Milan, Italy.
3Experimental Imaging Center, IRCCS San Raffaele Scientific Institute, 20132, Milan, Italy.
Abstract Cholangiocarcinoma (CC) is a malignancy with a very heterogeneous spectrum of morphopathological and prognostic characteristics. Diagnostic imaging is fundamental for early detection, preoperative staging, and resectability assessment, as well as early recognition of prognostic factors. Radical surgical treatment is limited by disease stage and technical feasibility. Interventional radiology has acquired a critical function in addressing disease control and survival improvement through loco-regional therapies, specifically in the setting of intrahepatic CC. In this review, we will describe the current state of art of diagnostic imaging, focusing on intrahepatic CC and proximal extrahepatic CC, and delineate the available loco-regional therapies strategies for unresectable intrahepatic CC.
Keywords: Cholangiocarcinoma, diagnostic imaging, loco-regional therapies, ablation, embolization
INTRODUCTION
Cholangiocarcinoma (CC) represents the most frequent malignant tumor of the biliary tract, accounting for 10% to 20% of all primary liver tumors[1]. These malignancies can be classified according to their morphological characteristics, anatomical site of origin, or based on histological classification. Even though histologically the vast majority are adenocarcinomas, with a high proportion of fibrous stroma, CCs represent a very diverse disease spectrum.Based on their anatomical localization, CCs are subdivided into intrahepatic cholangiocarcinoma (ICC)(i.e., originating from intrahepatic bile ducts) and extrahepatic cholangiocarcinoma (ECC). ECCs are further subdivided into perihilar- (peCC) and distal-extrahepatic cholangiocarcinoma (deCC) depending on its proximal or distal origin with respect to the confluence of the cystic duct into the main bile duct.
Morphologically, CCs are subdivided into mass-forming, periductal-infiltrating, or intraductal-growth, with intrinsic imaging findings and prognostic outcome. Intraductal-growth CCs tend to have a better prognosis,while mass-forming and periductal infiltrating types appear to have a poor outcome.
The global incidence of CC varies from 0.3 to 6 per 100,000 inhabitants per year[2], and similarly, the mortality ranges from 1 to 6 inhabitants per 100,000 per year[3]. Both CC incidence and mortality have been increasing in the past few decades, representing a relevant health burden.
Risk factors for development of CC are present in approximately 50% of cases. These risk factors vary according to geographical distribution as well as anatomical location. Diseases affecting the peripheral bile ducts, such as choledocholithiasis and primary sclerosing cholangitis, are more strongly associated to ECC;whereas, intrahepatic conditions, such as cirrhosis (i.e., viral or NASH-related) and intrahepatic lithiasis, are more typically linked to ICC[4]. Accordingly, patient age at diagnosis shows extreme variability, ranging from 2nd decade of life in the case of Cairoli disease[5]to the 6th decade of life in NASH-related forms[6].Comorbidities typical of CC patients, including but not limited to reduced liver function and cholestasis,may influence prognosis[7], as well as treatment selection whenever they contribute to patient unfitness for surgery.
CC imaging requires in-depth knowledge of disease specific characteristics, as cases may present with atypical imaging features[8]or “conventionally” described characteristics may mimic other malignancies or benign lesions[9,10]. Imaging evaluation of specific sub-group characteristics is therefore of paramount importance for disease diagnosis and therapeutic approach assessment. To date, computed tomography(CT) and magnetic resonance imaging (MRI), aided by magnetic resonance cholangiopancreatography(MRCP) sequences, remain the most commonly used imaging modalities. Positron emission tomography(PET)[11,12]may be useful for detection of metastases and may be of aid in further personalizing treatment options. In addition to diagnosis and resectability assessment, imaging may aid in providing information about prognosis of CCs, by combining information deriving from morphology, enhancement pattern, and texture analysis along with clinical and histopathological data.
Regarding treatment, surgical resection with negative margins is recognized as the only curative treatment for both ICC and ECC[13]. Several limitations, including disease stage, inadequate future liver remnant,presence of metastases, and technical contraindications, lower resectability rate to 20%-30%[14,15]. In patients with unresectable ICC, loco-regional treatment options are valid alternatives to systemic chemotherapy and have been shown to increase overall survival[16,17]. The most common techniques are ablative and intraarterial therapies. Recent evidence also supports these approaches and suggests a role for loco-regional treatments in the adjuvant and neo-adjuvant setting. Combined approaches can also be used when imaging and histopathology identify the need to confirm negative margins in patients with non-upfront surgical resection[18]. For ECC, the role of the interventional radiologist is mostly limited to the palliative and ancillary setting (i.e., biliary drainage/stent positioning and hypertrophy-inducing techniques to allow surgical resection), without direct effects on disease burden[19].
