GSH与NO协同处理对番茄贮藏品质及抗氧化性的影响
钟心怡 张文霞 邓嘉欣 陈紫婷 刘锴栋 周艳



摘要:【目的】探究還原型谷胱甘肽(GSH)与一氧化氮(NO)供体硝普钠(SNP)协同处理对番茄贮藏品质及抗氧化能力的影响,为番茄贮藏保鲜提供理论依据。【方法】以千禧番茄为试验材料,采用外源2 mmol/L GSH和0.25 mmol/L SNP分别单独处理及协同处理浸泡采后成熟期番茄果实5 min,以蒸馏水浸泡5 min为对照,进行贮藏期番茄果实品质、抗氧化酶活性和抗氧化物质含量分析。【结果】与对照相比,外源GSH与SNP协同处理下番茄果实在整个贮藏期失重率显著降低52.86%~56.07%(P<0.05,下同),原果胶含量显著增加29.67%~39.34%,从而延缓果实硬度的下降;贮藏前中期(5~10 d)果实的可溶性固形物含量显著增加23.36%~46.25%,整个贮藏期间的可滴定酸和可溶性糖含量分别显著增加93.95%~96.78%和8.32%~11.21%;番茄果实的总抗氧化能力显著提高17.25%~53.82%,果实中的丙二醛(MDA)含量、过氧化氢(H2O2)含量和超氧阴离子(O[-2]·)产生速率分别显著降低49.28%~52.27%、34.71%~42.75%和23.82%~53.60%;果实中的超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和过氧化物酶(POD)活性分别显著增加12.73%~143.80%、37.11%~95.24%和20.73%~91.45%,GSH、还原型抗坏血酸(AsA)和NO含量分别显著增加16.01%~44.63%、38.49%~39.73%和48.56%~73.96%。【结论】外源GSH与SNP协同处理能通过保持果实硬度、可溶性固形物、可滴定酸和可溶性糖含量,提高SOD、CAT和POD活性,以及GSH和AsA含量,维持果实体内的活性氧平衡,减少贮藏期产生的氧化损伤,从而提高其耐贮性。
关键词: 番茄;还原型谷胱甘肽;硝普钠;抗氧化能力;贮藏品质
中图分类号: S641.209.3 文献标志码: A 文章编号:2095-1191(2022)01-0157-09
Synergistic effect of glutathione and nitric oxide treatment on storage quality and antioxidant activity in tomato
ZHONG Xin-yi, ZHANG Wen-xia, DENG Jia-xin, CHEN Zi-ting,
LIU Kai-dong*, ZHOU Yan*
(Life Science and Technology School, Lingnan Normal University, Zhanjiang, Guangdong 524048, China)
Abstract:【Objective】Synergistic effects of reduced glutathione (GSH) and donor sodium nitroprusside(SNP) of nitric oxide(NO) treatment on storage quality and antioxidant capacity of tomato,which can provide a theoretical basis for improving the storage quality and prolonging the shelf life of tomato fruit. 【Method】Qianxitomato was used as the experimental material,and the tomato fruits at post-harvest maturity stage were soaked for 5 min with exogenous 2 mmol/L GSH and 0.25 mmol/L SNP,respectively,and soaked in distilled water for 5 min as control. The fruit quality,antioxidant enzyme activity and antioxidant substance content of tomato during storage period were analyzed. 【Result】Compared to control,the weight loss rate of tomato fruits were significantly decreased by 52.86%-56.07%(P<0.05,the same below),and the content of propectin were significantly increased by 29.67%-39.34% under the synergistic treatment of exogenous GSH and SNP,which delayed the decrease of fruit hardness. Compared to control,the content of total soluble solid (TSS) in the early/middle stage of storage(5-10 d)were significantly increased by 23.36%-46.25%,titratable acid(TA) and soluble sugar contents during the whole storage period were significantly increased by 93.95%-96.78% and 8.32%-11.21%,respectively,under the synergistic treatment of exogenous GSH and SNP in tomato fruits. Compared to control,the total an-tioxidant capacity of tomato fruits was significantly increased by 17.25%-53.82%,and the content of malondialdehyde (MDA),hydrogen peroxide (H2O2) and the production rate of superoxide anion(O[-2]·) in tomato fruits were significantly decreased by 49.28%-52.27%,34.71%-42.75% and 23.82%-53.60%,respectively,under the synergistic treatment of exo-genous GSH and SNP. The synergistic treatment of exogenous GSH and SNP also increased the activities of superoxide dismutase(SOD),catalase(CAT) and peroxidase(POD) in tomato fruits by 12.73%-143.80%,37.11%-95.24% and 20.73%-91.45%,and the contents of GSH,ascorbic acid(AsA) and NO in tomato fruits were significantly increased by 16.01%-44.63%,38.49%-39.73% and 48.56%-73.96%,respectively,compared with control. 【Conclusion】The synergistic treatment of exogenous GSH and SNP synergistic treatment can maintain fruit firmness,increase TSS,TA and soluble sugar content,improve SOD,CAT and POD activities,and GSH and AsA contents,maintain the balance of reactive oxygen species in fruit,reduce oxidative damage during storage,and thus enhance the storage tolerance of the tomato fruit.
