CoIP-MS法筛选CHCHD2互作蛋白及其功能的初步分析*
刘烜汋, 王莹莹, 樊馨蔓, 王芳, 徐安定, 徐晓红
CoIP-MS法筛选CHCHD2互作蛋白及其功能的初步分析*
刘烜汋, 王莹莹, 樊馨蔓, 王芳, 徐安定, 徐晓红△
(暨南大学附属第一医院神经内科及卒中中心,广东 广州 510630)
筛选氧化应激状态下CHCHD2 (coiled-coil-helix-coiled-coil-helix domain-containing 2)的互作蛋白,以期挖掘出CHCHD2保护神经细胞对抗氧化应激损伤的潜在机制。通过Lipofectamine 2000分别在人神经母细胞瘤细胞系SH-SY5Y中转染含有Flag标签的对照质粒及CHCHD2过表达质粒,用100 μmol/L过氧化叔丁醇(tert-butyl hydroperoxide, TBHP)或蒸馏水处理24 h后,采用免疫共沉淀(CoIP)的方法富集各组细胞中与CHCHD2相结合的蛋白,SDS-PAGE跑浓缩胶,切取条带,胶内酶解后进行液相色谱-质谱联用(LC-MS/MS)分析、数据库检索及生物信息分析,筛选与CHCHD2互作的蛋白,并对功能进行初步分析。(1)CHCHD2具有保护SH-SY5Y细胞对抗TBHP诱导的氧化应激损伤作用;(2)CoIP-MS结果提示,不同于生理状态,在氧化应激状态下共有64个蛋白是与CHCHD2互作的特有差异表达蛋白(differentially expressed proteins, DEPs);(3)通过GO功能注释和KEGG富集分析,我们发现氧化应激状态下特有DEPs主要在外泌体和细胞浆中发挥作用,参与蛋白翻译及翻译起始等生物过程,在蛋白及poly(A) RNA结合方面发挥分子功能,并参与糖代谢过程;(4)DEPs还参与了负性调控活性氧生物合成过程和对过氧化氢的反应等抗氧化应激相关生物过程,其中肿瘤坏死因子受体相关蛋白1(tumor necrosis factor receptor-associated protein 1, TRAP1)和热休克蛋白家族D成员1(heat shock protein family D member 1, HSPD1)是抗氧化应激过程中重要的候选蛋白;(5)通过蛋白互作网络分析,我们发现在氧化应激状态下特异性存在3个蛋白[Y盒结合蛋白1(Y-box-binding protein 1, YBX1)、含TCP1分子伴侣亚基6A(chaperonin containing TCP1 subunit 6A, CCT6A)和细胞色素C氧化酶装配因子4同源物(cytochrome C oxidase assembly factor 4 homolog, COA4)]与CHCHD2有直接相互作用,也是后续需要重点关注的蛋白。利用CoIP-MS法成功筛选出生理状态及氧化应激状态下CHCHD2的互作蛋白,并挖掘出与其抗氧化应激过程密切相关的2个候选蛋白(TRAP1和HSPD1)及另外3个与其直接作用的候选蛋白(YBX1、CCT6A和COA4),为进一步深入探索CHCHD2抗氧化应激作用中的生物过程及分子机制奠定基础。
CHCHD2蛋白;氧化应激;免疫共沉淀;质谱法;神经退行性疾病
神经退行性疾病包括阿尔茨海默病(Alzheimer disease, AD)、帕金森病(Parkinson disease, PD)、亨廷顿舞蹈病(Huntington disease, HD)和肌萎缩性侧索硬化(amyotrophic lateral sclerosis, ALS)等一系列疾病,是一类严重威胁人类健康和生活质量的老年疾病,目前国际上尚无有效治疗神经退行性疾病或延缓其病程的药物。虽然各种神经退行性疾病间的发病机制各不相同,但氧化应激导致的神经损伤被认为是其共有的重要发病机制之一[1]。因此,探寻神经细胞中氧化应激的具体调控机制及寻找抗氧化应激相关蛋白和信号通路,对明确神经退行性疾病的发病机制和探索相关的干预措施具有重要科研价值和社会意义。