In this short review, we will describe the current state of art diagnostic methods and the prognostic value of imaging in ICC and peCC, as well as the loco-regional therapeutic strategies for unresectable ICC.
DIAGNOSTIC IMAGING
Imaging choice is essential for correct tumor assessment and for the best patient management prior to,during, and after therapy. A multimodality approach, which includes CT, MRI with MRCP and PET, is usually necessary to reach a final diagnosis and correctly identify and select patients who may undergo surgery [Figures 1-3]. Imaging has a high negative predictive value with respect to resectability[20,21].Additional information may be collected using endoscopic retrograde cholangiopancreatography for completion of histological diagnosis if not already available.
Multidetector computerd tomography
Multiphasic contrast-enhanced CT scan has a well-documented role in diagnosis, tumor staging, and preoperative assessment of both peCC and ICC[9,22,23]. It also offers clinical information regarding local staging (e.g., relationship with hepatic ducts and vessels) and assessment of metastatic disease. The scan(s)should be obtained in the four phases: pre-contrast, arterial, portal, and delayed (3-5 min) post-contrast phases for evaluating enhancement patterns of the mass. The pre-contrast phase is useful in identifying predisposing peCC conditions such as intrahepatic stones, while post-contrast imaging[24]is useful for differential diagnosis of ICC given its abundance in fibrous stroma.
CT is generally the most commonly used imaging for upfront staging, and it is the modality used to describe vascular and intrahepatic involvement such as relationship with hepatic ducts and vessels. Fusion imaging techniques and three-dimensional CT angiography are useful in planning surgery, reducing time of intervention[25].
MRI - MRCP
Currently, MRI is the most common imaging method used to diagnose perihilar CC. Two-dimensional and three-dimensional (3D) MR cholangiography is considered to be the best non-invasive modality to evaluate the biliary system and correctly assess intraductal lesions. Heavily T2-weighted sequences, such as half-Fourier acquisition single-shot turbo spin echo or single-shot fast spin echo, can be used to distinguish the hyperintense signal in the bile ducts from the remaining suppressed signal when performing MRCP. MRI protocol should include axial and coronal T2-weighted sequences, dynamic study using T1-fat suppressed sequences such as DIXON, and diffusion imaging. MRI is more sensitive than CT for the detection of intrahepatic metastasis and extremely useful in pre-operative assessment[26,27]. Gadoxetic acid-enhanced MRI, in particular, is particularly sensitive in the hepatobiliary phase[28,29], due to hepatocyte uptake leading to clear parenchymal enhancement. In addition, diffusion-weighted (DWI) sequences, performed using 0-100 s/mm2and 800-1000 s/mm2for low- and high-b values, increases sensitivity of detection[30].
Preoperative MRI is commonly performed in patients with CC in order to assess the extent, resectability,and vascular involvement of the tumor. It is also used to map hepatic vessels and identify vascular anatomic variants since an accurate preoperative assessment of liver vasculature has been shown to significantly affect the surgical outcome in patients with CC[31].