Key words: tomato; reduced glutathione; sodium nitroprusside; antioxidant capacity; storage quality
Foundation items: Basic and Applied Basic Research Fund of Guangdong(2019A1515110138);Guangdong Natural Science Foundation(2019A1515012180);School-level Talents Project of Lingnan Normal University(ZL2032,ZL2021003)
0 引言
【研究意義】番茄(Solanum lycopersicum L.)属茄科番茄属一年生或多年生草本植物,富含维生素和矿物质,具有预防癌症、降血压和胆固醇、补血消炎、美容护肤等功效,能产生较高的经济效益(王玉凤等,2009)。但番茄属于呼吸跃变型果实,采摘后易发生后熟,不完善的采后贮藏条件和技术也会加剧番茄的软化、腐烂和变质,使其受到多种病菌侵染,生理品质下降,从而逐渐失去商品价值,对经济造成巨大的损失(颉博杰等,2021)。因此,如何提高番茄的贮藏品质成为亟需解决的重要问题。【前人研究进展】已有研究表明,低温贮藏、气调贮藏、果品辐射保鲜、化学药剂处理保鲜、涂膜保鲜和采后臭氧处理等均可在不同程度上对番茄果实起到贮藏保鲜的效果,但同时存在成本高、化学残留和营养成分流失等问题(Tsaniklidis et al.,2014;宋耀和张静,2016;王素朋等,2020;刘枫等,2021;王玉佳和韩爱云,2021)。谷胱甘肽(Glutathione,GSH)是一种富含巯基的低分子肽,以还原型谷胱甘肽(Reduced glutathione,GSH)和氧化型谷胱甘肽(Oxidized glutathione,GSSG)2种形式存在于植物的细胞质、线粒体和叶绿体(贾贞等,2009)。研究发现,GSH处理采后鸭梨(林琳等,2006)、荔枝(莫亿伟等,2010)和草莓(Ge et al.,2018)能提高果实的还原型抗坏血酸(AsA)和GSH含量,降低总酚含量、多酚氧化酶(PPO)和苯丙氨酸解氨酶(PAL)活性,以降低果实内活性氧(ROS)含量,提高果实的抗氧化能力,抑制病菌侵染,从而降低果实的腐烂率,增强其贮藏品质。一氧化氮(NO)是一种有效延缓果蔬衰老的天然植物生长调节剂,在延缓果实软化及衰老中起着重要的作用(李顺民等,2009) 。研究发现,NO处理杨梅(杨虎清等,2010)和大五星枇杷(任艳芳等,2016)能提高果实内超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性及丙二醛(MDA)含量,通过抑制膜脂过氧化加剧和提高果实的抗氧化能力,清除过量的ROS,从而有效缓解果实衰老,提高其贮藏品质。硝普钠(SNP,NO供体)处理也能通过降低茄子的花青素、总酚和AsA含量,维持其贮藏品质(范林林等,2017) 。【本研究切入点】GSH和NO单独处理均能提高果实的贮藏品质及抗氧化能力,但2种物质协同处理对果实贮藏期间的作用鲜见相关报道。【拟解决的关键问题】以千禧番茄成熟期果实为试材,用外源GSH和SNP(NO供体)对采后番茄果实进行处理,通过研究不同处理对贮藏期番茄果实品质、抗氧化酶活性及抗氧化物质含量的影响,探讨GSH与NO协同影响采后番茄的保鲜生理机制,为提高采后番茄果实耐贮性提供理论依据。
1 材料与方法
1. 1 试验材料
以千禧番茄成熟期果实为试材,果实采摘自岭南师范学院苗圃,将挑选好的果实使用0.05%次氯酸钠溶液(有效氯≥10%)表面灭菌3 min后,采用流水清洗干净用于试验。还原型谷胱甘肽(GSH)购自Sigma公司,SNP购自上海源叶生物科技有限公司,试验测定指标所用试剂盒均购自苏州科铭生物技术有限公司。主要仪器设备:GY-4硬度计(山东莱恩德智能科技有限公司)、紫外可见分光光度计(UV-8453型,美国Agilent公司)和离心机(Centrifuge 5427 R型,德国Eppendorf公司)。
1. 2 试验方法
1. 2. 1 试验设计 试验设4个处理:(1)蒸馏水(对照,CK);(2)2 mmol/L GSH(GSH处理);(3)0.25 mmol/L SNP(SNP处理);(4)2 mmol/L GSH+0.25 mmol/L SNP(GSH+SNP处理)。GSH浓度通过前期试验筛选所得,SNP浓度参考任艳芳等(2021)的研究结果。将番茄果实随机分成4组,按试验设计分别浸果处理5 min,取出自然晾干后放入铺有多层软纸的塑料盒中,于20 ℃下贮藏。每处理30个果实,重复3次,分别于贮藏后第5、10和15 d取样,用于相关指标的测定。
