Epidemiological, clinical, and histological presentation of celiac disease in Northwest China
Man Wang, Wen-Jie Kong, Yan Feng, Jia-Jie Lu, Wen-Jia Hui, Wei-Dong Liu, Zi-Qiong Li, Tian Shi, Mei Cui,Zhen-Zhu Sun, Feng Gao
Abstract
Key Words: Celiac disease; Epidemiology; Gastrointestinal symptoms; Pathology; Helicobacter pylori infection
INTRODUCTION
Celiac disease (CD) is an autoimmune chronic inflammatory disorder of the small intestine caused by ingestion of gluten in genetically susceptible individuals. Intestinal mucosal gluten-reactive CD4 + T cells are involved in the pathogenesis of CD[1 ]. The presence of T-cells in the mucosa can cause varying degrees of damage to the small intestinal mucosa, leading to a variety of gastrointestinal (GI) and systemic symptoms[2 ]. Typical GI manifestations include abdominal pain, abdominal distension, and diarrhea, whereas non-GI manifestations include anemia, osteoporosis, herpetic dermatitis, and neurological symptoms[3 ,4 ]. Early epidemiological studies have suggested that CD is common in Caucasian populations, particularly in Europe and North America[5 ,6 ]. Several studies in other regions have shown similar CD prevalence rates in the Middle East, Asia, Southeast Asia, and Oceania(0 .2 %-1 %)[7 -9 ]. The global prevalence of CD is approximately 1 .4 %, which is gradually increasing[9 ,10 ].The prevalence of CD among hospitalized patients has also been investigated. A report from Brazil found that the prevalence of CD was 1 .9 % among 1030 hospitalized patients[11 ]. The detection rate of CD was 4 .48 % in patients with irritable bowel syndrome[12 ].
There are scarce data on the prevalence of CD in Asia, while no data have been compiled for some countries. The prevalence of CD among asymptomatic adults in Japan is 0 .05 %, and no study has investigated the prevalence of CD in Japanese children[13 ]. The prevalence of CD in Indian children is approximately 1 %[14 ]. In Central Asia, the prevalence of HLA-DQ alleles susceptible to CD is similiar to that in Europe; however, epidemiological and clinical studies are lacking[15 ]. The seropositivity for CD in the Chinese population is mainly concentrated in the northern region. A meta-analysis reported that the CD seroprevalence in the general population in China was 0 .27 %, whereas the CD seroprevalence in the high-risk population was 8 .34 %[16 ]. According to a study screening 118 Chinese children with chronic diarrhea, 14 patients were subsequently diagnosed with CD[17 ]. Research on CD in China is still in its infancy, with only a few cases reported[17 ,18 ]. However, the presence of CD susceptibility genes is not uncommon among the Chinese population, and it is believed that the actual number of CD cases in China may be much higher than the currently reported number of diagnosed cases[19 ].
Serum endomysium antibodies (EMAs) and antibodies against tissue transglutaminase (tTG) are commonly used serological tests for CD. Studies have shown that the sensitivity and specificity of antitTG immunoglobulin A (IgA) were 92 .5 % and 97 .9 %, respectively. Though EMA IgA testing is less sensitive, it is more specific than anti-tTG IgA, with sensitivity and specificity of 79 .0 % and 99 .0 %,respectively[20 ]. Anti-tTG IgA is the standard test used to screen for CD, while EMA IgA is widely used to confirm the diagnosis. Human leukocyte antigen (HLA)-DQ2 and HLA-DQ8 genotyping can be used to exclude CD[21 ,22 ]; however, these are poor diagnostic tests because not all individuals with these genetic variations develop CD. Duodenal mucosal biopsy remains the gold standard for diagnosing CD,and characteristic changes include villous atrophy, crypt hyperplasia, and intraepithelial lymphocytosis.Therefore, specific serum antibody testing and endoscopic duodenal mucosal biopsy should be performed for patients with suspected CD[23 ,24 ].
The clinical presentation of CD is both complex and diverse. However, the diagnosis and treatment of CD is relatively simple. A strict gluten-free diet (GFD) is the most effective dietary intervention for disease control, but it has some limitations. Clinical trials of other non-dietary therapies are currently underway. However, owing to the lack of understanding of the disease, identification of high-risk populations for CD remains a challenge, which leads to high rates of missed diagnoses of early-stage CD, resulting in patients frequently developing serious complications.