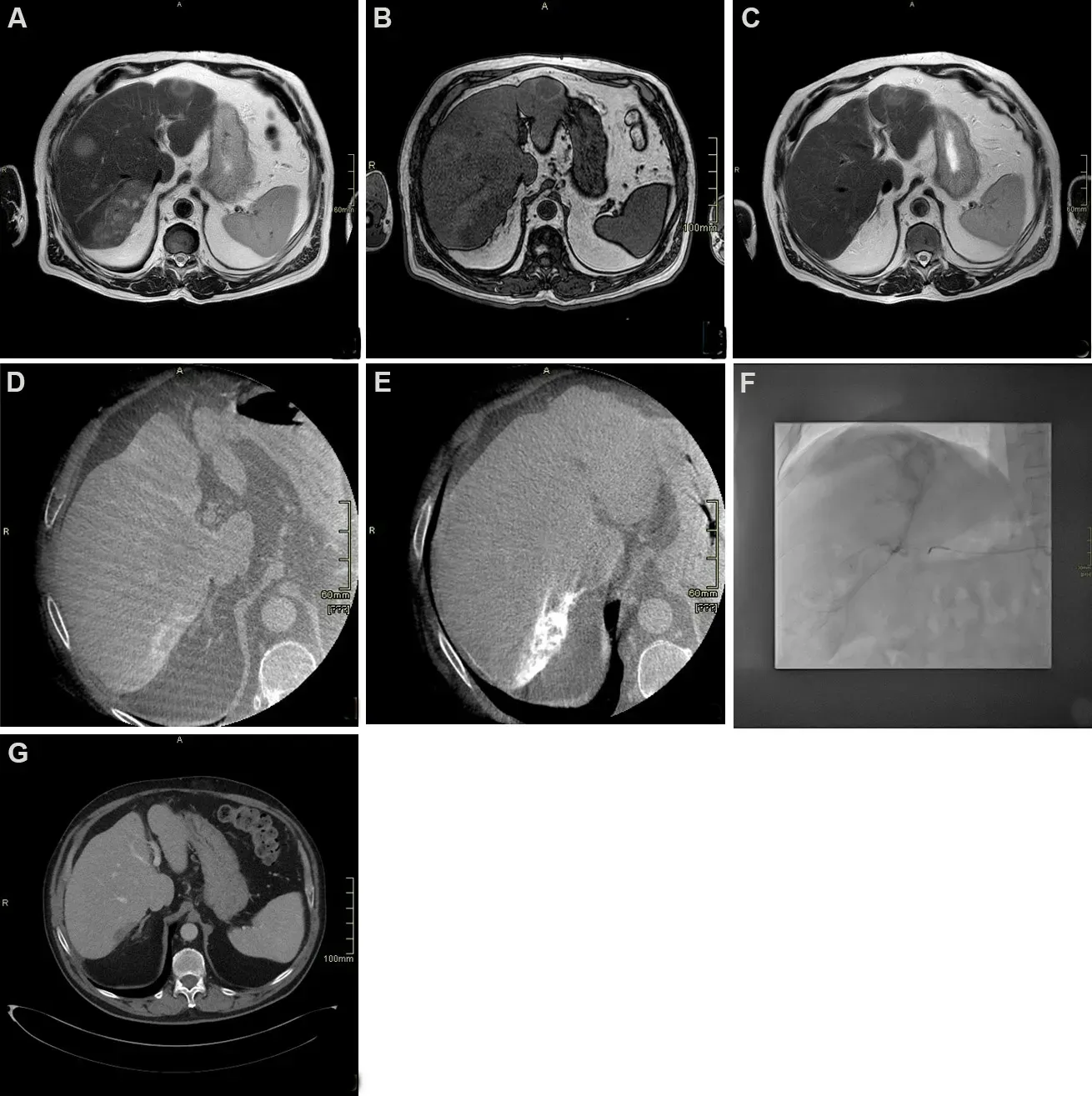
Figure 1. Seventy-year-old male patient with diagnosis of intrahepatic cholangiocarcinoma (ICC) undergoing chemotherapy and drugeluting beads transarterial chemoembolization. (A) T2 weighted axial scan demonstrating multifocal hyperintense ICC; (B) T1 weighted axial scan demonstrating multifocal hypointense ICC; (C) T2 weighted axial scan after chemotherapy showing significant reduction; (D)cone beam computed tomography (CT) arterial phase demonstrating target lesion for transarterial chemoembolization showing peripheral vascular “rim” enhancement; (E) cone beam CT after treatment showing uptake of embolizing beads; (F) angiogram during transarterial chemoembolization treatment, showing lesion uptake; (G) delayed post-contrast CT scan phase 1 month after treatment demonstrating complete devascularization of the lesion.
Parket al.[32]nearly two decades ago compared the diagnostic performance of MRCP with that of combined MDCT-angiography and found comparable results between the two techniques. MRCP sequences are usually acquired using both (radial) thick-slab- and thin 3D T2 sequences; the former provides a comprehensive overview of the biliary system with a good suppression of the surrounding tissue. Thin T2 sequences, driven by thick slab sequences, provide detailed spatial resolution of complex anatomical structures and detection of small abnormalities such as small masses or stenosis[33]. In addition to MRCP,which is useful for depicting intraductal CC tumor growth, DWI-MRI, dynamic contrast-enhanced MRI,and late-phase sequences with hepatocyte-specific contrast agents can describe extra-ductal tumor growth as well as identify tumor masses located within dilated bile ducts[33,34].