1. 2. 2 果实硬度和失重率测定 使用GY-4硬度计测定番茄果实硬度。利用称重法(李洁,2015)测定番茄果实重量,并使用差量法计算果实失重率。
1. 2. 3 可溶性固形物、可滴定酸和可溶性糖含量测定 可溶性固形物含量采用手持式糖量计测定;可滴定酸含量采用NaOH滴定法测定(Ren et al.,2017);可溶性糖含量采用蒽酮比色法于630 nm下进行测定(曹建康等,2007)。
1. 2. 4 总酚和原果胶含量测定 总酚和原果胶含量测定根据试剂盒说明书进行操作,分别于760和530 nm比色测定吸光值。
1. 2. 5 总抗氧化能力测定 根据试剂盒说明书进行操作,于593 nm测定吸光值(周培禄等,2018) 。
1. 2. 6 MDA、过氧化氢(H2O2)含量和超氧阴离子(O[-2]·)产生速率测定 根据试剂盒说明书进行操作,MDA含量于532和600 nm测定吸光值,H2O2含量和O[-2]·产生速率分别于530和415 nm测定吸光值(Wang et al.,2017;董守坤等,2018)。
1. 2. 7 SOD、POD和CAT活性测定 根据试剂盒说明书进行操作,SOD和CAT分别于560和405 nm下记录吸光值,POD于470 nm下记录1和2 min吸光值(董守坤等,2018)。
1. 2. 8 AsA和GSH含量测定 AsA和GSH含量根据试剂盒说明书分别于420和412 nm比色测定吸光值。
1. 2. 9 NO含量测定 根据试剂盒说明书于550 nm波长测定吸光值(Zhang et al.,2017)。
1. 3 统计分析
采用SPSS 19.0和Duncan’s法对试验数据进行处理,并利用Origin 2019b制图。
2 结果与分析
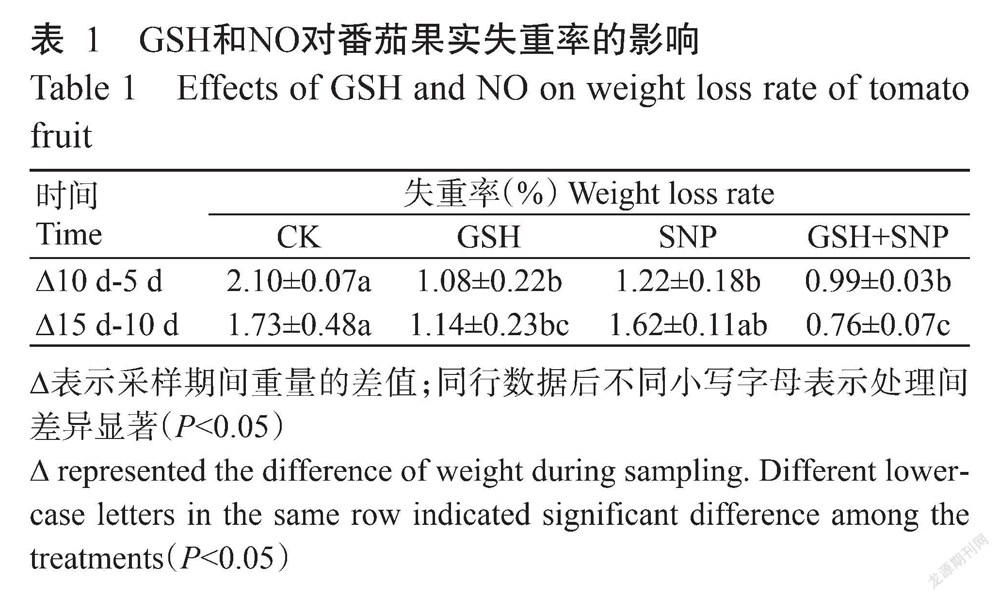
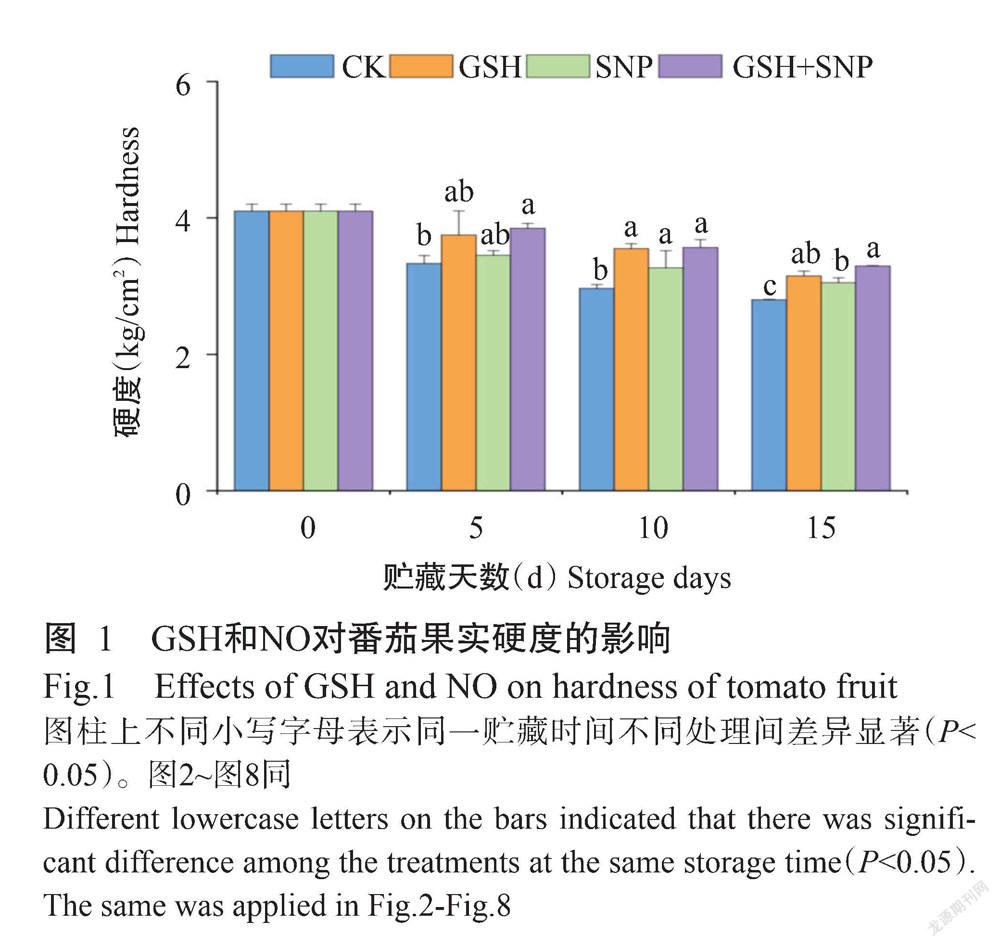
2. 1 GSH和NO对番茄果实失重率和硬度的影响
由表1可知,与CK相比,外源GSH使番茄果实的失重率在10 d-5 d和15 d-10 d分别显著降低48.57%和34.10%(P<0.05,下同),外源SNP处理使番茄果实的失重率在贮藏10 d-5 d显著降低41.90%,外源GSH+SNP使番茄果实的失重率在10 d-5 d和15 d-10 d分别显著降低52.86%和56.07%。由图1可知,与CK相比,外源GSH使番茄果实的硬度在贮藏第10 d显著增加19.66%,外源SNP使番茄果实硬度在贮藏第10和15 d分别显著增加10.11%和9.12%,外源GSH+SNP使番茄果实硬度在整个贮藏期间显著增加15.50%~20.22%。
2. 2 GSH和NO对番茄果实可溶性固形物、可滴定酸和可溶性糖含量的影响
由图2可知,与CK相比,外源GSH使番茄果实的可溶性固形物、可滴定酸和可溶性糖含量在整个贮藏期间分别显著增加19.23%~31.25%、64.32%~85.71%和5.00%~19.63%;外源SNP使番茄果实中可溶性固形物含量在贮藏第5和10 d分别显著增加25.83%和23.36%,可滴定酸和可溶性糖含量在整个贮藏期间分别显著增加19.78%~22.50%和8.21%~9.80%;外源GSH+SNP使番茄果实中可溶性固形物含量在贮藏第5和10 d分别显著增加46.25%和23.36%,可滴定酸和可溶性糖含量在整个贮藏期间分别显著增加93.95%~96.78%和8.32%~11.21%。与外源GSH处理相比,外源GSH+SNP使番茄果实中可滴定酸在贮藏第5 d显著增加18.83%,可溶性糖含量在贮藏第15 d显著增加5.91%。
2. 3 GSH和NO对番茄果实总酚和原果胶含量的影响
由图3可知,与CK相比,外源GSH使番茄果实中的总酚和原果胶含量在整个贮藏期间分别显著增加30.83%~44.63%和12.90%~23.67%;外源SNP使番茄果实中总酚含量在整个贮藏期间显著增加25.51%~41.27%,原果胶含量在贮藏第5 d显著增加14.60%;外源GSH+SNP使番茄果实中总酚和原果胶含量在整个贮藏期间分别显著增加37.01%~104.39%和29.67%~39.34%。与外源GSH处理相比,外源GSH+SNP使番茄果实中总酚含量在贮藏第10和15 d分别显著增加13.81%和41.31%,原果胶含量在整个贮藏期间显著增加12.67%~18.59%。