线粒体相关蛋白CHCHD2(coiled-coil-helix-coiled-coil-helix domain-containing 2)是“CHCH(coiled-coil-helix-coiled-coil-helix)”功能结构域蛋白质家族中的一员。基因突变已被鉴定与PD、AD、额颞叶痴呆、路易体痴呆等神经退行性疾病密切相关[2]。在不同的情况下,CHCHD2蛋白可以在线粒体和核中发挥不同的功能。生理条件下,CHCHD2在线粒体中发挥作用,作为氧化呼吸链的应激调节器,与细胞色素C氧化酶(cytochrome C oxidase, COX)结合,维持线粒体嵴结构的稳定;而在低氧环境时,CHCHD2移位到细胞核中,作为自身和COX亚基4异构体2(COX subunit 4 isoform 2, COX4I2)的核转录因子,促进自身和COX4I2的表达[3],从而稳定氧化磷酸化(oxidative phosphorylation, OXPHOS)过程,并补偿了活性氧(reactive oxygen species, ROS)产生而造成的能量缺乏[4],维持了线粒体呼吸链的稳定。在果蝇中的突变或缺失会破坏呼吸链中细胞色素C的稳定性,导致电子泄漏和ROS生成,进而导致氧化应激的产生、多巴胺能神经元缺失及运动功能障碍[5]。而过表达外源性CHCHD2蛋白能够降低细胞内ROS水平[3],并挽救由突变而导致的PD相关表型[5]。提示了突变是通过其正常功能缺失而致病,同时说明CHCHD2可能具有保护细胞对抗氧化应激损伤的功能。但在氧化应激状态下,CHCHD2蛋白可以与哪些蛋白相互作用、通过哪些信号通路起到神经保护作用仍然不清楚。
本研究发现,过表达CHCHD2可保护人神经母瘤细胞系SH-SY5Y对抗过氧化叔丁醇(tert-butyl hydroperoxide, TBHP)诱导的氧化应激损伤,并进一步通过免疫共沉淀(co-immunoprecipitation, CoIP)联合质谱(mass spectrometry, MS)的方法挖掘氧化应激状态下与CHCHD2相互作用的特有蛋白及相关信号通路,为寻找神经退行性疾病及其它神经系统疾病中抗氧化应激的治疗靶点提供了新的思路和方向。
材料和方法
1 细胞
SH-SY5Y细胞购自American Type Culture Collection (ATCC)。
2 主要药物和试剂
CHCHD2-Flag和vector-Flag质粒均购自上海吉凯基因科技有限公司;转染试剂Lipofectamine 2000购自Invitrogen;DMEM高糖培养基、胎牛血清及青-链霉素均购自Gibco;TBHP购自上海麦克林生化科技公司;caspase-3(激活型)抗体(兔单抗)、Flag抗体(小鼠单抗)、RIPA裂解液和PMSF购自上海碧云天生物技术公司;过氧化物酶标记的山羊抗小鼠IgG(H+L)和过氧化物酶标记的驴抗兔IgG(H+L)均购自上海翌圣生物科技公司;蛋白A/G磁珠购自Thermo Fisher。PBST为PBS中加入0.1% Tween 20;等渗裂解液为在pH 7.4的50 mmol/L Tris-HCl中加入150 mmol/L NaCl、1% Triton X-100和1% cocktail蛋白酶抑制剂。
3 主要方法