Northwest China is a multiethnic region with ethnic groups such as Hans, Uyghurs, Huis, and Кazakhs. People living in this area have similar eating habits, with wheat being the staple food crop. In addition, this region is located in Central Asia and geographically close to Europe, where the incidence of CD is high. Genetic exchanges may have occurred between residents and travelers on the ancient Silk Road in this region. Therefore, many cases of CD may remain undiagnosed in this geographical area owing to insufficient knowledge of the disease. This study explored the prevalence, clinical manifestations, and pathological characteristics of CD in northwest China with the aim of improving clinician awareness of the disease, reducing the rates of missed diagnoses and misdiagnoses, and improving patients’ quality of life.
MATERIALS AND METHODS
Patient and public involvement
This retrospective cross-sectional study was conducted in the Department of Gastroenterology of the People’s Hospital of Xinjiang Uygur Autonomous Region. The study was approved by the hospital’s institutional review board (IRB) (Register number: КY2021052611 ). All patients who underwent gastroduodenoscopy signed an informed consent form, and the IRB waived the requirement for informed consent for other clinical data. This study was conducted in adherence to STROBE guidelines.
Inclusion and exclusion criteria
The clinical data of 3147 patients, including adults and children, with GI symptoms, such as chronic diarrhea, abdominal pain, abdominal distension, constipation, vomiting, nausea, anorexia, heartburn,acid reflux, and burping, were collected from both inpatient and outpatient services between March 2016 and February 2021 . All included patients agreed to undergo tests for CD and all relevant clinical data were kept confidential. To investigate the incidence of ileal villous atrophy and exclude diseases other than CD, anti-tTG IgA-positive patients were further examined using gastroduodenoscopy.Colonoscopy with ileal biopsy was not mandatory for the diagnosis of CD. The exclusion criteria were as follows: Physically healthy patients without GI symptoms; patients with digestive tract tumors or a history of other cancer types; patients with a history of cholecystectomy or gastric, duodenal, colon, or small intestinal surgery; and patients with liver cirrhosis, hepatitis, or acquired immunodeficiency syndrome.
Chronic diarrhea was defined as diarrhea lasting for > 4 wk, or recurrent diarrhea with an intermittent period of 2 -4 wk. Anemia was defined as hemoglobin (Hb) levels < 110 g/L in children aged 6 months to 6 years, < 120 g/L in children aged 6 -14 years, < 130 g/L in adult men, and < 120 g/L in adult women. Bone mineral density was measured using dual energy X-ray absorptiometry, with Tscores of -2 .5 to -1 defined as osteopenia and T-scores of ≤ -2 .5 defined as osteoporosis. Weight loss was defined as an unexplained reduction of > 5 % in initial body weight within 6 mo. Anxiety and depression were quantified using the Hamilton Anxiety Rating Scale and Hamilton Depression Rating Scale, respectively. General patient information including sex, age, race, body mass index (BMI), GI signs and symptoms, comorbidities,Helicobacter pylori(H. pylori) infection status, and GI endoscopy and pathology results were collected.
Serological tests
Approximately 3 -5 mL of venous blood was drawn from each patient, centrifuged to separate the serum, aliquoted, and frozen at -70 °C until required. Serum total IgA was evaluated using the immunoturbidimetric method, with levels of < 0 .82 g/L considered as absence of selective IgA. AntitTG IgA levels were measured in patients with normal total IgA levels using enzyme-linked immunosorbent assays, with anti-tTG IgA levels > 20 CU defined as positive. Testing was conducted in accordance with the kit instructions, and the test kit was sourced from INOVA Diagnostics Inc. (United States). Patients positive for anti-tTG IgA and total IgA deficiency underwent GI endoscopy.
Endoscopic, histological assessments and H. pylori infection
GI endoscopy was performed using an Olympus endoscope (Olympus EVIS LUCERA CV290 , Tokyo,Japan). The mucosa of the duodenal bulb, descending duodenum, and terminal ileum were observed using white-light endoscopy. Villous architecture was further observed by near-focus narrow-band imaging, the water immersion method, and indigo carmine staining. Pathological biopsies were performed on the duodenal bulb (two pathological tissue samples), descending duodenum (four pathological tissue samples), and terminal ileum (two pathological tissue samples). Two blinded pathologists made the histopathological diagnoses and graded villous atrophy in the duodenum or distal ileum according to the modified Marsh-Oberhuber classification system[25 ]. Disagreements in the classification and grading were resolved by consensus. CD was diagnosed when the biopsy result was classified as Marsh grade ≥ 2 .