2. 4 GSH和NO对番茄果实总抗氧化能力的影响
由图4可知,与CK相比,外源GSH使番茄果实的总抗氧化能力在整个贮藏期间显著提高8.44%~39.15%;外源SNP使番茄果实的总抗氧化能力在贮藏第5和15 d分别显著提高10.91%和15.10%;外源GSH+SNP使番茄果实的总抗氧化能力在整个贮藏期间显著提高17.25%~53.82%;与外源GSH处理相比,外源GSH+SNP使番茄果实的总抗氧化能力在整个贮藏期间显著提高8.12%~10.55%。
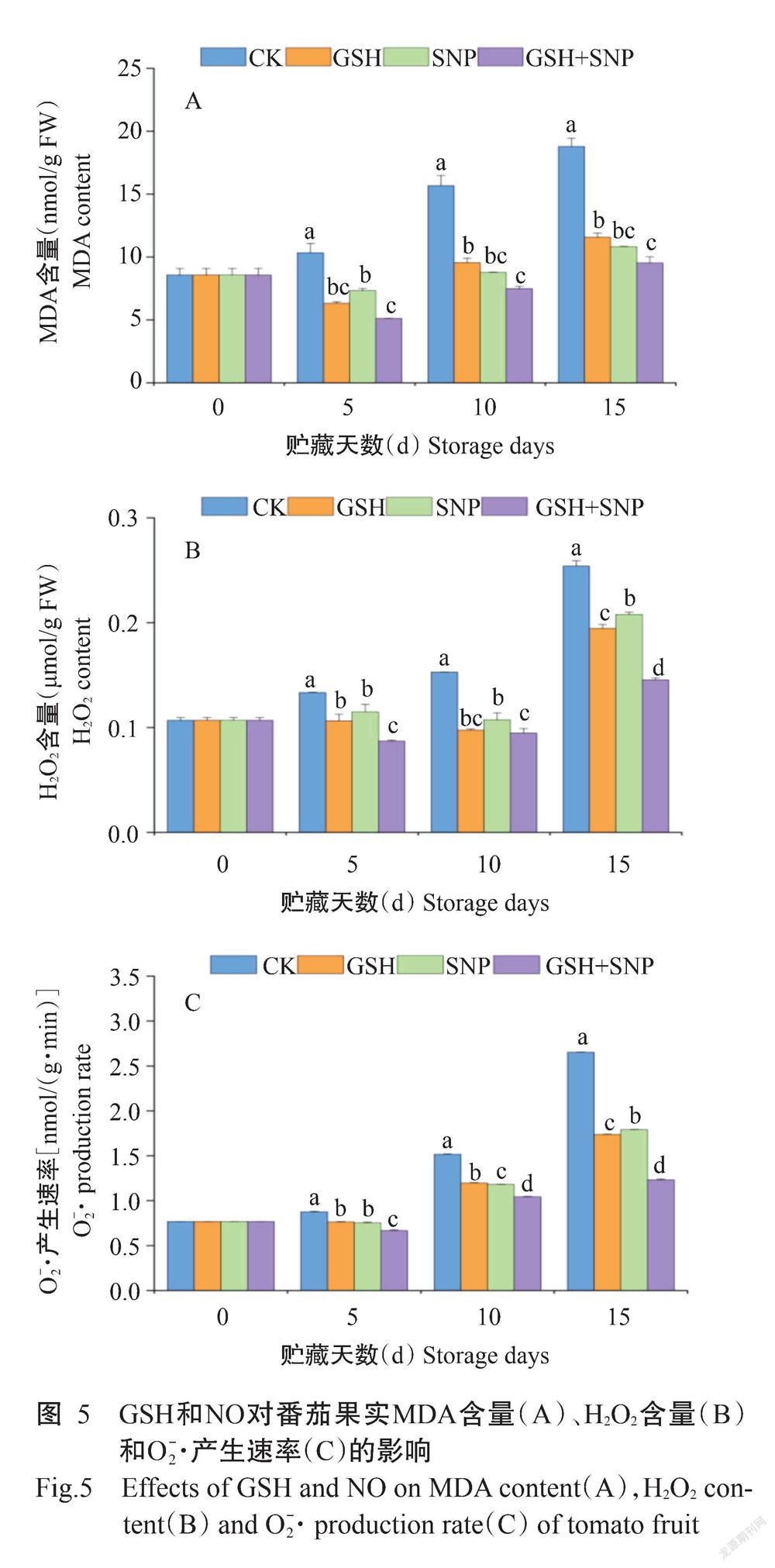
2. 5 GSH和NO对番茄果实MDA、H2O2含量和O[-2]·产生速率的影响
由图5可知,与CK相比,外源GSH使番茄果实中MDA、H2O2含量和O[-2]·产生速率在整个贮藏期间分别显著降低38.69%~39.09%、20.35%~36.18%和12.83%~34.48%,外源SNP使其分別显著降低29.09%~44.08%、13.81%~29.72%和14.03%~32.53%,外源GSH+SNP使其分别显著降低49.28%~52.27%、34.71%~42.75%和23.82%~53.60%。与外源GSH处理相比,外源GSH+SNP使番茄果实中MDA含量在贮藏第10和15 d分别显著降低21.63%和17.70%,H2O2含量在贮藏第5和15 d分别显著降低18.04%和25.24%,O[-2]·产生速率在整个贮藏期间显著降低12.60%~29.17%。
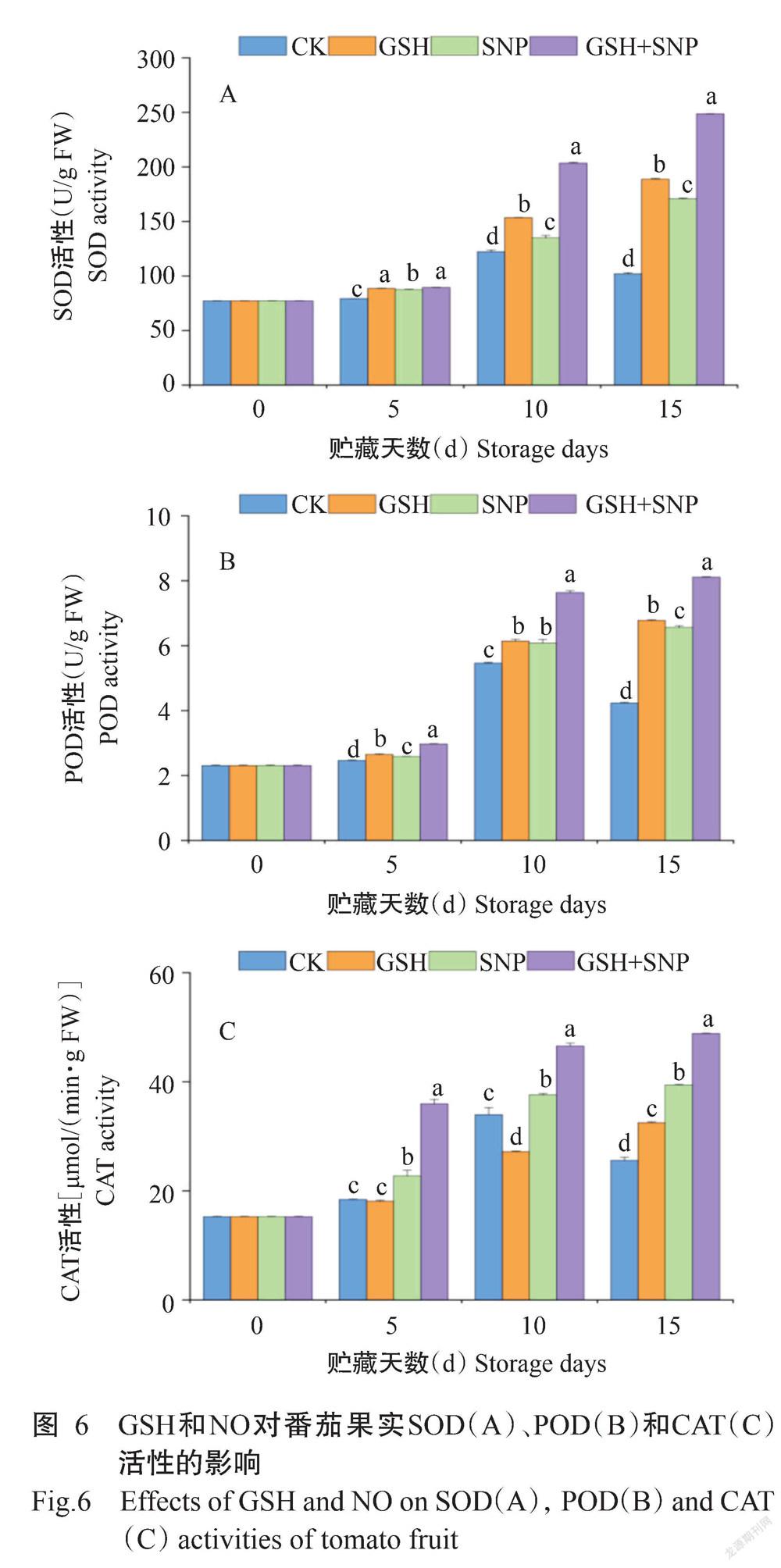
2. 6 GSH和NO对番茄果实SOD、POD和CAT活性的影响
由图6可知,与CK相比,外源GSH使番茄果实中SOD和POD活性在整个贮藏期间分别显著增加11.66%~85.16%和7.72%~59.94%,CAT活性在贮藏第15 d显著增加27.08%;外源SNP使番茄果实中SOD、CAT和POD活性在整个贮藏期间分别显著增加10.35%~67.39%、10.74%~54.18%和5.06%~54.86%;外源GSH+SNP使番茄果实中SOD、CAT和POD活性在整个贮藏期间分别显著增加12.73%~143.80%、37.11%~95.24%和20.73%~91.45%。与外源GSH处理相比,外源GSH+SNP使番茄果实中SOD活性在贮藏第10和15 d分别显著增加32.51%和31.67%,CAT和POD活性在整个贮藏期间分别显著增加50.37%~98.43%和12.08%~24.43%。
2. 7 GSH和NO对番茄果实AsA和GSH含量的影响
由图7可知,与CK相比,外源GSH使番茄果实中AsA和GSH含量在整个贮藏期间分别显著增加14.49%~35.04%和21.27%~31.55%,外源SNP使其分别显著增加4.95%~33.15%和17.54%~21.16%,外源GSH+SNP使其分别显著增加16.01%~44.63%和38.49%~39.73%。与外源GSH处理相比,外源GSH+SNP使番茄果实中AsA和GSH含量在整个贮藏期间分别显著增加1.33%~7.11%和1.42%~7.19%。
2. 8 GSH和NO对番茄果实NO含量的影响
由图8可知,与CK相比,外源GSH使番茄果实的NO含量在贮藏第5 d显著增加18.49%;外源SNP使番茄果实的NO含量在整个贮藏期间显著增加35.55%~37.21%;外源GSH+SNP使番茄果实的NO含量在整个贮藏期间显著增加48.56%~73.96%。与外源GSH处理相比,外源GSH+SNP使番茄果实的NO含量在整个贮藏期间显著增加25.38%~77.76%。
3 讨论
果实硬度与细胞壁强度和组织的膨压有关,是评定果实贮藏品质的物理标准之一(Duran et al.,2016;刁倩楠等,2019)。果实硬度由果肉细胞壁完整程度决定,果肉细胞壁的主要物质是果胶和纤维素,果胶降解和酶活性直接影响果实硬度(罗静等,2018)。本研究中,外源GSH单独处理和GSH+SNP协同处理使番茄果实失重率降低、原果胶含量在整个贮藏期间显著增加,外源GSH和SNP单独处理或协同处理均能使番茄果实硬度在不同程度上增加,说明GSH和SNP单独处理能通过降低番茄果实水分损失和增加原果胶含量来增加果实硬度,与石玲等(2019)研究得出NO能使甜瓜采后果实维持较高的硬度和原果胶含量来延缓细胞壁代谢,从而提高贮藏品质的结论一致。GSH与SNP协同处理对番茄果实软化的抑制作用能达到最佳。但有关GSH与SNP协同抑制番茄果实软化的机理还需进一步研究。
