Glutathione Transferase Omega 1 Promotes The Expression of MHCII via Inhibiting Its Ubiquitination*
LIU Ji-Wei,LI Shu-Hang,XIE Di,ZHANG Hui-Min,LI Qing,BAI Li**
(1)Hefei National Laboratory for Physical Sciences at Microscale,the CAS Key Laboratory of Innate Immunity and Chronic Disease,School of Life Sciences,Division of Molecular Medicine,University of Science and Technology of China,Hefei 230026,China;2)The First Affiliated Hospital of the University of Science and Technology of China,Hefei 230001,China)
Abstract Objective We investigated the role of glutathione transferase omega 1 (GSTO1) in regulating function of bone marrow-derived dendritic cells (BMDCs). Methods The expression of MHCII on the surface of Gsto1-/- BMDCs and GSTO1 inhibitor-treated BMDCs was detected by flow cytometry and confocal microscope. To investigate the role of BMDCs, they were cocultured with CD4 T cells from OTII mice in the presence of OVA and IFN-γ production was measured. The recycling and internalization of MHCII molecule in BMDCs treated with GSTO1 inhibitor were measured by flow cytometry. The transcriptional level of MHCII molecule in BMDCs treated by GSTO1 inhibitor was detected by real-time quantitative PCR.The total protein level of MHCII molecule in BMDCs treated by GSTO1 inhibitor was detected by Western blot. The ubiquitination level of MHCII after GSTO1 inhibitor treatment was detected by co-immunoprecipitation assay. Results GSTO1 deficiency and GSTO1 inhibitor did not affect proliferation and apoptosis of BMDCs, but reduced the surface expression of antigen presentation molecule MHCII.Gsto1-/-BMDCs and BMDCs treated with GSTO1 inhibitor showed impaired CD4 T cell-activating capability.In GSTO1 inhibitortreated BMDCs, the recycling and internalization of MHCII molecule were normal. GSTO1 inhibitor showed no influence on the transcription of MHCII but reduced its total protein level. Furthermore, BMDCs treated with GSTO1 inhibitor showed higher level of ubiquitinated MHCII molecule and treatment with proteasomes inhibitor MG132 recovered the surface MHCII expression in these cells. Conclusion These data demonstrate that GSTO1 in BMDCs promotes MHCII expression and thus CD4 T cell-activating capacity by inhibiting ubiquitination of MHCII molecule.
Key words GSTO1,MHCII,dendritic cell,ubiquitination
Glutathione (GSH) is the main non-protein tripeptide antioxidant substance in cells, the balance between GSH and its oxidize form GSSG determines the redox state of cells. GSH binds cysteine in proteins and thereby leads to protein glutathioneylation to modulate protein function[1].Glutathionylation is a reversible post-translational modification catalyzed by different cellular oxidoreductases, which promote the formation of mixed disulfides between protein cysteine and glutathione (GSH) cysteine[2]. This process is catalyzed by glutathione transferase (GST) family,which consist of three super-families: the cytosolic GST, mitochondrial GST, and microsomal GST. The cytosolic GST family contains a variety of types,such as alpha,zeta,theta,mu,pi,sigma,and omega class[3].Glutathione transferase omega 1 (GSTO1) is a constitutively active deglutathionylating enzyme[4].As an atypical GST isoform, GSTO1 is overexpressed in several tumors and has been implicated in drug resistance[5-6]. Recent studies suggest that GSTO1 is involved in activation of NLRP3 inflammasome and inflammatory diseasesin vivo[7]. It remains unclear that whether GSTO1 regulates functions of dendritic cells(DCs)and DC-related immune responses.