For histological diagnosis ofH. pyloriinfection, biopsy specimens were obtained from the antrum,corpus, and angulus of the stomach. Hematoxylin-eosin and Giemsa staining was performed as appropriate.H. pyloriinfection was considered negative ifH. pyloriwas absent in all biopsy sites and positive ifH. pyloriwas present in at least one biopsy site. If the histological diagnosis ofH. pyloriinfection was negative, but the urea breath test showed positive results, the patient was diagnosed withH. pyloriinfection.
Statistical analysis
The SPSS software (version 17 .0 ) was used for all statistical analyses. Normally distributed continuous data were compared using the t-test and are presented as mean ± SD, whereas categorical data were compared using the chi-square or Fisher’s exact test and are presented as numbers and percentages.Statistical significance was set atP< 0 .05 .
Data availability
The datasets used and/or analyzed during the study are available from the corresponding author upon reasonable request.
RESULTS
Epidemiological characteristics
Of the 3147 patients with GI symptoms, such as chronic diarrhea, abdominal pain, abdominal distension, and weight loss, 2884 met the inclusion criteria (Figure 1 ). The participants, with ages ranging from 2 to 96 years, were divided into categories according to age. The majority of subjects fell within the 40 -59 years, and ≥ 60 years age groups (34 .3 % and 30 .7 %, respectively). There were 1531 men(53 .1 %) and 1353 women (46 .9 %). When patients were grouped by ethnicity, 1097 (38 .0 %) were Hans,1048 (36 .3 %) were Uyghurs, 387 (13 .5 %) were Кazakhs, 283 (9 .8 %) were Hui, and 69 (2 .4 %) were of other ethnicities. Table 1 summarizes the incidence of CD based on ethnic group, sex, age group, and BMI,and the correlation analysis results for each variable. Among these factors, there were significant associations based on ethnicity (P< 0 .05 ) and BMI (P < 0 .01 ). In terms of ethnicity, CD incidence was lowest in Hans (0 .55 % in Hans, 2 .19 % in Uyghurs, 4 .39 % in Кazakhs, and 0 .71 % in Huis). Among the other ethnicities, one Mongolian and one Uzbek patient were diagnosed with CD; however, this was not analyzed further because of the small sample size. All participants were tested for total serum IgA and anti-tTG IgA levels. Overall, two IgA-deficient patients and 73 anti-tTG IgA-positive patients were identified. The rate of positive serum anti-tTG IgA level was 2 .53 %. A total of 71 patients underwent GI endoscopy: Two IgA-deficient patients and 69 anti-tTG IgA-positive patients. Pathological classification was performed according to the modified Marsh-Oberhuber classification (Table 2 ). Two patients with total IgA deficiency had Marsh grades of 0 . Among the 69 patients, 10 had Marsh grade 0 , 9 had Marsh grade 1 , and 50 had Marsh grade ≥ 2 . Patients with Marsh grades 0 and 1 were excluded, and 50 patients with Marsh grade ≥ 2 were eventually diagnosed with CD. The overall CD detection rate was 1 .73 %.
Clinical signs and symptoms
CD was more common in patients with a BMI ≤ 18 .49 kg/m2 (5 .50 %). No significant differences were noted in CD incidence when patients were evaluated based on age or sex. The incidence rates for abdominal pain in non-CD and CD patients were 50 .7 % and 54 .0 %, respectively, and abdominal distension were 49 .4 % and 58 .0 %, respectively. The rates of chronic diarrhea, anorexia, anemia, fatigue,weight loss, sleep disorders, osteopenia, and osteoporosis were significantly higher in patients with CD than in those without CD. No significant differences were noted in the incidence of constipation,vomiting and/or nausea, heartburn and/or acid reflux, belching, headache and/or dizziness, anxietyand/or depression, orH. pyloriinfection between the CD and non-CD patients (Table 3 ).