可溶性固形物含量是衡量果实成熟度和内在品质的重要指标(Wang and Zhu,2017)。可滴定酸含量与水果中有机酸浓度直接相关。可溶性糖是影响热带水果营养价值、风味和甜度的主要成分。本研究中,单独使用外源GSH和SNP处理能使番茄果实的可溶性固形物、可滴定酸和可溶性糖含量在一定程度上增加,而GSH+SNP协同处理较GSH单独处理进一步增加贮藏前期番茄果实中的可滴定酸含量和贮藏后期的可溶性糖含量。前人研究结果也表明,GSH和SNP处理可延缓猕猴桃和苹果果实可溶性固形物、可滴定酸和可溶性糖含量下降,一定程度上抑制在贮藏过程中果实品质的劣变(张晓平等,2007;Xu et al.,2019)。由此说明,外源GSH与SNP协同处理番茄果实能通过增加可溶性固形物、可滴定酸和可溶性糖含量,来保持贮藏期的果实品质。
果实在成熟和衰老过程中不断产生ROS,破坏细胞膜结构和功能的完整性,导致膜脂过氧化,引发一系列反应,使得细胞完整性丧失和MDA含量增加。因此,MDA通常被视为水果成熟的一个指标(Gill and Tuteja,2010;Chen et al.,2015) 。ROS(O[-2]·、H2O2和·OH)会导致植物细胞氧化损伤,植物衰老(Xia et al.,2016) 。在本研究中,单独使用外源GSH和SNP能使番茄果实的总抗氧化能力增强,MDA、H2O2含量和O[-2]·产生速率降低,而GSH+SNP协同处理使番茄果实的总抗氧化能力在整个贮藏期间提升幅度最大,MDA、H2O2含量和O[-2]·产生速率在贮藏期间不同程度地显著降低。前人研究发现,NO处理或GSH处理均能延缓黄瓜幼苗的O[-2]·产生速率和H2O2含量,降低MDA含量(杨志峰等,2020)。GSH与SNP协同处理进一步降低细胞膜的膜脂过氧化程度,提高番茄的抗氧化能力,防止果实发生氧化损伤。
植物有复杂的抗氧化系统来应对ROS,包括酶促系统(如SOD、CAT和POD)和非酶促系统(如AsA和GSH)(Gill and Tuteja,2010)。当植物中的ROS增加时,连锁反应被激发以防止细胞损伤:SOD催化超氧自由基生成O2和H2O2,生成的H2O2被CAT分解成水和氧气(Yi et al.,2010)。POD和CAT是果实中清除H2O2的主要保护酶。POD具有多种生理效应,在H2O2存在下,其可催化多种底物的氧化,如GSH、AsA和酚类,从而降低内源ROS清除剂的含量。SOD、CAT和POD协同作用能维持果实中较低水平的ROS,降低自由基毒性,以延缓果实衰老,延长果实贮藏期(Gill and Tuteja,2010)。在本研究中,单独使用外源GSH和SNP能使番茄果实的SOD、CAT和POD活性在不同程度上升高,而GSH+SNP协同处理升高的趋势更显著。前人研究表明,外源GSH和SNP单独处理分别不同程度提高荔枝和树莓的抗氧化能力(莫亿伟等,2010;王俊文,2020);楊志峰等(2020)研究发现,SNP与GSH协同处理能显著增强黄瓜幼苗SOD、POD和CAT活性以抵抗低温胁迫。说明GSH与SNP协同处理能进一步提高番茄果实中的SOD、CAT和POD活性,从而增强果实的抗氧化能力,维持果实贮藏期间的品质。
番茄果实采后的一些生理生化指标[抗氧化物质(GSH、AsA和总酚)和信号转导物质(NO水平)]会随着采后贮藏时间的推移而不断变化,逐渐使番茄失去其自身的商品价值及食用价值(徐福乐,2010)。总酚是果实中的重要色素物质,具有较强的抗氧化功能。AsA含量是衡量采后果实品质的重要指标之一。GSH和AsA是2种重要的非酶促抗氧化物质,能在AsA-GSH循环系统中协同清除ROS(任小林等,2004)。NO作为一种植物信号分子,可通过抑制植物组织中乙烯的生成及其效应来延长果实的贮藏期,并改善果实采后贮藏品质(Ren et al.,2017)。在本研究中,外源SNP单独处理能显著提高番茄果实的总酚含量,与刘娜(2013)研究得出NO溶液处理通过提高采后肥城桃果实的总酚、类黄酮和木质素含量来提高果实贮藏期间抗病性的结论一致。外源GSH单独处理与GSH+SNP协同处理在不同程度上促进番茄果实中AsA含量的积累,GSH和SNP分别单独处理亦能在不同程度上加快番茄果实中GSH的合成,与林琳等(2006)研究得出GSH处理能增强鸭梨果实抗氧化能力的结论一致。在采后贮藏期间,外源GSH和SNP分别单独处理能不同程度地加快番茄果实中NO的积累以延缓果实衰老,且GSH与SNP协同处理效果最佳,与Leshem等(1998)研究得出内源NO含量的增多能延缓果实成熟和衰老的结论一致。由此可知,外源GSH与SNP协同处理番茄果实能显著抑制果实GSH和AsA含量下降,提高果实的抗氧化能力,亦可通过促进果实中NO合成,加快果实中氧化还原信号转导,从而提高果实的贮藏品质。