Imaging plays a central role in the preoperative staging and follow-up of CC, as well as in the surveillance of patients with an increased risk of CC. The lifetime incidence of CC in patients with primary sclerosing cholangitis ranges from 5% to 10%[33]. According to recent recommendations, MRI including MRCP is appropriate for the surveillance of these patients[35].
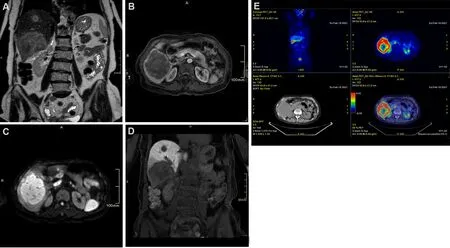
Figure 2. Seventy-one-year-old female patient with hepatitis C cirrhosis. (A) T2 weighted coronal scan demonstrating multifocal hyperintense intrahepatic cholangiocarcinoma; (B) T1 fat suppressed axial scan arterial phase showing peripheral rim enhancement; (C)diffusion-weighted scan demonstrating hyperintense signal; (D) T1 fat suppressed coronal scan showing hypointense signal on hepatobiliary phase: note the hypointense rim surrounding a relatively hyperintense cloud-like area; (E) 18F-FDG PET scan demonstrating lesions uptake.
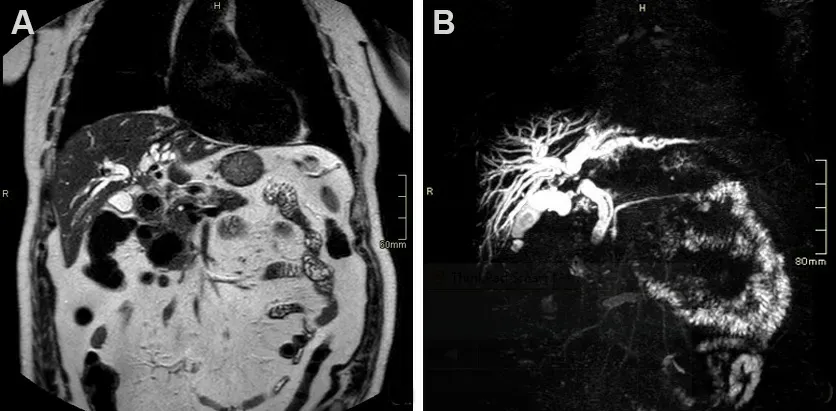
Figure 3. Seventy-nine-year-old male patient presenting with cholestasis. Coronal single shot fast spin echo T2 images (A) and magnetic resonance cholangiopancreatography (B) show an infiltrating mass in the common bile duct (CBD), proximal to cystic duct insertion, leading to imaging diagnosis of perihilar cholangiocarcinoma. The patient was deemed resectable and successfully underwent right hepatectomy with CBD resection.
PET-CT/PET-MRI
Combined modalities, such as PET/CT and PET/MRI, give both functional and morphological information on involvement and extension of disease. These modalities may be useful in pre-operative assessment as they allow detection of both distant and nodal metastasis using 18F-FDG. Potential caveats may be false positives in cases with biliary inflammation and false negatives in mucinous CC[36].
peCC
The most common peCC is the periductal infiltrating type characterized by elongated strictures with irregularly visible walls on imaging and delayed enhanced duct dilation. Imaging assessment of tumor infiltration in the deeper mucosal layers of the bile ducts is challenging[37]. In addition, imaging characteristics of peCC are similar to those of other hepatic diseases, benign and malignant, and differential diagnosis becomes cardinal for correct patient management. In these cases, evaluation using contrast enchanced-CT or MRI-MRCP is recommended. DWI seems to be able to differentiate malignant strictures in extrahepatic disease[38], with contrasting views of T1 sequences in peCC. 18F-FDG PET/CT has shown to be able to differentiate between benign and malignant strictures[39,40]in larger lesions[41,42].
Surgical assessment on imaging, even more in the light of new advances in surgical techniques and perioperative management which allow a larger cohort of patients to pursue this curative indication, is also challenging. Extent of involvement of the bile ducts (proximal margin involvement and longitudinal extent)and patient intrinsic patient characteristics are major differentials. Imaging using different CT modalities(i.e., CT alone, CT with direct cholangiography, or CT with reconstruction) have been able to confirm the documented ability of MRCP-dynamic MRI to identify secondary confluence involvement with comparable results[37]. The description of local extent of tumor invasion, anatomy of the biliary tree and association of tumor extent within the liver is mandatory for optimal resectability assessment.