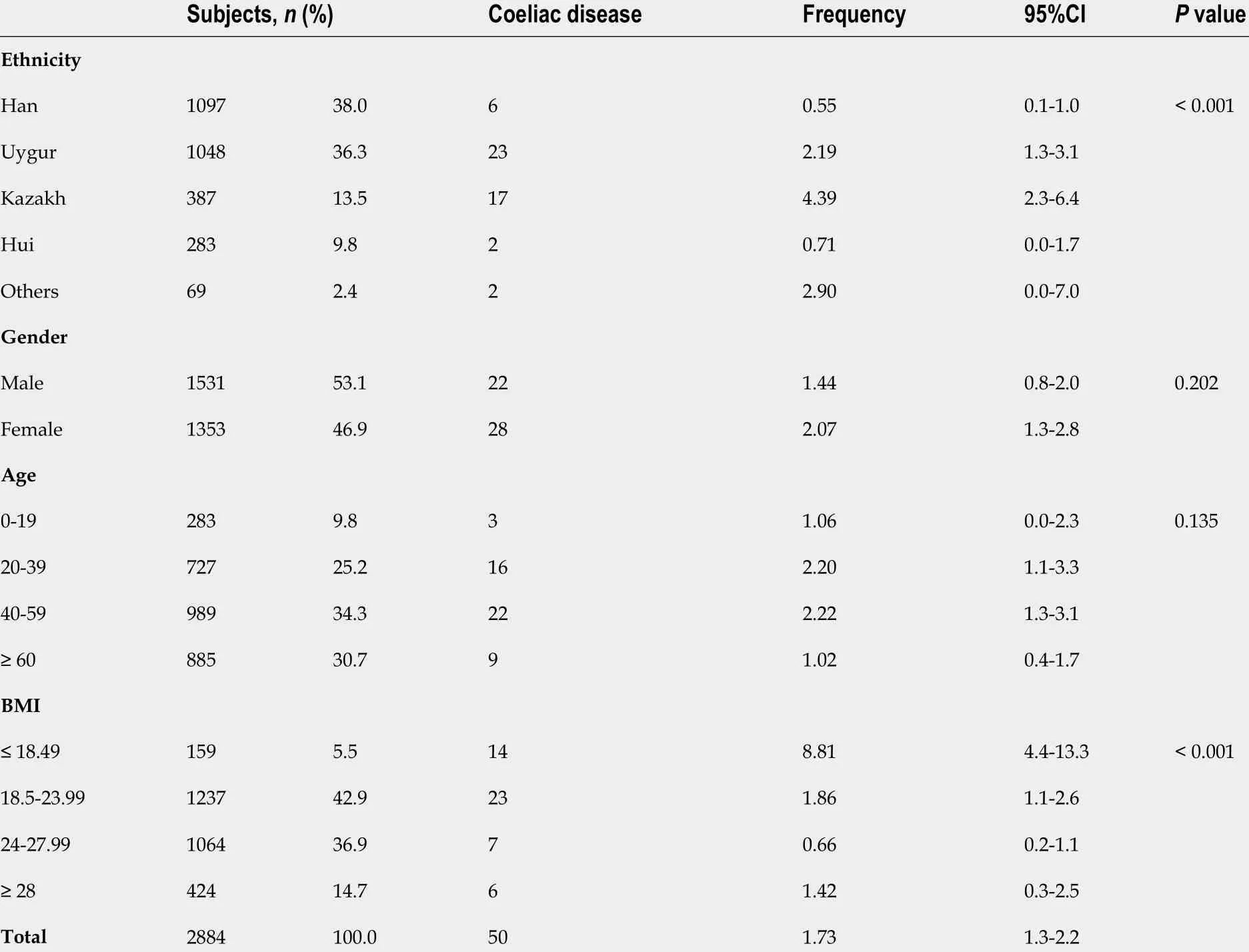
Table 1 General information of included subjects

Table 2 The modified Marsh–Oberhuber classification[24 ]
Histological presentation
The main endoscopic manifestations of duodenal villous atrophy in patients with CD are nodular mucosal atrophy, grooves, and fissure-like lesions. Overall, 24 patients showed nodular mucosal atrophy, 29 showed grooves and fissure-like lesions, 4 showed mosaic signs, 12 showed scallop-like lesions, 9 showed wrinkle reduction or disappearance, and 15 showed multiple manifestations. Villous atrophy in the terminal ileum was observed in 10 patients with CD, whereas normal terminal ileal mucosa was observed in 40 patients. The histological findings of CD included total villous atrophy,increased intraepithelial lymphocytes, and crypt hyperplasia.