4 结论
外源GSH与SNP协同处理通过降低果实失重率和提高细胞壁原果胶含量,抑制果实软化,延缓果实可溶性固形物、可滴定酸和可溶性糖含量下降,一定程度上抑制在贮藏过程中果实品质的劣变;亦可通过降低细胞膜的膜脂过氧化程度,提高番茄果实中SOD、CAT和POD活性,有效清除果实中的ROS,防止果实发生氧化损伤;还能通过抑制果实GSH和AsA含量下降,增强果实的抗氧化能力,同时促进果实中NO的合成,加快果实中氧化还原信号转导,以提高果实耐贮性。
参考文献:
曹建康,姜微波,赵玉梅. 2007. 果蔬采后生理生化实验指导[M] . 北京:中国轻工业出版社. [Cao J K,Jiang W B,Zhao Y M. 2007. Guidance of physiological and biochemical experiments after fruit and vegetable harvesting[M]. Beijing:China Light Industry Press.]
刁倩楠,田守波,陈幼源,熊浩楠,张永平. 2019. 甜瓜幼苗叶片内源一氧化氮和蔗糖代谢对低温胁迫的响应[J]. 西北植物学报,39(3):498-505. [Diao Q N,Tian S B,Chen Y Y,Xiong H N,Zhang Y P. 2019. Response of endogenous nitric oxide and sucrose metabolizing to chi-lling stress in melon seedlings[J]. Acta Botanica Boreali-Occidentalia Sinica,39(3):498-505.] doi:10.7606/j.issn. 1000-4025.2019.03.0498.
董守坤,马玉玲,李爽,董娜,刘丽君. 2018. 干旱胁迫及复水对大豆抗坏血酸—谷胱甘肽循环的影响[J]. 东北农业大学学报,49(1):10-18. [Dong S K,Ma Y L,Li S,Dong N,Liu L J. 2018. Effect of drought stress and re-watering on ascorbate-glutathione cycle of soybean[J]. Journal of Northeast Agricultural University,49(1):10-18.] doi:10.19720/j.cnki.issn.1005-9369.2018.01.002.
范林林,王清,左进华,高丽朴,史君彦,王倩. 2017. 外源NO处理对茄子贮藏品质的影响[J]. 中国食品学报,17(1):186-192. [Fan L L,Wang Q,Zuo J H,Gao L P,Shi J Y,Wang Q. 2017. The effect of exogenous NO treatment on eggplant quality during storage[J]. Journal of Chinese Institute of Food Science and Technology,17(1):186-192.] doi:10.16429/j.1009-7848.2017.01.024.
贾贞,王丹,游松. 2009. 谷胱甘肽的研究进展[J]. 沈阳药科大学学报,26(3):238-242. [Jia Z,Wang D,You S. 2009. Progress on glutathione and its preparation[J]. Journal of Shenyang Pharmaceutical University,26(3):238-242.] doi:10.14066/j.cnki.cn21-1349/r.2009.0301.
李洁. 2015. 外源NO和乙烯处理对番茄采后品质及乙烯合成相关基因表达的影响[D]. 乌鲁木齐:新疆农业大学. [Li J. 2015. The effect of exogenous NO and ethylene on quality and ethylene related synthesis genes expression of postharvest potato[D]. Urumqi:Xinjiang Agricultural University.]