ICC
Mass-forming ICCs are the most common morphological subtype of ICCs. On imaging they appear as large non-capsulated masses with associated peripheral dilation of bile ducts and may appear hypervascular similarly to HCC. Further characteristic imaging features include capsule retraction. Depending on their stromal component, generally characterized by fibrosis following desmoplastic reaction in the central portion, they may exhibit a typical late central enhancement with peripheral hypoenhancement. In several cases, additional nodules in proximity of the lobulated or irregular mass forming nodules and hepatic metastases are also visible. Since pattern of enhancement can vary, differential diagnosis with HCC is often challenging even when gadoxetic acid is used as contrast agent[43-45]. However, MRI may address towards diagnosis of ICC when “target appearance” is noted on diffusion weighted imaging or in post-contrast late hepatospecific phase. In the former, DWI images at high b-value will show an hyperintense rim surrounding a central area of hypointensity. In the latter, a central cloud-like hyperintensity will be surrounded by a rim of peripheral hypointensity. In both cases, these typical appearances reflect the histological components of ICC, characterized by a peripheral area of hypercellularity and a central hypocellular area dominated by fibrosis. Histopathology is considered mandatory in order to confirm imaging features which may resemble other benign or malignant (combined) lesions.
Prognostic value of CT and MRI
Recently, studies have demonstrated that preoperative CT and MRI features - of ICC may predict overall survival and/or disease-free survival after surgical resection or loco-regional therapies.
In ICC, studies suggest that extended areas of arterial hyperenhancement and hyperintense signal on DWI[46-48]show a favorable prognosis as opposed to tumors characterized by hyper- or isointense signal on the hepatobiliary phase[49]or with extended areas of delayed phase enhancement reflecting a higher fibrous content[50]. This is consistent with the histopathological observation that patients with scirrhous type ICC,characterized by abundant fibrous stroma, show higher rates of lymph node metastasis and poorer survival outcomes than those with non-scirrhous type ICC[51].
A study by Pandeyet al.[52]assessing preoperative characteristics of ICC candidate to trans-arterial chemoembolization demonstrated that overall survival (OS) was higher in patients with lower values of baseline apparent diffusion coefficient, percentage of viable tumor volume > 90%, and viable tumor burden> 6.6% independently from clinical confounders (age and sex). Data suggest that tumors characterized by lower viable tumor burden, hence a greater degree of fibrosis and necrosis, are associated to a hostile tumor microenvironment, where hypoxia, acidosis, and inadequate perfusion limit the efficacy of intra-arterial drug delivery.
In peCC, a recent study by Yooet al.[53]on prognostic performance of preoperative MRI demonstrated that imaging evidence of peritumoral fat stranding, common bile duct involvement, and Bismuth type III/IV disease were independent predictors of residual disease after surgery, which was in turn associated to poorer survival.
INTERVENTIONAL RADIOLOGY
Loco-regional treatments are gaining increasing interest in the field of ICC as technological advances guarantee an effective disease control while maintaining the safety of a minimally invasive approach.Knowledge of CC disease presentation as well as clinical characteristics of the patients are essential to select the most appropriate interventional tool based on a personalized approach. Promising results from combination of loco-regional treatments and systemic therapies of unresectable ICCs are also briefly discussed in this article.
ABLATION
Ablation is the physical destruction of tumor cells via delivery of thermal energy through a percutaneous needle placement. The low incidence of ICC compared to HCC, makes the availability of literature on the efficacy of ablation scarce. First reported in 2002 by Slakeyet al.[54]for the treatment of non-resectable ICC,ablation is now recognized by guidelines as a palliative option for non-resectable ICC measuring ≤ 3 cm without evidence of extrahepatic disease[55]. Kolarichet al.[16], through a population analysis of 4374 patients undergoing non-surgical management of ICC, produced robust evidence in demonstrating that ablation was associated with a statistically significant survival benefit over no local therapy only in stage I disease(i.e., without extrahepatic disease).
Another setting where ablation has shown very interesting results is the management of post-surgical intrahepatic recurrence of ICC. In a recent study by Xuet al.[56]comparing repeated resection (n= 65)vs.microwave ablation (n= 56) of recurrent ICC, with comparable tumor size among the two groups, ablation had a similar efficacy in terms of overall survival and progression free survival with a much lower complication rate.