DCs are professional antigen-presenting cells(APCs), and link the innate immunity and adaptive immunity.Viaantigen presentation, DCs play essential roles in activating T cells[8-9]. Antigens presented by MHCI molecules induce activation of CD8 T cells, whereas antigens presented by MHCII molecules activate CD4 T cells. Antigenic peptide-MHCII complexes (p-MHCII) expressed on the surface of DCs are critical to antigen presentation and the initiation of CD4 T cell activation, therefore the surface expression and transport of these complexes are tightly regulated in DCs[10].The newly synthesized MHCII binds to a chaperone protein called invariant chain (li), which remains associated with MHCII while transporting to the plasma membraneviathe trans-Golgi network. Once on the plasma membrane,the MHCII-li complex is rapidly internalized by clathrin-mediated endocytosis and transported from the early endosome to the late endosome[11],where the MHCII-associated li is degraded by proteases and removed by H2-DM (a mouse MHCII-like αβ dimer which has very limited polymorphism). Then,immunogenic peptides will bind to MHCII and the newly formed p-MHCII complexes recycle back to the plasma membrane[11]. Large amounts of MHCII are stored in intracellular antigen-processing compartment and are transported to cell surface when DCs are activated[12-13].
Ubiquitination of MHCII has been reported as an important strategy to regulate MHCII expression in APCs[14]. Oligo-ubiquitination of MHCII at a single lysine residue has been identified in the cytoplasmic tail of MHCII β-chain. It has been reported that ubiquitination of MHCII influences its internalization,transportation to antigen-processing compartments,and delivery to lysosomes for degradation[15-16].Ubiquitination of MHCII is promoted by the E3 ubiquitin ligase membrane-associated RING-CH 1(March-1), and that reduces surface expression and half life of MHCII in DCs[17]. On the other hand,activation signal blocks the ubiquitin-dependent turnover of MHCII by promoting MHCII recycling and preventing its delivery to lysosomes, thereby enhancing MHCII stability and favoring antigen presentation to CD4T cells[17].
In this study, we investigate the role of GSTO1 in regulating functions of DCs. We show that GSTO1 activity promotes surface expression of MHCII molecule in bone marrow-derived dendritic cells(BMDCs), and thus enhances the antigen presenting function of BMDCs. Although GSTO1 does not influence the recycling or internalization or transcription of MHCII molecule, it inhibits ubiquitination of MHCII. Our results propose that GSTO1 enhances antigen presentation in BMDCs by inhibiting MHCII degradation.
1 Materials and methods
1.1 Mice
Gsto1+/-andGsto1-/-mice were bred and maintained in specific pathogen-free conditions.Gsto1-/-mice were provided by Prof. XU Tao’s laboratory of Institute of Biophysics, Chinese Academy of Sciences. OTII CD45.1 mice were provided by Prof. ZHU Shu’s laboratory of University of Science and Technology of China(USTC).All animal procedures were approved by the University of Science and Technology of China(USTC) Institutional Animal Care and Use Committee. And all experiments were performed in accordance with the approved guidelines.
1.2 Flow cytometry
BMDCs were induced with 20µg/L GM-CSF for 7 d in complete RPMI 1640 medium[18],and then were collected and seeded in 96-well flat plate at 1×109cells/L in the presence or absence of 5 μmol/L C1-27(MCE, 568544-03-6) or 4 μmol/L ML175(AOBIOUS) for 24 h. For surface staining, cells were preincubated with anti-mouse CD16/32 antibody(BioLegend,101302)for 15 min on ice at 2 mg/L,and then were stained with monoclonal antibodies against BV421-CD11b (BioLegend, 101236), PE/cy7-CD11c(BioLegend, 117318), Percp/cy5.5-CD40 (BioLegend,124624),APC-CD80 (BioLegend, 104714),APC/cy7-CD86 (BioLegend, 105030), PE-CD1d (BioLegend,140805), FITC-MHCII (BioLegend, 107606) for 45 min on ice at 1 mg/L. For cell proliferation analysis, BMDCs were stained with PE mouse anti-Ki67 set (BD Pharmingen, 556027), according to the manufacturer’s method. For cell apoptosis analysis,BMDCs were stained with FITC Annexin V Apoptosis Detection kit (Vazyme, A211-01),according to the manufacturer’s method. For intracellular staining, cells finishing surface staining were fixed with 4% paraformaldehyde (PFA, Sigma,158127) for 15 min and then were permeabilized with 0.1% saponin (Sigma, s7900) for 30 min and blocked with 5% FBS (Biological Industries, 04-001).Antibodies against intracellular molecules were used to stain the cells for 1 h on ice. To measure the amount of intracellular MHCII, surface MHCII was blocked with purified antibody against MHCII(Abcam, ab139365), before intracellular staining with PE-conjugated antibody from same clone. Cells were washed with PBS twice and subsequently analyzed by flow cytometry.