3.1细胞培养及模型构建SH-SY5Y细胞的培养基为DMEM高糖基础培养基加上10%胎牛血清及青链霉素双抗,细胞培养在37 ℃、5% CO2培养箱里。药物实验(CHCHD2-OE+TBHP)组:CHCHD2-Flag质粒瞬时转染SH-SY5Y细胞,转染24 h后加入100 μmol/L TBHP处理24 h,收集细胞;药物对照(vector+TBHP)组:vector-Flag质粒瞬时转染SH-SY5Y细胞,转染24 h后加入100 μmol/L TBHP处理24 h,收集细胞;无药物实验(CHCHD2-OE)组:CHCHD2-Flag质粒瞬时转染SH-SY5Y细胞,转染24 h后加入与药物组TBHP等体积的蒸馏水处理24 h,收集细胞;无药物对照(vector)组:vector-Flag质粒瞬时转染SH-SY5Y细胞,转染24 h后加入与药物组TBHP等体积的蒸馏水处理24 h,收集细胞。
3.2总蛋白的提取用预冷的D-PBS洗细胞3次;每皿加入500 μL裂解液;用细胞刮铲轻轻将细胞刮下,收集在1.5 mL EP管中,4 ℃旋转裂解1~2 h;随后在4 ℃、12 000 r/min离心15 min,提取上清液。
3.3CoIP每反应体系采用50 μL磁珠,去掉Buffer,用500 μL PBST洗磁珠2~3次;用150 μL PBST重悬磁珠,加4 μg抗体,在4 ℃旋转孵育过夜。用PBST清洗3次后,加入提取的蛋白上清,4 ℃旋转过夜孵育。用等渗裂解液轻轻清洗3次,加适量1× Loading Buffer,100 ℃煮10 min,进行Western blot实验。
3.4Western blot蛋白经过SDS-PAGE分离后,以290 mA、1 h的条件转移至硝酸纤维素膜;随后用5%脱脂奶粉室温封闭1 h,加入Ⅰ抗[caspase-3(激活型)抗体(兔单抗)和Flag抗体(小鼠单抗),1∶1 000],4 ℃摇床孵育过夜;然后加入辣根过氧化物酶标记的对应Ⅱ抗[过氧化物酶标记的山羊抗小鼠IgG(H+L)和过氧化物酶标记驴抗兔IgG(H+L),1∶5 000],室温孵育1 h,用显影仪进行显影。。
3.5LC-MS/MS分析
3.5.1蛋白浓缩制备8%的浓缩胶[ddH2O 4.78 mL,30% Acry/Bis 2.6 mL,Tris-HCl (pH 8.8) 2.5 mL,10% SDS 100 μL,10% AP 25 μL,TEMED 10 μL],80 V恒压跑30 min。
3.5.2蛋白酶解(1)切胶:将蛋白条带切出后放入EP管中;(2)水洗:加入MilliQ水清洗1 min,离心去上清液,水洗2次;(3)脱色:加入50% MeOH/50 mmol/L NH4HCO3于37 ℃恒温箱内脱色30 min,离心去上清液;(4)脱水:加入100%乙腈,震荡30 s等胶粒变白后吸出液体;(5)烷基化:往干燥胶粒中加入25 mmol/L DTT/50 mmol/L NH4HCO3,于56 ℃反应30 min,吸出DTT,加入55 mmol/L IAA/50 mmol/L NH4HCO3,室温暗处反应30 min;(6)水洗:将IAA吸出,加入MilliQ水清洗3次;(7)脱水:100%乙腈脱水至胶粒变白;(8)酶切:用25 mmo/L NH4HCO3稀释胰酶至20 mg/L;于每管加入适量胰酶,冰浴30 min;再补加适量25 mmol/L NH4HCO3至覆盖胶粒,放入37 ℃恒温箱酶切过夜;(9)肽段提取:将EP管盒整个放入超声仪中超声15~20 min,吸出肽段于新的EP管。
3.5.3质谱检测肽段用样品溶解液(0.1%甲酸和2%乙腈)溶解,4 ℃、13 200 r/min离心20 min,取上清,进行质谱鉴定。液相色谱为Dionex Ultimate 3000 RSLCnano(Thermo Scientific),与色谱相连的质谱仪为Q Exactive(Thermo Scientific)。
3.5.4数据库检索质谱原始文件经过MM File Conversion软件处理转换,得到MGF格式文件,然后用MASCO(http://www.matrixscience.com/)检索Uniprot数据库。