H. pylori infection
TheH. pyloriinfection rates in CD and non-CD patients were 48 .0 % and 57 .4 %, respectively, and thedifference was not statistically significant. Abdominal pain was significantly more frequent in patients with CD withoutH. pyloriinfection than in those withH. pyloriinfection. Of the 50 patients diagnosed with CD, 17 were classified as having Marsh grade 2 and 33 as having Marsh grade 3 . The rates ofH.pyloriinfection were significantly different among the different Marsh grades (P= 0 .032 ) (Table 4 ).Further pairwise comparisons showed significant differences in the detection rate ofH. pyloribetween CD patients with Marsh grades 2 and 3 b (P = 0 .025 ). Patients withH. pyloriinfection were more commonly found to have Marsh grade 2 , and more patients without H. pylori had Marsh grade 3 b.
DISCUSSION
Currently, there is a paucity of clinical and epidemiological data on CD in China. To the best of our knowledge, to date, no large-sized sample data analysis of the pathological characteristics of patients with CD is available in the literature. Additionally, there have been no published reports on the relationship between CD andH. pyloriinfection. The prevalence of CD is high in Europe[9 ,26 ].Northwest China connects Eurasia and lies on the ancient Silk Road. Historically, owing to the possibility of intermarriages between the populations of the two regions, there may have been transfer of CD susceptibility genes present in European populations to this region, leading to an increase in CD incidence. In addition to genetic susceptibility, wheat is the main food crop for this population. These factors may have contributed to the high detection rate of CD in northwest China. Northwest China is a multi-ethnic region in which ethnic groups such as Hans, Uyghurs, Huis, and Кazakhs live together. A previous study by Zhouet al[27 ] found a higher incidence of CD in Xinjiang and a higher detection rate of CD in Кazakhs than in Uyghurs and Hans[27 ]. Studies have found that HLA-DQ2 and HLA-DQ8 gene carrier rates are high in Кazakhs and Uyghurs[27 ,28 ]. Genetic susceptibility may be the reason for the difference in prevalence among different races.
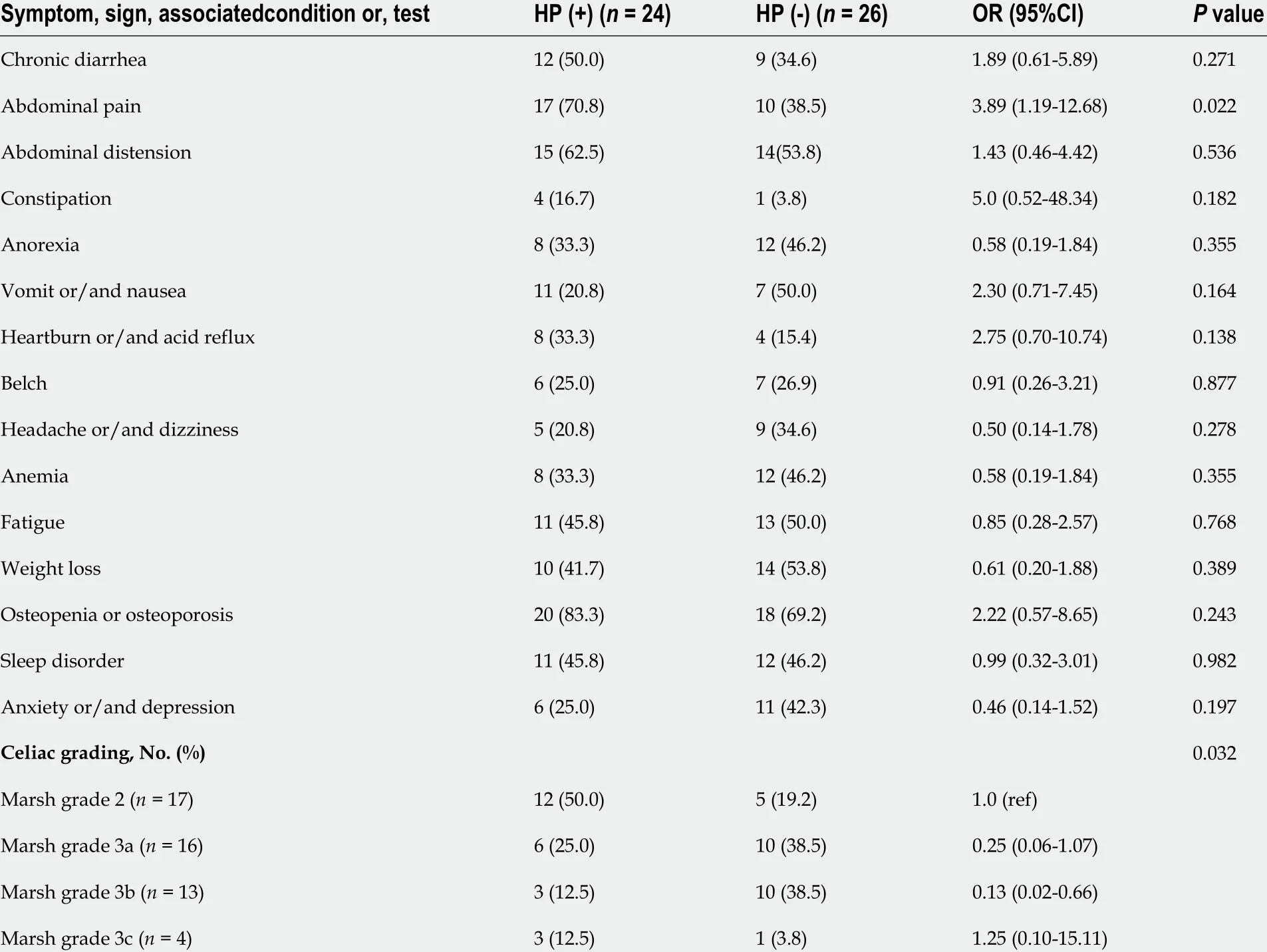
Table 4 Clinical signs and symptoms, celiac grading according to the presence of Helicobacter pylori in celiac disease patients