李顺民,明建,曾凯芳. 2009. 一氧化氮对果蔬采后成熟衰老及抗病性的影响[J]. 食品工业科技,30(10):330-332. [Li S M,Ming J,Zeng K F. 2009. Effect of nitric oxide on postharvest maturation and disease resistance of fruit and vegetable[J]. Science and Technology of Food Industry,30(10):330-332.] doi:10.13386/j.issn1002-0306. 2009.10.032.
林琳,姜微波,曹健康,王宝刚,赵玉梅. 2006. 谷胱甘肽处理对采后鸭梨果實黑心病和抗氧化代谢的影响[J]. 农产品加工(学刊),(8):4-7. [Lin L,Jiang W B,Cao J K,Wang B G,Zhao Y M. 2006. Effects of postharvest GSH treatment on core browning and antioxidant metabolism in Yali pear fruit[J]. Academic Periodical of Farm Pro-ducts,(8):4-7.] doi:10.3969/j.issn.1671-9646-B.2006. 08.001.
刘枫,张新宪,高振峰,陈园园,张晓宇. 2021. 番茄采后自发气调贮藏技术[J]. 科学技术与工程,21(12):4870-4874. [Liu F,Zhang X X,Gao Z F,Chen Y Y,Zhang X Y. 2021. Modified atmosphere storage technology of tomato after harvest[J]. Science Technology and Engineering,21(12):4870-4874.] doi:10.3969/j.issn.1671-1815.2021. 12.017.
刘娜. 2013. 外源NO诱导采后肥城桃果实抗褐腐病的效果及机理研究[D]. 泰安:山东农业大学. [Liu N. 2013. Effect and mechanisms of exogenous nitric oxide on di-sease resistance against Monilinia fracticola in postharvest ‘Feicheng’ peach fruit[D]. Tai’an:Shandong Agricultural University.]
罗静,郭琳琳,黄玉南,王超,乔成奎,谢汉忠,方金豹. 2018. 猕猴桃PG基因在果实贮藏过程中的表达及其与硬度的关系[J]. 园艺学报,45(5):865-874. [Luo J,Guo L L,Huang Y N,Wang C,Qiao C K,Xie H Z,Fang J B. 2018. Relationship between PG gene expression and fruit firmness during kiwifruit storage[J]. Acta Horticulturae Sinica,45(5):865-874. doi:10.16420/j.issn.0513-353x. 2017-0580.
莫亿伟,郑吉祥,李伟才,牛铁荃,谢江辉. 2010. 外源抗坏血酸和谷胱甘肽对荔枝保鲜效果的影响[J]. 农业工程学报,26(3):363-368. [Mo Y W,Zheng J X,Li W C,Niu T Q,Xie J H. 2010. Effects of ascorbic acid and gluta-thione treatments on litchi fruits during post harvest sto-rage[J]. Transactions of the Chinese Society of Agricultural Engineering,26(3):363-368.] doi:10.3969/j.issn.1002-6819.2010.03.061.
任小林,张少颖,于建娜. 2004. 一氧化氮与植物成熟衰老的关系[J]. 西北植物学报,24(1):167-171. [Ren X L,Zhang S Y,Yu J N. 2004. Nitric oxide and its role in maturation and senescence in plant[J]. Acta Botanica Boreali-Occidentalia Sinica,24(1):167-171.] doi:10.3321/j.issn:1000-4025.2004.01.030.
任艳芳,何俊瑜,刘冬,刘进平. 2016. 一氧化氮对“大五星”枇杷贮藏期间果实抗氧化酶的影响[J]. 北方园艺,(3):121-124. [Ren Y F,He J Y,Liu D,Liu J P. 2016. Effect of nitric oxide on antioxidant enzymes of ‘Dawuxing’ loquat during storage[J]. Northern Horticulture,(3):121-124.] doi:10.11937/bfyy.201603034.
任艳芳,薛宇豪,田丹,何俊瑜,张黎明,吴情,刘树. 2021. 水杨酸和硝普钠协同处理对芒果贮藏品质及抗氧化活性的影响[J]. 食品科学,42(9):151-159. [Ren Y F,Xue Y H,Tian D,He J Y,Zhang L M,Wu Q,Liu S. 2021. Synergistic effect of salicylic acid and nitric oxide treatment on quality and antioxidant activity in postharvest mango fruit[J]. Food Science,42(9):151-159.] doi:10.7506/spkx1002-6630-20200507-065.
石玲,吴斌,敬媛媛,李亚玲,李玲,何欢,廖海慧,朱璇. 2019. 一氧化氮熏蒸处理对甜瓜采后细胞壁代谢及黑斑病控制的影响[J]. 食品科学,40(23):239-245. [Shi L,Wu B,Jing Y Y,Li Y L,Li L,He H,Liao H H,Zhu X. 2019. Effects of nitric oxide fumigation on cell wall metabolism and black spot control of postharvest melon[J]. Food Science,40(23):239-245.] doi:10.7506/spkx1002-6630-20181201-006.
宋耀,張静. 2016. 樱桃番茄采后贮藏保鲜技术研究进展[J]. 保鲜与加工,16(5):116-120. [Song Y,Zhang J. 2016. Research progress on storage technology of postharvest cherry tomato[J]. Storage and Process,16(5):116-120.] doi:10.3969/j.issn.1009-6221.2016.05.025.
王俊文. 2020. NO对采后树莓贮藏品质及苯丙烷和花青素代谢的影响[D]. 泰安:山东农业大学. [Wang J W. 2020. Effects of NO on the quality and the metabolism of phenylpropane and anthocyanin in raspberries during storage[D]. Tai’an:Shandong Agricultural University.]
王素朋,马利华,赵功伟. 2020. 超声波辅助涂膜保鲜对番茄贮藏品质的影响[J]. 食品科技,45(8):44-50. [Wang S P,Ma L H,Zhao G W. 2020. Effect of ultrasonic assisted film preservation on storage quality of tomato[J]. Food Science and Technology,45(8):44-50.] doi:10.13684/j.cnki.spkj.2020.08.009.
王玉凤,徐暄,孙其文. 2009. 硒浸种对番茄种子萌发的影响[J]. 湖北农业科学,48(10):2461-2463. [Wang Y F,Xu X,Sun Q W. 2009. The effect of selenium on seed germination of Lycopersicon esculentum[J]. Hubei Agricultu-ral Sciences,48(10):2461-2463.] doi:10.14088/j.cnki.issn0439-8114.2009.10.059.
王玉佳,韩爱云. 2021. 番茄的保鲜技术与方法[J]. 农产品加工,(2):40-42. [Wang Y J,Han A Y. 2021. Technology and method of tomato preservation[J]. Farm Products Processing,(2):40-42.] doi:10.16693/j.cnki.1671-9646(X).2021.01.045.