3.6生物信息学分析根据生物学意义,得到3组差异表达蛋白(differentially expressed proteins, DEPs):(1)生理状态下的DEPs:CHCHD2-OE组减去vector组;(2)氧化应激状态下的DEPs:CHCHD2-OE+TBHP组减去vector+TBHP组;(3)氧化应激损伤条件下特有的DEPs:仅存在于用氧化应激状态中而不存在于生理状态中的DEPs。进一步利用Fisher精确检验(单侧),分别对这些蛋白进行基于Gene Ontology (GO)和KEGG的功能富集分析,筛选所有值不超过设定阈值的功能节点和通路进行后续分析。同时,基于STRING数据库(https://string-db.org),得到不同分组中DEPs的蛋白质-蛋白质互作网络。
4 统计学处理
用SPSS 28.0软件进行统计学分析。数据均采用均数±标准误(mean±SEM)表示。多组间比较采用双因素方差分析(two-way ANOVA)。以<0.05为差异有统计学意义。
结果
1 CHCHD2保护SH-SY5Y细胞对抗氧化应激损伤
为了模拟神经退行性疾病中氧化应激损伤的病理状态,我们建立了外源性氧化应激诱导剂TBHP诱导人神经母细胞瘤SH-SY5Y细胞损伤的体外细胞模型。接下来为了明确CHCHD2是否具有保护人神经细胞对抗氧化应激损伤的作用,CHCHD2标签质粒及对照标签质粒被转染至SH-SY5Y细胞内。我们用Western blot检测4个分组中Flag标签蛋白的表达情况,结果显示CHCHD2-OE组及CHCHD2-OE+TBHP组均成功检测到Flag标签蛋白的表达;cleaved caspase-3是细胞凋亡的重要标志物,我们用Western blot检测了4个分组中cleaved caspase-3蛋白的表达情况,结果显示,相比于无药物组,药物组的cleaved caspase-3蛋白表达量均显著上升,但是相比于对照组,CHCHD2-OE实验组中cleaved caspase-3蛋白表达量均显著下降,见图1。该结果证明CHCHD2具有保护SH-SY5Y细胞对抗氧化应激损伤的作用。
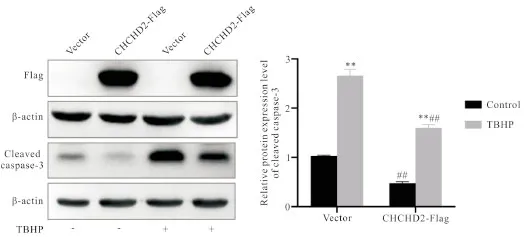
Figure 1.CHCHD2 protected SH-SY5Y cells against oxidative stress-induced neuronal damage. The levels of Flag tag protein and cleaved caspase-3 protein in SH-SY5Y cells were detected by Western blot. Mean±SEM. n=3. **P<0.01 vs control group; ##P<0.01 vs vector group.
2 CoIP实验
为了探索生理状态及氧化应激状态下CHCHD2可以与哪些蛋白相互作用,我们进行了CoIP实验。用Western blot检测总细胞蛋白液(Input)中及免疫沉淀(IP)洗脱液中诱饵蛋白CHCHD2的表达,结果显示在Input组中均可以检测到CHCHD2蛋白的表达,而在IP组中,仅CHCHD2-OE组及CHCHD2-OE+TBHP组可以检测到,见图2。这说明我们已经成功富集CHCHD2及其互作蛋白,可以进行后续质谱实验。
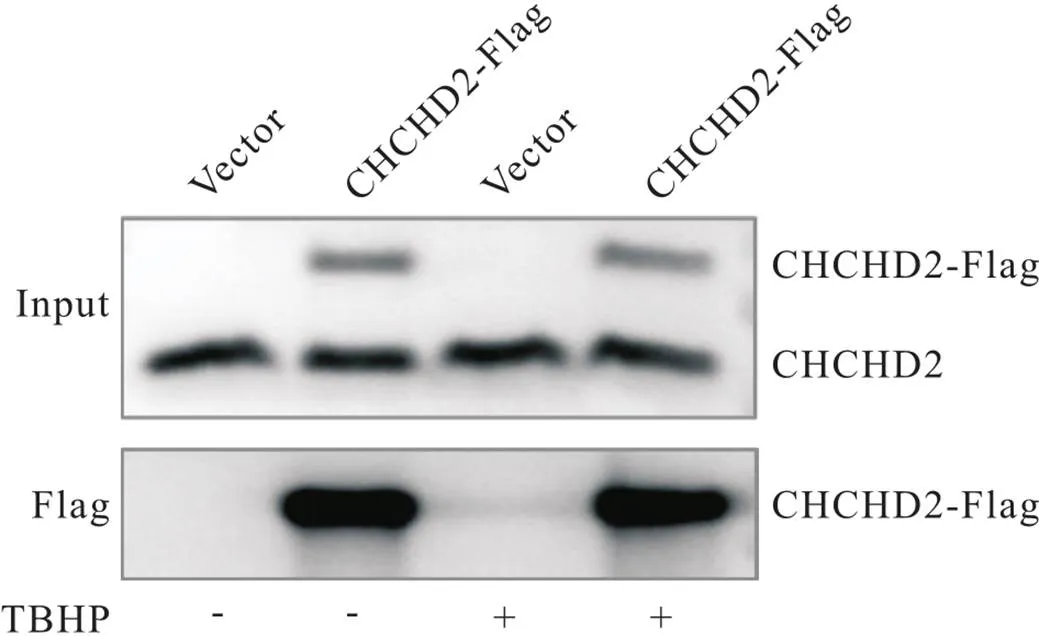
Figure 2.Co-immunoprecipitation assay. The expression of bait protein CHCHD2 in the Input (up) and IP elute (bottom) was detected by Western blot.