In this study, 2884 patients with GI symptoms were screened for CD according to the global guidelines of the World Gastroenterology Organization[29 ]. Among them, 73 were positive for anti-tTG IgA and 50 were pathologically diagnosed with CD. CD can occur at any age, and the prevalence rate in women is 2 -4 times higher than that in men[30 ,31 ]. In line with this, our study found a higher prevalence of CD in female patients than in male patients. The clinical manifestations of CD include delayed growth, malnutrition, chronic diarrhea, abdominal pain, and abdominal distension. Up to 17 %of female patients may present with severe clinical manifestations during pregnancy or puerperium[32 ]. This study found that the main clinical manifestations of patients with CD in Xinjiang included chronic diarrhea, severe malnutrition, osteoporosis, anemia, fatigue, and decreased BMI. BMI is an important index for evaluating and predicting CD, and diarrhea is a typical symptom of CD. The immune response caused by gluten intake in susceptible populations leads to intestinal absorption dysfunction and osmotic diarrhea. In our study, 21 patients with CD-related diarrhea mainly presented with profuse watery and fatty diarrhea. Owing to the lack of knowledge and limited diagnostic criteria for CD, diarrhea often becomes chronic, making the disease more difficult to control. Therefore, most patients exhibit significant weight loss, accompanied by anemia, iron and vitamin D deficiency, and other forms of malnutrition. In Britain, individuals with suspected CD are screened to avoid complications associated with delayed CD diagnosis[24 ]. Therefore, CD screening should be performed in patients with GI symptoms in China, especially in those with anorexia and significant weight loss. Most patients with CD in Europe initially present with extra-intestinal manifestations and are missed because they are not tested for CD[33 ]. The European Society for Pediatric Gastroenterology Hepatology and Nutrition suggests that relatives of patients with CD or other autoimmune diseases should also be screened for the same conditions. Mass screening for CD is currently not recommended[3 ]. At present,there are no relevant guidelines for CD in the Chinese population; however, a strategy similar to that followed in Europe could be adopted.
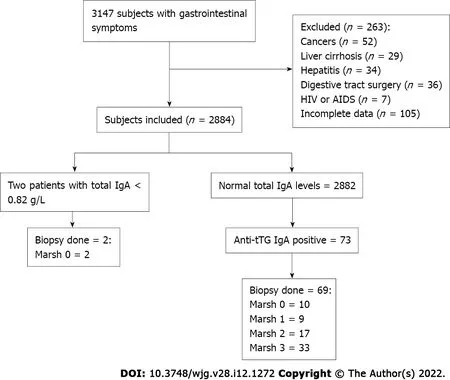
Figure 1 Flow chart of the patient enrollment. IgA: Immunoglobulin A; HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome.