1.3 Cytokine analysis
Cytokines, including IL-6 and TNF-α, in cell supernatants were analyzed with mouse Th1/Th2/Th17 CBA kit (BD Biosciences, 560485), according to the manufacturer’s instructions.
1.4 Immunofluorescent staining
BMDCs were collected and seeded into confocal dish at 2×109cells/L with or without C1-27(5 μmol/L)for 24 h. Then, cells were blocked with anti-mouse CD16/32 antibody for 15 min at 4℃before staining with Alexa Fluor 488 anti-mouse MHCII antibody(Santa Cruz Biotechnology, sc-23940) overnight at 4℃. Cells were washed 3 times with 0.1% Tween-20(Sigma,P1379)and sealed with DAPI Fluoromount-G(SouthernBio, 0100). Images were taken with Olympus inverted fluorescence microscope(Olympus)and analyzed with Image J software.
1.5 RT-qPCR
Total RNA was extracted from BMDCs with ReliaPrep RNA Cell Miniprep System (Promega,Z6011), according to the manufacturer’s protocols.cDNA was synthesized with a high-capacity cDNA reverse transcription kit (Applied Biosystems,4368814), according to the manufacturer’s instructions. Primers for qPCR were as follows:H2-Ab(forward: AAGGCATTTCGTGTACCAGTTC;reverse: CCTCCCGGTTGTAGATGTATCTG);Ciita(forward: AGACCTGGATCGTCTCGTG; reverse:AGTGCATGATTTGAGCGTCTC);β-actin(forward:GTGACGTTGACATCCGTAAAGA;reverse:GCCGGACTCATCGTACTCC). qPCR was performed with the Roche LightCycler 96 using TB Green Premix Ex Taq ™II (Takara, RR82WR). Relative expression values were normalized to a control gene(β-actin).
1.6 MHCII recycling and internalization assay
For recycling assay, BMDCs pre-treated with or without C1-27 were incubated with 10 μg mouse anti-MHCII antibody (Abcam, ab139365) for 30 min at 4℃to block the MHCII molecule on the surface.After washing and resuspending cells with RPMI 1640 complete medium, cells were incubated in 37℃water bath for different times and then cells were stained with FITC-conjugated anti-mouse MHCII antibody (Biolegend, 107606) for 45 min. The newly recycled MHCII stained with FITC-conjugated antimouse MHCII antibody was detected by flow cytometry. The percentage of recycled MHCII was measured using the equation (Tx-T0)/T×100, whereT0was the mean fluorescence intensity (MFI) of FITC at 0 min, andTxwas the MFI of FITC at the indicated times, andTwas the total MFI of FITC in cells that were not blocked with anti-MHCII antibody.
For internalization assay, BMDCs pre-treated with or without C1-27 were surface-labeled with FITC-conjugated anti-mouse MHCII antibody for 45 min at 4℃.After washing with RPMI 1640 media containing 0.5% FBS, cells were incubated for different times at 37℃in complete medium to allow internalization of MHCII. Then, surface antibody was stripped with buffer containing 0.5 mol/L NaCl and 0.5% acetic acid (pH 3.0) for 10 min on ice. Cells were then fixed with 4% PFA, and the internalized MHCII was measured by flow cytometry. The percentage of internalized MHCII was measured using the equation (Tx-T0)/T×100, whereT0was the MFI of FITC at 0 min,andTxwas the MFI of FITC at the indicated times, andTwas the total MFI of FITC in cells that not treated with strip solution.