3 DEPs质谱结果
针对质谱结果,我们将DEPs分成以下4种情况(图3)进行初步分析;(1)生理状态组:用CHCHD2-OE组减去背景vector组,一共得到52个蛋白(表1),表明在生理状态下除CHCHD2本身外,共有51个蛋白在SH-SY5Y细胞中与CHCHD2功能存在联系;(2)氧化应激状态组:用CHCHD2-OE+TBHP组减去vector+TBHP组共得到75个蛋白(表2),表明存在74个CHCHD2相关蛋白在病理状态下参与了对抗外源性氧化剂损伤的氧化应激过程;(3)生理状态与氧化状态共有组:生理状态组与病理状态组取交集共得出11个共有蛋白可与CHCHD2相互作用(表3);(4)氧化应激状态特有组:在氧化应激状态下存在64个与CHCHD2相互作用的特有蛋白参与对抗氧化应激过程(表4)。
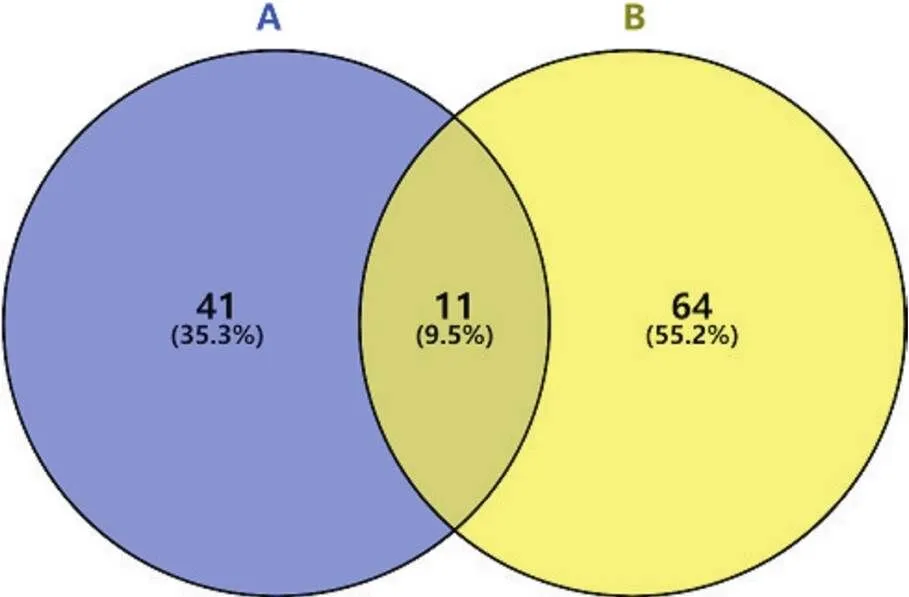
Figure 3.The distribution of differentially expressed proteins between physiological condition group (A) and oxidative stress condition group (B).