Ablation can be performed under ultrasound or CT guidance as per local expertise, and several technical options exist for reaching tumor tissue destruction. Among these, radiofrequency ablation is the most extensively studied, with proven technique efficacy in tumors up to 5 cm[57]. Microwave ablation have the advantage of lower susceptibility to heat-sink effects, and with higher temperatures in a short time, it can achieve larger ablation zones[58]. However, these theoretical advantages have not effectively translated to an improvement in prognosis[59], leaving the choice among radiofrequency ablation and microwave ablation to local expertise and availability.
Alternative ablative strategies are cryoablation and irreversible electroporation, both of which may be an option in central liver tumors and tumors adjacent to sensitive structures (e.g., gallbladder, major bile ducts,and bowel loops) due to their safer profile. However, data regarding their efficacy on ICC are extremely scarce[60].
Factors affecting local tumor progression after ablation of ICC are size[61]and superficial location[59]. Higher rates of local tumor progression in these settings are the result of under treatment due to technical limitations (larger lesions) and possible damages to neighboring structures (in subcapsular lesions). Factors affecting survival after ablation are albumin-bilirubin grade[62], tumor size, and presence of more than one tumor[59].
One very frequently encountered complication after ablation of ICC is the development of a biliary abscess,with an incidence rate ranging between 7% and 20%[63]. Risk factors for the occurrence of this complication are cholangiectasis (since thermal injury to the bile duct leads to contamination of ablation zone by enteric bacteria) and presence of bilioenteric anastomosis (due to retrograde enteric bacterial contamination of the biliary tract). For these reasons, pre-procedural care of these patients includes prophylactic antibiotic therapy and palliation of biliary dilation whenever possible.
Transarterial chemoembolization
Conventional transarterial chemoembolization (cTACE) is the emulsion of chemotherapeutics and an oilbased contrast agent (lipiodol) followed by an embolizing agent into the tumor-feeding artery[64].Doxorubicin, cisplatin, mitomycin-C, and gemcitabine are the most commonly used drug combinations[65,66]. In order to be eligible for TACE, good hepatic function (Child-Pugh Class A or B) and performance status ECOG 0-2) are required. As for ablation, in patients with biliary dilation or bilioenteric anastomosis, the increased risk of procedure-related development of biliary abscess justifies a preprocedural prophylactic antibiotic regimen[67]. The ideal candidate for TACE is a patient with nonresectable, multifocal disease not tolerating systemic chemotherapy. Another typical indication during MDTs may be the presence of particularly hypervascular disease localization, wherein a good response to intraarterial therapy may be expected.
Survival of ICC after cTACE has been shown to range from 12 to 25.2 months from diagnosis, and from 9.1 to 16.3 months from the procedure[68]. The extreme variability in the results is a reflection of the variability in patient-related variables, including tumor burden and previous therapies, as well as technical variables including type of chemotherapeutic drug and number of treatment sessions. cTACE has a safe profile with few adverse events. Post-embolization syndrome (i.e., transient nausea, abdominal pain, fever, and selflimited increase in liver enzymes)[69]is the most common side-effect.
Drug-eluting beads transarterial chemoembolization (DEB-TACE) is a relatively novel advance of TACE,where local release of chemotherapeutic agents is mediated by pre-loaded beads obtaining both a therapeutic and embolizing effect, with lower systemic drug exposure compared cTACE[70]. Also in DEBTACE, efficacy of the procedure is extremely variable, with survival ranging from 8.6 to 30 months[68]with the same limitations described for cTACE.
DEB-TACE has been described with both doxorubicin and irinotecan; a study by Venturiniet al.[71]on 10 patients comparing DEB-TACE with doxorubicin and DEB-TACE with irinotecan showed no significant differences between the two groups in terms of safety and efficacy.
Both in cTACE and DEB-TACE, treatment design may be lobar, segmental, or subsegmental, according to the disease extension and distribution. Underlying liver function must be taken into account when planning the treatment strategy, since lower volume of ischemia may be tolerated in cirrhotic patients; for this reason,treatment schedule may be organized in sequential sessions (at least 2 weeks apart) if a large volume of liver is involved, in order to allow healthy parenchyma to withstand the ischemic injury and preserve function.
Trans-arterial radioembolization
Trans-arterial radioembolization (TARE) consists in the intra-arterial injection of radioactive microspheres,in order to selectively release a high radiation dose to liver tumor cells while maintaining an acceptable dose to the healthy parenchyma[72]. Inclusion criteria for TARE include, but are not limited to, ECOG performance status of 0-2 and adequate liver function with bilirubin < 2.0 mg/dL. Some of the exclusion criteria are the presence of shunts with gastrointestinal arteries not feasible for embolization and an estimated radiation dose to the lungs > 30 Gy. Similar to TACE, the ideal candidates for TARE are patients with unresectable liver-only or liver-dominant tumors[73], particularly if hypervascularization is evident on preoperative imaging.