1.7 Western blotting and coimmunoprecipitation(CoIP)
BMDCs were treated with GSTO1 inhibitor C1-27 (10 μmol/L) for 24 h.To detect total protein of MHCII, cells were harvested and lysed with sample buffer and were boiled for 15 min. Proteins were separated by electrophoresis and were detected by western blotting. Antibodies against MHCII(Invitrogen, 14-5321-82, 1∶1 000 dilution), and β-actin (Transgen Biotech, HC201-02, 1∶1 000 dilution)were used in our experiments.
To detect ubiquitination of MHCII, cells were harvested and washed with ice-cold PBS and then lysed with a NP-40 (Biotec, P0016F) buffer containing 1% protease inhibitor cocktail(Trans, N21024, 1∶100 dilution) and EDTA(ethylenediaminetetraacetic acid) (Trans, 10622,1∶100 dilution) on ice for 1 h.Half of cell lysates mixed with 5×lane marker loading buffer (Biotec,P0015) was heated at 101℃for 15 min and used as input sample. The rest cell lysates were incubated with 2 μg anti-MHCII antibody (Invitrogen, 14-5321-82) and 15 μl protein G beads (Invitrogen, 10004D)and rotated at 4℃ overnight. Antibody against ubiquitin was used to detect ubiquitination of MHCII in co-immunoprecipitated proteins.
1.8 Antigen presentation and activation of CD4 T cells
BMDCs (5×108cells/L) were loaded with or without 500 mg/L OVA (Sigma-Aldrich, A5503)overnight in the presence or absence of 5 μmol/L C1-27, and then were washed and were co-cultured with CD4 T cell enriched from splenocytes of OTII transgenic mice for 6 d in the presence of rhIL-2(1×105U/L). IFN-γ in supernatant was measured with CBA flex set(BD,558296).
1.9 Data analysis
Error bars represent standard error of the mean(SEM). The statistical significance of differences between two groups was determined by the unpaired two-tailed student’sttest.
2 Results
2.1 GSTO1 deficiency reduces surface MHCII expression in BMDCs
Glutathionylation of protein is an important strategy to response to external signals, including sensing energy and metabolic changes, cell apoptosis,signal transduction, and protein folding[2]. GSTO1, an atypical GST, is significant in the glutathionylation cycle[19]. In order to study the functions of GSTO1 in DCs,we generatedGsto1-/-mice andGsto1+/-mice as littermate controls. The levels of surface markers,including CD80, CD86, CD40, CD1d, and MHCII, in BMDCs fromGsto1+/-andGsto1-/-mice were compared by flow cytometry. The expression of CD80, CD40, and MHCII were significantly reduced in GSTO1 deficient BMDCs, whereas CD86 and CD1d expressions were not influenced by deletion of GSTO1 (Figure 1a). To further prove the reduction of surface MHCII molecule, we stained BMDCs with Alex Fluor 488-conjugated anti-MHCII and imaged these cells with laser confocal microscopy. In comparison withGsto1+/-BMDCs,Gsto1-/-BMDCs showed lower level of surface MHCII(Figure 1b).On the other hand, GSTO1 deficiency did not influence the proliferation or apoptosis of BMDCs (Figure 1c,d). These results suggest that GSTO1 promotes the surface expression of MHCII in BMDCs.
2.2 GSTO1 inhibitors reduce surface MHCII expression in BMDCs
To investigate whether GSTO1 promoted surface expression of MHCIIviaits catalytic activity, we inhibited activity of GSTO1 with two inhibitors ML175 and C1-27, respectively. Both ML175 and C1-27 reduced the surface expression of CD80,CD86, and MHCII, but exhibited no influence on CD40 or CD1d in BMDCs (Figure 2a, b). Again,confocal images demonstrated reduced surface MHCII in C1-27 treated BMDCs (Figure 2c). In line with the normal proliferation and apoptosis inGsto1-/-BMDCs, GSTO1 inhibitor C1-27 showed no influence on proliferation and apoptosis of BMDCs.These results confirm that GSTO1’s catalytic activity is required for surface expression of MHCII in BMDCs.