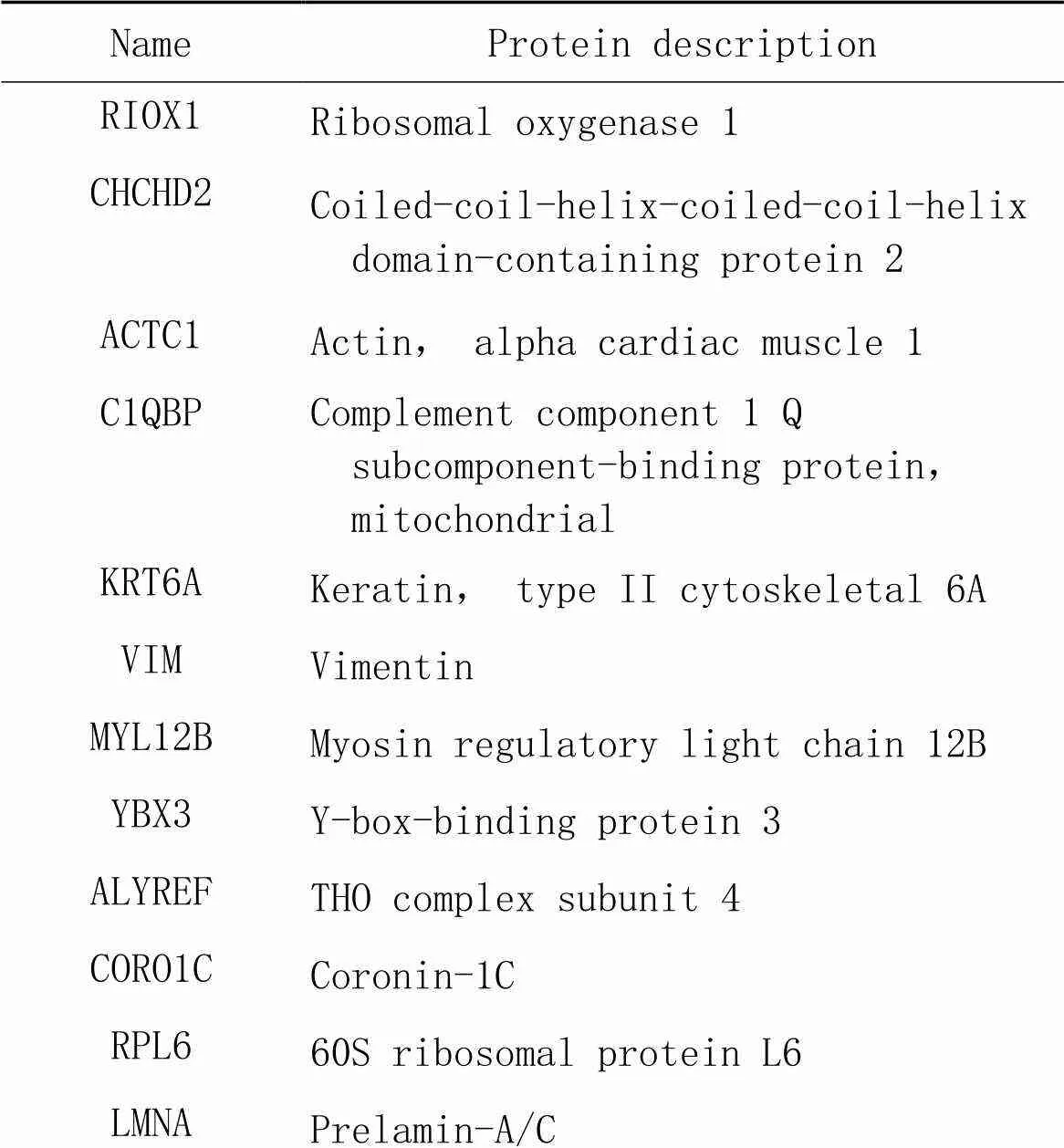
表1 生理状态组差异表达蛋白

RPS2440S ribosomal protein S24 KPNB1Importin subunit beta-1 RPS640S ribosomal protein S6 RPA1Replication protein A 70 kDa DNA-binding subunit ITIH2Inter-alpha-trypsin inhibitor heavy chain H2 POLDIP2Polymerase delta-interacting protein 2 CLPXATP-dependent Clp protease ATP-binding subunit clpX-like, mitochondrial MTHFD1C-1-tetrahydrofolate synthase, cytoplasmic RPS3A40S ribosomal protein S3a MAGED2Melanoma-associated antigen D2 LSM14BProtein LSM14 homolog B G3BP2Ras GTPase-activating protein-binding protein 2 U2AF2Splicing factor U2AF 65 kDa subunit GNAI2Guanine nucleotide-binding protein Gi subunit alpha-2 BAZ1ABromodomain adjacent to zinc finger domain protein 1A PRMT5Protein arginine N-methyltransferase 5 CHN1N-chimaerin FUSRNA-binding protein FUS RBM39RNA-binding protein 39 SPATA7Spermatogenesis-associated protein 7 KPNA2Importin subunit alpha-1 HBDHemoglobin subunit delta COL19A1Collagen type XIX alpha-1 chain