The procedure is divided in a work-up session and a treatment session, generally a week apart. In the former, an accurate angiographic study, possibly aided by cone-beam-CT technology, is performed to identify and possibly embolize extrahepatic feeders and shunts between hepatic and gastrointestinal circulation. Following a good targeting of the lesion(s), albumin macroaggregates marked with technetium-99 (99mTc-MAA) are injected intraarterially and the patient undergoes a single photon emission CT within the same day to map 99mTc-MAA distribution and validate treatment feasibility. If the work-up has a positive outcome, the treatment session consists in targeting the same vessels previously injected with 99mTc-MAA and infuse of yttrium-90 (Y-90) microspheres. In case of work-up failure, either a new workup procedure is scheduled or other treatment strategies are considered. TARE may be performed with either glass or resin Y-90 charged microspheres, without significant differences in terms of safety or efficacy[74]. In both cases, bead size range is 20-30 microns, which allows radioisotope entrapment within the sinusoids exerting its therapeutic effect by localized radiation rather than by ischemia as seen in TACE. The lower ischemic effect allows the theoretical possibility of treating larger liver volumes than TACE, without altering liver function as much.
Studies show median survivals ranging from 9 to 22 months[67,75]; factors associated to worse survival after TARE were having ECOG performance 1 or 2[76-78]and infiltrative disease[76,78]. However, a considerable heterogeneity of baseline data mainly regarding the inclusion of patients with both peripheral and infiltrative disease, varying tumor burden, and patients who received previous systemic chemotherapy constitute the main limit in drawing conclusions regarding its efficacy.
A meta-regression study by Cucchettiet al.[79]showed that naïve patients had a 2-year survival of 50.4%compared to 23.6% in patients previously treated with systemic CT. Survival was found to be longer in patients with non-infiltrative type ICC; however, these data are not confirmed by the largest single center cohort of patients (n= 85) available to date[80]. The recent CIRT prospective trial[81]enrolled 120 patients with ICC and showed a median OS of 14.7 months and severe adverse events at thirty days (grade 4 and 5)observed in less than 2.5% of patients. Of note is that 60.8% of these patients had received systemic CT.
In addition, TARE seems to have a favorable safety profile: post-radioembolization syndrome, hepatic dysfunction, biliary complications, portal hypertension, radiation pneumonitis, gastrointestinal ulceration,vascular injury, and lymphopenia are the most frequent adverse events[82]even though they rarely occur,especially when treatment planning and execution are properly performed.
Another fairly recent interesting application of TARE is in the neoadjuvant setting (i.e., in downstaging an initially unresectable ICC rendering the surgical option feasible). A study by Ribyet al.[83]showed that patients undergoing preoperative TARE could undergo resection with a comparable prognosis to patients undergoing upfront surgery; furthermore, TARE showed benefit as downstaging treatment in terms of survival compared with patients treated with chemotherapy.
HEPATIC ARTERY INFUSION CHEMOTHERAPY
Hepatic arterial infusion chemotherapy (HAIC) (i.e., injecting chemotherapeutic agents into the hepatic artery without embolization) is an option to obtain effective drug delivery without the systemic side effects seen with systemic chemotherapy. HAIC consists of a continuous infusion of floxuridine into the hepatic arterial circulation administered through a surgically implanted pump at a predetermined flow rate[84].Previous studies have demonstrated that HAIC is a promising option for advanced ICC and has shown better tumor control than systemic chemotherapy[85].
Recently, studies involving agents other than floxuridine have been published; a study describing intraarterial epirubicin and cisplatin combined with systemic 5-fluorouracil demonstrated an objective response rate and median survival time of 36% and 15.4 months, respectively[86].
A study by Caiet al.[87]compared the outcome of mFOLFOX-HAIC and TACE, demonstrating higher oneyear overall survival rates after the HAIC treatment compared with those after TACE treatment (1-year OS rates: 60.2%vs.42.9%, 2-year OS rates: 38.7%vs.29.4%,P= 0.028).
HAIC was shown to have the highest rate of adverse events and liver-related toxicity among intra-arterial therapies for ICC[17], therefore it has questionable safety profile. In general, its application is reserved to extended bilobar disease, in case of non-response or poor compliance to systemic chemotherapy.