2.3 Deficiency of GSTO1 in BMDCs reduces the activation of CD4 T cells
DCs promote the activation and differentiation of CD4 T cellsviapresenting antigens through MHCII molecule. Hence, we investigated whether the reduction of surface MHCII as a result of GSTO1 deficiency would impair the capability of DCs to activate CD4 T cells. We pulsedGsto1+/-BMDCs,Gsto1-/-BMDCs, and C1-27 treatedGsto1+/-BMDCs with OVA, and co-cultured them with OTII CD4 T cells, respectively. We found that presence of OVA was required for activating CD4 T cells, as indicated by the IFN-γ production in supernatants. Both deletion ofGsto1and inhibition of its activity in BMDCs reduced OVA-induced CD4 T cell activation(Figure 3).Therefore, GSTO1 promotes the capability of BMDCs to activate CD4 T cells.

Fig.1 GSTO1 deficiency reduces the surface expression of MHCII in BMDCs
2.4 GSTO1 promotes surface expression of MHCII via inhibiting its ubiquitination
It is well-known that MHCII molecule undergoes internalization and recycling. Next, we investigated whether the C1-27-induced reduction of surface MHCII in BMDCs was due to alteration in MHCII internalization or recycling. We found that C1-27 displayed no influence on recycling rate or internalization rate of MHCII in BMDCs (Figure 4a).In line with the reduced surface MHCII but normal recycling and internalization rate, we found that the surface and intracellular MHCII was reduced in C1-27 treated BMDCs (Figure 4b). Besides, the protein level of total MHCII in BMDCs was also decreased in C1-27 treated BMDCs (Figure 4c). Our results indicated a decrease of total MHCII protein in GSTO1 activity deficient BMDCs.Notably,the C1-27 treatment did not influence the transcription of MHCII, as indicated by normal mRNA levels ofH2-AbandCiita, which encodes a key regulator for MHCII, in C1-27 treated BMDCs (Figure 4d).Therefore,C1-27 induced reduction of MHCII protein would likely be due to increased protein degradation.It has been reported that ubiquitination regulates the retention and degradation of MHCII in DCs[20]. In C1-27 treated BMDCs, we detected increased ubiquitination of MHCII molecule, in comparison with untreated cells (Figure 4e). Protein that has been ubiquitinated will be degraded by proteasome, and proteasome inhibitor could interfere with the protein degradation. Next, we added MG132, an inhibitor of proteasome, to C1-27 treated BMDCs, and showed that MG132 abrogated the inhibitory effect of C1-27 on MHCII surface expression (Figure 4f). Together,our results indicate that GSTO1 promotes the surface expression of MHCIIviainhibiting its ubiquitination and degradation.