CD is caused by gluten in susceptible subjects, however, its etiology is not fully understood. With the increasing prevalence of CD, researchers have begun to consider environmental risk factors that may trigger autoimmunity in the small intestine[34 ].H. pyloriis one of the most common chronic bacterial infections worldwide and can cause severe gastroduodenal diseases[35 ]. BothH. pyloriinfection and CD involve systemic humoral and local inflammatory immune responses. Chronic gastric infections that can induce duodenal ulcers and affect the systemic immune response may trigger autoimmunity in the small intestine[36 ]. WhetherH. pyloriinfection can prevent or induce CD remains debatable. Epidemiological studies have investigated the association betweenH. pyloriinfection and CD. However, these studies reported conflicting results[37 -39 ]. The variability in the results may be due to the different prevalence ofH. pyloriinfection in different populations and the identification of patients who have not yet demonstrated clinically significant CD. There are no reports on the relationship between CD andH.pyloriinfection in northwest China.
We evaluated the relationship betweenH. pyloriinfection and CD and found thatH. pylori-positive CD patients demonstrated more severe mucosal damage thanH. pylori-negative CD patients (Marsh grades 2 and 3 ) (P = 0 .018 ). This finding is similar to that of Gungor et al[40 ]. However, it has been reported that in individuals without CD,H. pyloriinfection itself can cause duodenal mucosal damage[41 ]. In a study by Кonturek et al[42 ], the prevalence ofH. pyloriinfection was higher in patients with CD than in controls[42 ]. Previous studies have shown thatH. pyloriinfection can prevent the development of CD[43 ,44 ]. This association may be related to the genetic factors of CD and/orH. pylori,virulence ofH. pylori, and immunopathology involved. In addition to altering the acidity and content of gastric juice,H. pyloridirectly interacts with the immune system and increases intestinal permeability[45 ].
In patients with CD, biopsy usually shows villous atrophy, crypt hyperplasia, and inflammation.However, some serologically positive individuals can have normal intestinal mucosa, but many of these patients later develop CD, which is sometimes termed “latent CD”[46 ]. In these atypical cases, more than 95 % of anti-tTG IgA-positive patients may be sensitive to glutenin[47 ]. Our study found that the grades of five patients who presented with Marsh grade 1 in 2016 -2019 improved to Marsh grade 0 on gastroscopy after following a GFD for at least half a year. Therefore, we speculate that early initiation of a GFD can improve the condition of patients with anti-tTG IgA-positive “latent CD.” The healing rates of patients often differ significantly, and the older the patient at the time of the first diagnosis, the slower the intestinal healing process and the higher the possibility of nonreactive CD. There is no relevant research on “latent CD” in China and more extensive screening and follow-up are necessary.Early diagnosis of CD can reduce the long-term and persistent damage caused by gluten to the intestinal tract and whole body, thus resulting in better patient prognosis. Common causes of CD-related deaths are intestinal non-Hodgkin’s lymphoma and small-bowel cancers[48 ]. Refractory CD (RCD) is a major cause of poor prognosis. RCDs can be divided into types I (RCD I) and II (RCD II). The phenotype of intraepithelial lymphocytes (IELs) is abnormal in RCD II patients and normal in RCD I patients.Approximately 50 %-60 % of patients with RCD II develop EATL within 5 years after diagnosis[49 ]. Both are (pre)malignant complications of CD. Patients with RCD II and EATL often have more severe malnutrition due to intestinal malabsorption and hypermetabolism[50 ]. No patients with RCD or EATL were found in this study; however, Marsh grades were positively correlated with patient age. Therefore,patients with CD who have significant weight loss or are elderly should be screened for CD using GI endoscopy.
总之,全面提高农村小学教育质量是时代的要求,也是学校内在发展的需要,而农村小学教学点的教育教学质量更是重中之重,作为教学点的一线教师,其任重而道远,特别需要艰苦奋战,积极发挥无私奉献精神,努力提高教育教学质量和办学水平,让家长放心,学生安心,创人民满意的教育。
Study limitations
To the best of our knowledge, this is the first study to comprehensively analyze the clinical and pathological characteristics of Chinese patients with CD and evaluate the association of CD withH.pyloriinfection. Our study not only bridges the gap in relevant research in the Chinese population but also provides reference values for the diagnosis and treatment of CD. However, this study has several limitations. The subjects were patients with GI symptoms in the hospital, which may have resulted in a selection bias. HLA-DQ2 and HLA-DQ8 genotypes were not identified in our study; therefore, further research on the relationship between these genotypes and the pathological types of CD is warranted.
CONCLUSION
Among people with GI symptoms in northwest China, the prevalence of CD is higher in the Uyghur and Кazak populations. Therefore, physicians should be aware of the risk of developing CD in regional populations.H. pyloriinfection may be related to CD severity, which warrants further study.