颉博杰,刘晓奇,张洋,张丹,吕剑,胡琳莉,郁继华,肖雪梅. 2021. 番茄果实采后贮藏期风味品质的动态变化[J]. 甘肃农业大学学报,56(1):96-101. [Xie B J,Liu X Q,Zhang Y,Zhang D,Lü J,Hu L L,Yu J H,Xiao X M. 2021. Dynamic changes of flavor quality of tomato fruits in postharvest storage period[J]. Journal of Gansu Agricultural University,56(1):96-101.] doi:10.13432/j.cnki.jgsau.2021.01.013.
徐福乐. 2010. 外源一氧化氮熏蒸对番茄果实采后品质的影响[J]. 福建农业学报,25(1):72-76. [Xu F L. 2010. Effect of NO fumigation on quality of post-harvest tomatoes[J]. Fujian Journal of Agricultural Sciences,25(1):72-76.] doi:10.19303/j.issn.1008-0384.2010.01.014.
楊虎清,吴峰华,周存山,王允祥. 2010. NO对杨梅采后活性氧代谢和腐烂的影响[J]. 林业科学,46(12):70-74. [Yang H Q,Wu F H,Zhou C S,Wang Y X. 2010. Effects of nitric oxide on active oxygen metabolism and fruit decay in postharvest Chinese Bayberry[J]. Scientia Silvae Sinicae,46(12):70-74.]
杨志峰,王小宇,崔金霞,刘慧英,张文博,吴佩. 2020. 低温胁迫下外源NO与GSH协同作用提高黄瓜幼苗耐冷性[J]. 植物生理学报,56(7):1573-1582. [Yang Z F,Wang X Y,Cui J X,Liu H Y,Zhang W B,Wu P. 2020. Synergistic effect of exogenous NO and GSH under chilling stress to improve cold tolerance of cucumber seedlings[J]. Plant Physiology Journal,56(7):1573-1582.] doi:10.13592/j.cnki.ppj.2020.0036.
张晓平,任小林,任亚梅,王小会,孙芳娟,白景祥. 2007. NO处理对采后猕猴桃贮藏性及叶绿素含量的影响[J]. 食品研究与开发,28(1):145-148. [Zhang X P,Ren X L,Ren Y M,Wang X H,Sun F J,Bai J X. 2007. Effects of nitric oxide treatment on storage ability and chlorophyll content of postharvest kiwifruit[J]. Food Research and Development,28(1):145-148.] doi:10.3969/j.issn.1005-6521.2007.01.044.
周培禄,刘光亮,王树声,李琦瑶,许娜,王程栋,杨银菊,曾文龙,陈爱国. 2018. 低温胁迫下烟苗多酚代谢及其抗氧化能力分析[J]. 中国烟草科学,39(5):33-39. [Zhou P L,Liu G L,Wang S S,Li Q Y,Xu N,Wang C D,Yang Y J,Zeng W L,Chen A G. 2018. Analysis of polyphenol metabolism and antioxidant capacity of tobacco seedlings under cold stress[J]. Chinese Tobacco Science,39(5):33-39.] doi:10.13496/j.issn.1007-5119.2018.05.005.
Chen H J,Gao H Y,Fang X J,Ye L,Zhou Y J,Yang H L. 2015. Effects of allyl isothiocyanate treatment on postharvest quality and the activities of antioxidant enzymes of mulberry fruit[J]. Postharvest Biology and Technology,108:61-67. doi:10.1016/j.postharvbio.2015.05.011.
Duran M,Aday M S,Zorba N N D,Temizkan R,Büyükcan M B,Caner C. 2016. Potential of antimicrobial active packaging ‘containing natamycin,nisin,pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry[J]. Food and Bioproducts Processing,98:354-363. doi:10.1016/j.fbp.2016.01.007.
Ge C,Lou Y,Mo F,Xiao Y H,Li N Y,Tang H R. 2018. Effects of glutathione on the ripening quality of strawberry fruits[J]. AIP Conference Proceedings,2079(1):020013. doi:10.1063/1.5092391.
Gill S S,Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry,48(12):909-930. doi:10.1016/j.plaphy.2010.08.016.
Leshem Y Y,Wills R B H,Veng-Va Ku V. 1998. Evidence for the function of the free radical gas—nitric oxide (NO·)—as an endogenous maturation and senescence regulating factor in higher plant[J]. Plant Physiology and Biochemistry,36(11):825-833. doi:10.1016/S0981-9428(99)80020-5.
Ren Y F,He J Y,Liu H Y,Liu G Q,Ren X L. 2017. Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘Tainong’ mango fruit during ripening[J]. Horticulture,Environment and Biotechnology,58(1):27-37. doi:10.1007/s13580-017-0001-z.
Tsaniklidis G,Delis C,Nikoloudakis N,Katinakis P,Aivalakis G. 2014. Low temperature storage affects the ascorbic acid metabolism of cherry tomato fruits[J]. Plant Physiology and Biochemistry,84(5):149-157. doi:10.1016/j.plaphy. 2014.09.009.
Wang B,Guo X,Zhao P J,Ruan M B,Yu X L,Zuo L P,Yang Y L,Li X,Deng D L,Xiao J X,Xiao Y W,Hu C J,Wang X,Wang X L,Wang W Q,Peng M. 2017. Molecular diversity analysis,drought related marker-traits association mapping and discovery of excellent alleles for 100-day old plants by EST-SSRS in cassava germplasms(Manihot esculenta Cranz)[J]. PLoS One,12(5):e0177456. doi:10.1371/journal.pone.0177456.
Wang B,Zhu S J. 2017. Pre-storage cold acclimation maintained quality of cold-stored cucumber through differentially and orderly activating ROS scavengers[J]. Postharvest Biology and Technology,129:1-8. doi:10.1016/j.postharvbio.2017.03.001.
Xia Y X,Chen T,Qin G Z,Li B Q,Tian S P. 2016. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit[J]. Postharvest Biology and Technology,111:15-24. doi:10.1016/j.postharvbio.2015.07.026.
Xu J N,Qi Y M,Zhang J,Liu M M,Wei X Y,Fan M T. 2019. Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation[J]. Food Chemistry,300(1):125130. doi:10.1016/j.foodchem.2019.125130.
Yi C,Jiang Y M,Shi J,Xia H X,Xue S,Duan X W,Shi J Y,Prasad N K. 2010. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process[J]. Food Chemistry,118(1):42-47. doi:10.1016/j.foodchem.2009.04.074.
Zhang H,Liu X L,Zhang R X,Yuan H Y,Wang M M,Yang H Y,Ma H Y,Liu D,Jiang C J,Liang Z W. 2017. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice(Oryza sativa L.)[J]. Frontiers in Plant Science,8:1580. doi:10.3389/FPLS.2017.01580.
(責任编辑 罗 丽)