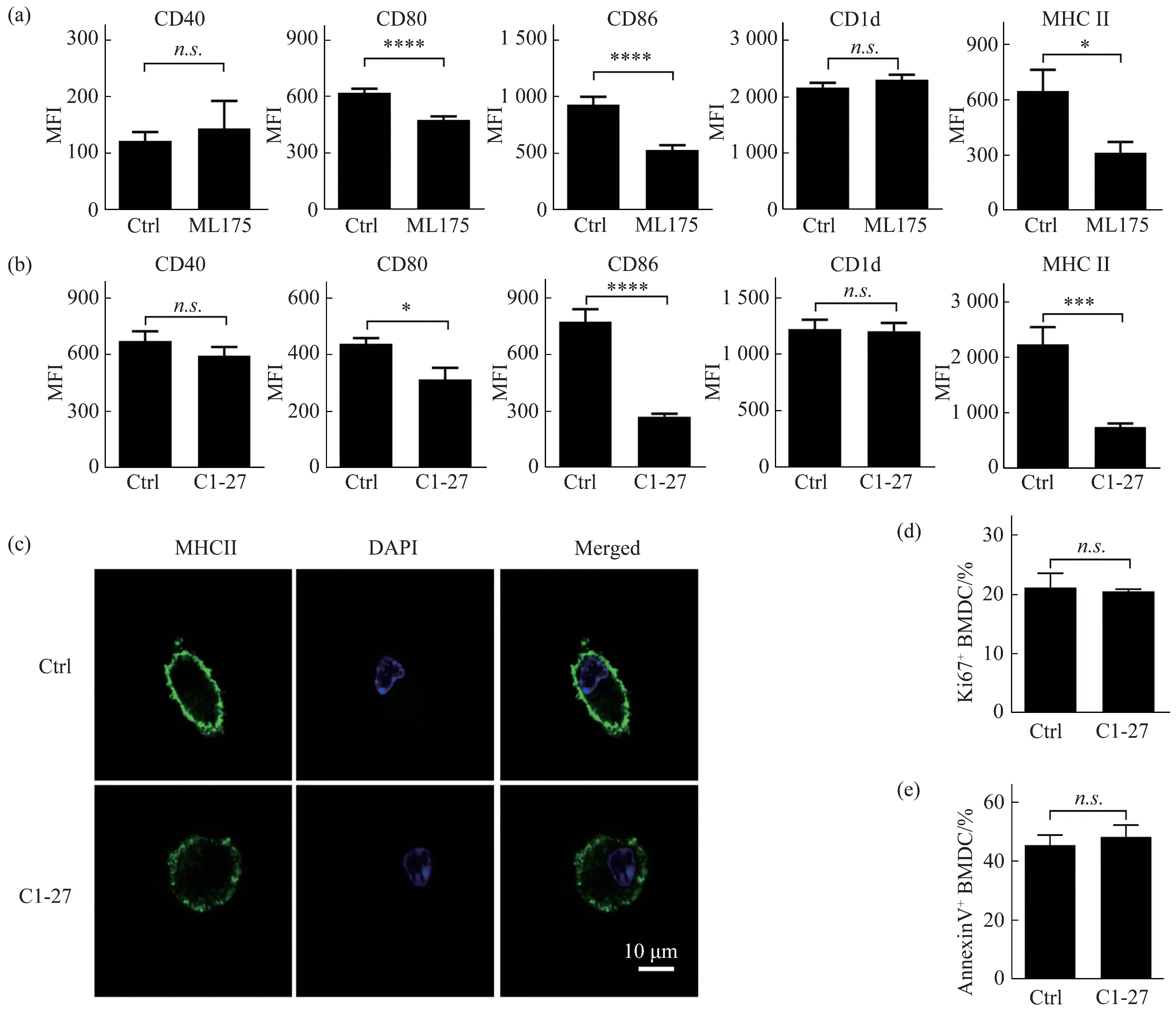
Fig.2 GSTO1 inhibitors reduce the surface expression of MHCII in BMDCs
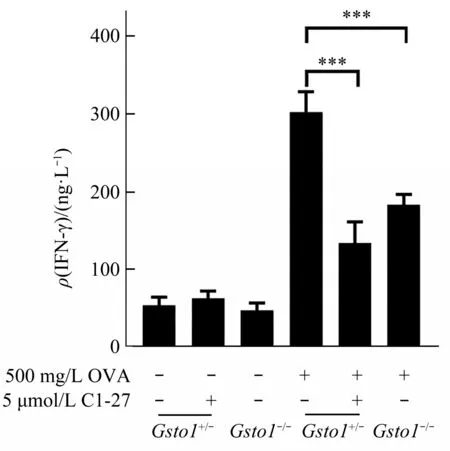
Fig.3 Deficiency of GSTO1 in BMDCs reduces the activation of CD4 T cells
3 Discussion
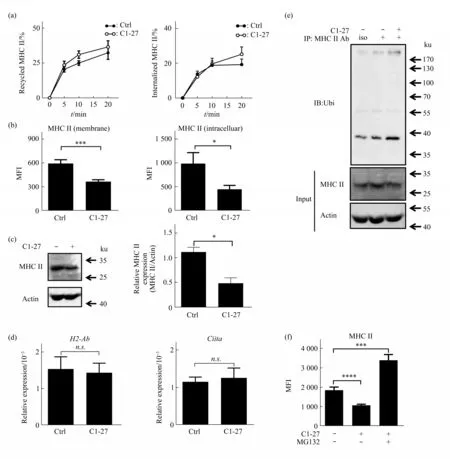
Fig.4 C1-27 promotes ubiqutination and degradation of MHCII
Published studies have shown that GSTO1 plays an important role in NLRP3 inflammasome activation and inflammatory diseases[21-22]. As the link of innate immunity and adapted immunity, DCs are important in presenting antigen, and activating naive T cells. It still unclear that whether GSTO1 regulates immune responses via modulating functions of DCs. In this study, we found that the expression of CD80, CD86,and MHCII but not CD40 and CD1d were downregulated by GSTO1 inhibitor ML175 and C1-27 in BMDCs.Similar reduction of MHCII was observed in GSTO1 deficient BMDCs, although CD80 expression was not changed and CD40 expression was decreased a little in these cells. On the other hand, BMDCs proliferation and apoptosis were not influenced by GSTO1 deficiency.
Antigen-specific CD4 T cells are activated by the antigens presented by MHCII on the surface of APCs.In line with the role of GSTO1 in promoting surface MHCII expression, GSTO1 is important for the antigen presenting function of BMDCs. Additionally,the activation of T cells also requires co-stimulation signal provided by APCs. Both MHCII and costimulation molecule play essential roles in the activation of T cells. Therefore, GSTO1 deficiency induced reduction of CD80 might also contribute to the impaired capability of BMDCs to activate CD4 T cells. Together, we propose that GSTO1 in BMDCs would influence the crosstalk between BMDCs and CD4 T cells. Thus, we investigated how GSTO1 regulated MHCII expression in DCs. We supposed that C1-27 damages the transcription of MHCII and CIITA. The results showed that MHCII expression reduction caused by C1-27 did not result from the effect of C1-27 on the transcription ofMHCIIandCiitagenes.