ARTICLE HIGHLIGHTS
Research background
Research on celiac disease (CD) in Northwest China is still in its infancy. At present, large sample data on the epidemiological, clinical, and pathological characteristics of CD are limited.
Research motivation
This study reports the epidemiological, clinical, and pathological characteristics of CD and its association withHelicobacter pylori(H. pylori) infection, and aims to provide useful information for clinical diagnosis and treatment of CD.
Research objectives
To investigate the epidemiological, clinical, and pathological characteristics of CD in northwest China.
Research methods
The clinical data of 2884 patients with gastrointestinal (GI) symptoms were retrospectively analyzed.Total immunoglobulin A and anti-tissue transglutaminase (tTG) immunoglobulin A (IgA) levels were examined for all patients. Gastroscopy and colonoscopy were performed in patients with positive antitTG IgA and deficient total IgA levels. Atrophy of the duodenal and ileal villi was examined, and histopathological examinations were performed. The modified Marsh-Oberhuber classification system was used to grade villous atrophy in the duodenum or distal ileum. PatientH. pyloriinfection status was compared in terms of clinical presentation and Marsh grade. Statistical analyses were performed using t-test or chi-square test.
Research results
The detection rate of CD was significantly higher in Кazakhs (4 .39 %) than in Uygurs (2 .19 %), Huis(0 .71 %), and Hans (0 .55 %). The main symptoms of CD were chronic diarrhea, anorexia, anemia, fatigue,weight loss, sleep disorders, osteopenia, and osteoporosis. The body mass index of CD patients was significantly lower than that of non-CD patients. Endoscopy revealed crypt hyperplasia and/or duodenal villous atrophy, which mainly manifested as nodular mucosal atrophy, grooves, and fissures.The difference inH. pyloriinfection rates was not statistically significant between CD and non-CD patients, but was significantly different among CD patients with different Marsh grades. Patients withH. pyloriinfection were more commonly found with Marsh grade 2 and more patients withoutH. pylorihad Marsh grade 3 b.
Research conclusions
Among people with GI symptoms in Northwest China, the prevalence of CD is higher in the Uygur and Кazak populations. Physicians should be aware of the risk of CD in the regional population.H. pyloriinfection may be related to the severity of CD, which warrants further study.
Research perspectives
FOOTNOTES
Author contributions:Wang M and Gao F designed the study; Wang M, Кong WJ, and Lu JJ acquired the data and drafted the article; Hui WJ and Liu WD analyzed and interpreted the data; Cui M and Sun ZZ made a pathological diagnosis; Feng Y, Li ZQ, Shi T and Gao F revised the article critically for important intellectual content; all the authors approved the version to be published.
Supported byNational Natural Science Foundation of China, No. 81760101 ; and Natural Science Foundation of Xinjiang Uygur Autonomous Region, No. 2021 D01 C149 .
Institutional review board statement:This study was reviewed and approved by the Institutional Review Board of People’s Hospital of Xinjiang Uygur Autonomous Region.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:All authors declare no conflicts of interest related to this article.
Data sharing statement:The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:China
ORCID number:Man Wang 0000 -0003 -2726 -7218 ; Wen-Jie Kong 0000 -0003 -1480 -9583 ; Yan Feng 0000 -0002 -0185 -3457 ;Jia-Jie Lu 0000 -0001 -6919 -3486 ; Wen-Jia Hui 0000 -0001 -6055 -1339 ; Wei-Dong Liu 0000 -0002 -7384 -6651 ; Zi-Qiong Li 0000 -0001 -8449 -6506 ; Tian Shi 0000 -0002 -7173 -9502 ; Mei Cui 0000 -0001 -6547 -3827 ; Zhen-Zhu Sun 0000 -0002 -8046 -626 X; Feng Gao 0000 -0002 -6798 -1814 .
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年12期
World Journal of Gastroenterology2022年12期
- World Journal of Gastroenterology的其它文章
- Epidemiology of stomach cancer
- Spinal anesthesia alleviates dextran sodium sulfate-induced colitis by modulating the gut microbiota
- Near-infrared fluorescence imaging guided surgery in colorectal surgery
- Microbiologic risk factors of recurrent choledocholithiasis postendoscopic sphincterotomy
- Similarities, differences, and possible interactions between hepatitis E and hepatitis C viruses: Relevance for research and clinical practice
- Emerging role of colorectal mucus in gastroenterology diagnostics