CHEMOSATURATION - PERCUTANEOUS HEPATIC PERFUSION
First studied for treatment of hepatic metastases from ocular melanoma[88], chemosaturation - percutaneous hepatic perfusion with intra-arterial melphalan injection represents an emerging technique for disease control in unresectable ICC. In this technique, a transfemoral catheterization of the hepatic artery is used for chemoperfusion of the liver with melphalan. A transfemoral venous double-balloon catheter is inflated to isolate the intrahepatic tract of the inferior vena cava. Venous blood from the liver, extracted through side holes of the double-balloon catheter, is filtered via an extracorporeal hemofiltration circuit, while a transjugular venous access is used for blood return.
A multi-institutional study by Marquardtet al.[89]on a cohort of 26 patients with unresectable ICC demonstrated an overall response rate of 20% and disease control in 53% of patients after first treatment session, with a median OS of 26.9 months from initial diagnosis and 7.6 months from first treatment, and median progression free survival of 4.1 months. Patients with liver-only disease exhibited a significantly longer median OS compared to patients with loco-regional lymph node metastases (12.9 monthsvs.4.8 months, respectively;P< 0.01). Regarding safety, no grade 3 or 4 adverse events occurred during the procedure; however, hematological toxicity including thrombocytopenia and anemia, requiring transfusions in the post-procedural period, were fairly common, and one patient with a tumor load > 40% of the liver developed acute multi-organ failure after the treatment. Given its good response coupled with a mild burden of systemic side effects, the application of this treatment strategy is limited to extended liver disease not responding to the previously mentioned therapies or systemic therapy.
COMBINED THERAPIES
Kochet al.[90]showed that combination of TACE and chemotherapy doubled median OS compared to TACE alone (26.3 months). Recently, Mosconiet al.[91]showed that TARE combined with chemotherapy had a median OS of 17.9 months (95%CI: 14.3-21.4 months) with significantly better median survival in the treatment-naive patients (52 monthsvs.16 months). A phase II clinical trial[92]evaluating TARE plus chemotherapy as first-line for locally advanced ICC in 41 patients, demonstrated a mean OS of 22 months.Interestingly, 9 patients were downstaged to surgery; of these, 8 received resection with R0 margins and 6 were still disease-free at 24 months follow-up. In this study, grade 3-4 hematologic toxicity was observed in 70% of patients, similarly to the toxicity observed in the ABC-02 trial[93,94], possibly due to gemcitabine/oxaliplatin more than due to addition of TARE. Looking at liver-specific toxicity, among patients with liver cirrhosis (n= 12), 9 had hepatic failure; of the 9 patients, 5 had undergone bilobar TARE.HAIC with floxuridine was studied in combination with systemic gemcitabine and oxaliplatin in patients with unresectable ICC[95]in a phase 2 clinical trial on 38 patients, with a mean OS of 25 months; 4 patients were downstaged to surgery. Liver-specific toxicity leading to discontinuation was observed in 4 patients;47% received full-dose of floxuridine at 6 months.
CONCLUSION
The frequency of ICCs, in particular due to its presence in the aging population, is becoming increasingly relevant. Its dismal prognosis, especially in non-resectable disease, makes the role of diagnostic radiology crucial; disease diagnosis at an early stage and the most detailed prognostic information derived from imaging may change the approach and final outcome of patients. As the knowledge of this disease advances,so do treatment strategies, with continuously evolving techniques and indications. In this setting,combination therapies, with the apparent ability to effectively downstage the disease to resectability, seem promising due to a critical improvement compared to both loco-regional treatments alone[17]and to systemic therapy[94]. Future perspectives include the introduction of novel systemic agents (i.e.,immunotherapeutic regimens and molecular targeted therapy with TACE or TARE). For both the diagnostic and the interventional setting, the multidisciplinary approach, including but not limited to,pathologists, oncologists, and surgeons, is fundamental to achieve a better characterization of the vast CC disease spectrum and identify the best personalized care possible.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design, data analysis, and interpretation: Della Corte A,Di Gaeta E, Steidler S, De Cobelli F
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
- Hepatoma Research的其它文章
- Robotic donor hepatectomy: a niche advancement or the way forward? A perspective from the world’s largest center
- Advances in Y-90 radioembolization for the treatment of hepatocellular carcinoma
- Molecular mechanisms of liver carcinogenesis related to metabolic syndrome
- Histopathology of hepatocellular carcinoma - when and what