It has been demonstrate that the rapid turnover of p-MHCII in immature DCs is regulated by ubiquitindependent p-MHCII degradation under the control of March-1[17]. MHCII that are transported to the cell surface are rapidly internalized and degraded in immature DCs[23-24]. It has been shown that ubiquitination of MHCII on Lys225 is responsible for the rapid turnover in immature DCs[17]. Here, our results demonstrate that GSTO1 inhibits ubiquitination of MHCII but shows no effect on internalization or recycling of MHCII. It is possible that GSTO1-regulated ubiquitination of MHCII has no influence on its internalization. Our results that GSTO1 promotes antigen presenting function of BMDCsviainhibiting MHCII ubiquitination are in line with previous findings that ubiquitination and deubiquitination are involved in regulating maturation and function of DCs[25]. However, it is still unclear how GSTO1 regulates the ubiquitination of MHCII,although our study suggests involvement of its catalytic activity. It has been reported that GSTO1 deglutathionylates proteins[4]. Whether GSTO1 inhibits ubiquitination of MHCIIviadeglutathionylating MHCII or other ubiquitinationrelated proteins remains to be explored.
4 Conclusion
The present study demonstrates that GSTO1 promotes the expression of MHCII in BMDCs by inhibiting its ubiquitination, and thus favors antigen presentation and CD4 T cell-activating capacity.
AcknowledgementsWe thank Prof. XU Tao and Prof. ZHU Shu for providing withGsto1-/-mice and OTII CD45.1 mice.