ARHGEF10LRho guanine nucleotide exchange factor 10-like protein HADHATrifunctional enzyme subunit alpha, mitochondrial RPL1960S ribosomal protein L19 SLC4A7Sodium bicarbonate cotransporter 3 VWFvon Willebrand factor CUL5Cullin-5 XRCC1DNA repair protein XRCC1 RBMXL1RNA binding motif protein, X-linked-like-1 LRRC59Leucine-rich repeat-containing protein 59 CDK13Cyclin-dependent kinase 13 GSNGelsolin RASIP1Ras-interacting protein 1 KLBBeta-klotho LAMA4Laminin subunit alpha-4 HNRNPA1Heterogeneous nuclear ribonucleoprotein A1 IPPActin-binding protein IPP SUCLA2Succinate-CoA ligase [ADP-forming] subunit beta, mitochondrial
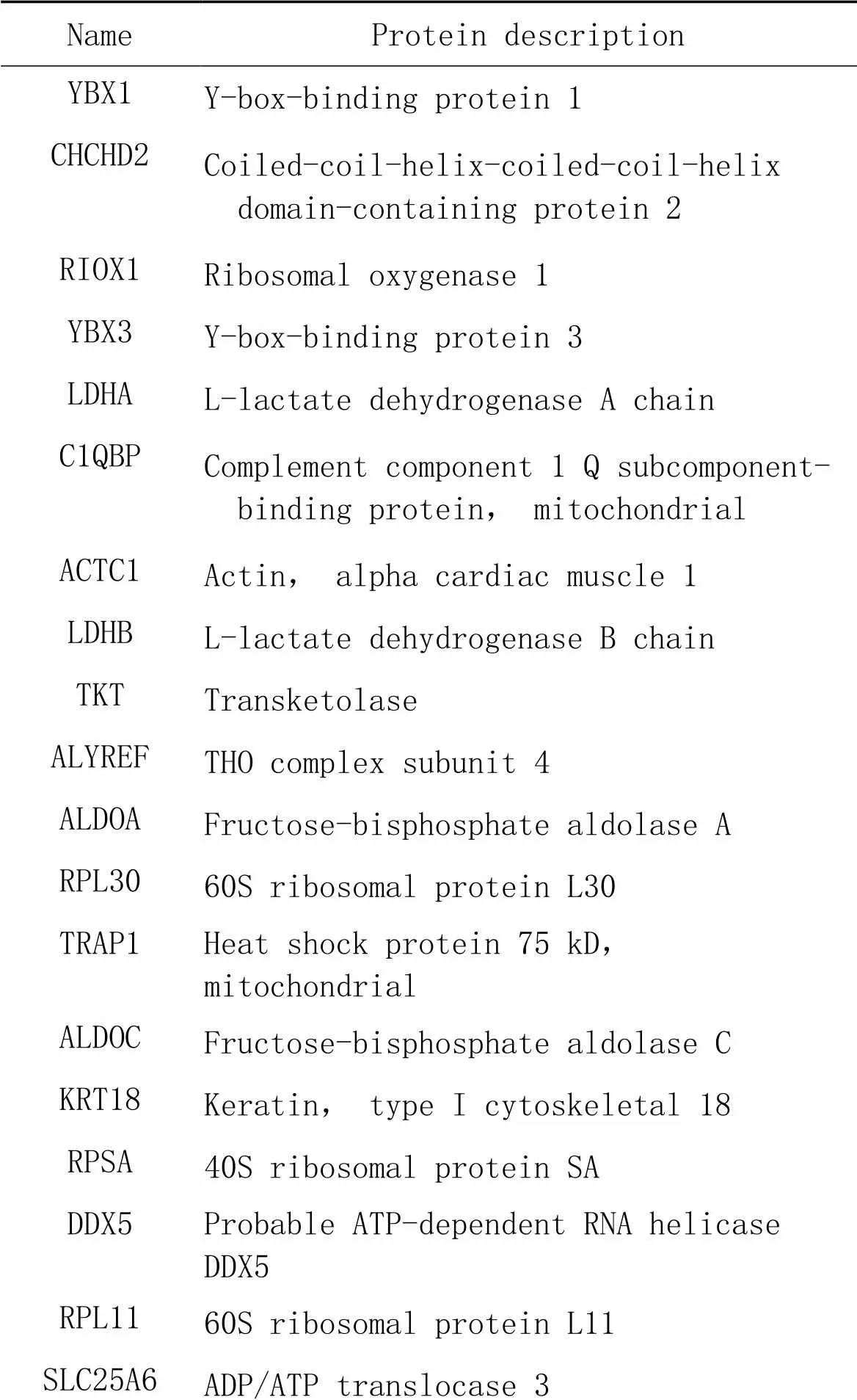
表2 氧化应激状态组差异表达蛋白
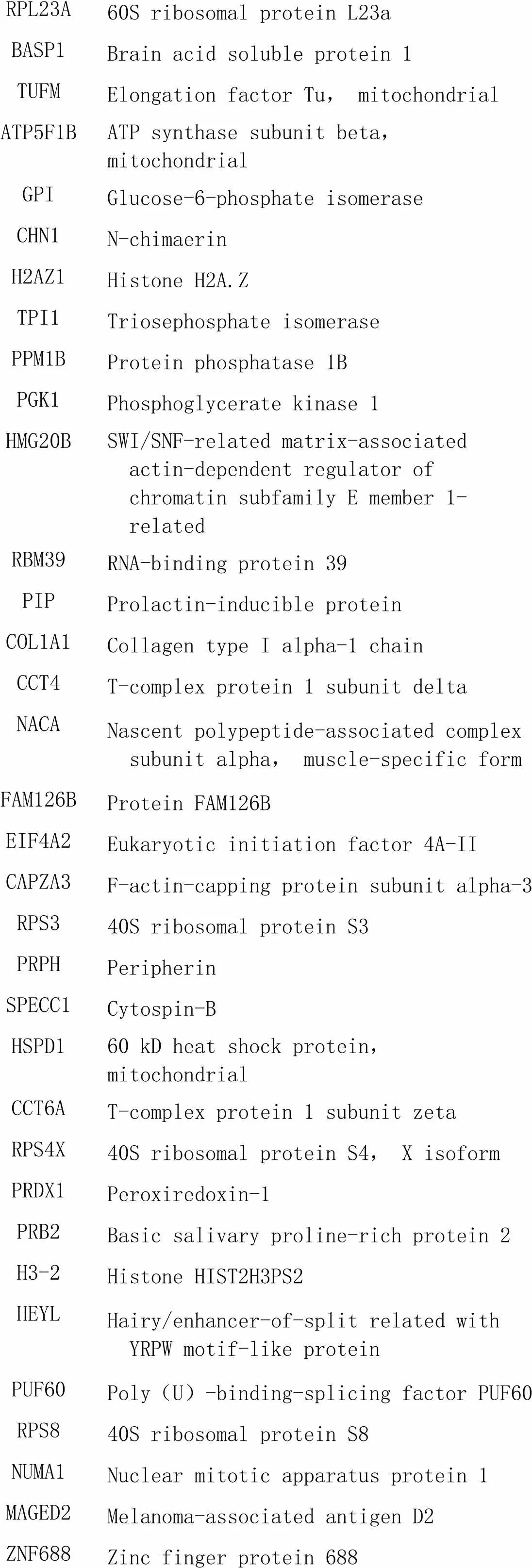
RPL23A60S ribosomal protein L23a BASP1Brain acid soluble protein 1 TUFMElongation factor Tu, mitochondrial ATP5F1BATP synthase subunit beta, mitochondrial GPIGlucose-6-phosphate isomerase CHN1N-chimaerin H2AZ1Histone H2A.Z TPI1Triosephosphate isomerase PPM1BProtein phosphatase 1B PGK1Phosphoglycerate kinase 1 HMG20BSWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1-related RBM39RNA-binding protein 39 PIPProlactin-inducible protein COL1A1Collagen type I alpha-1 chain CCT4T-complex protein 1 subunit delta NACANascent polypeptide-associated complex subunit alpha, muscle-specific form FAM126BProtein FAM126B EIF4A2Eukaryotic initiation factor 4A-II CAPZA3F-actin-capping protein subunit alpha-3 RPS340S ribosomal protein S3 PRPHPeripherin SPECC1Cytospin-B HSPD160 kD heat shock protein, mitochondrial CCT6AT-complex protein 1 subunit zeta RPS4X40S ribosomal protein S4, X isoform PRDX1Peroxiredoxin-1 PRB2Basic salivary proline-rich protein 2 H3-2Histone HIST2H3PS2 HEYLHairy/enhancer-of-split related with YRPW motif-like protein PUF60Poly(U)-binding-splicing factor PUF60 RPS840S ribosomal protein S8 NUMA1Nuclear mitotic apparatus protein 1 MAGED2Melanoma-associated antigen D2 ZNF688Zinc finger protein 688
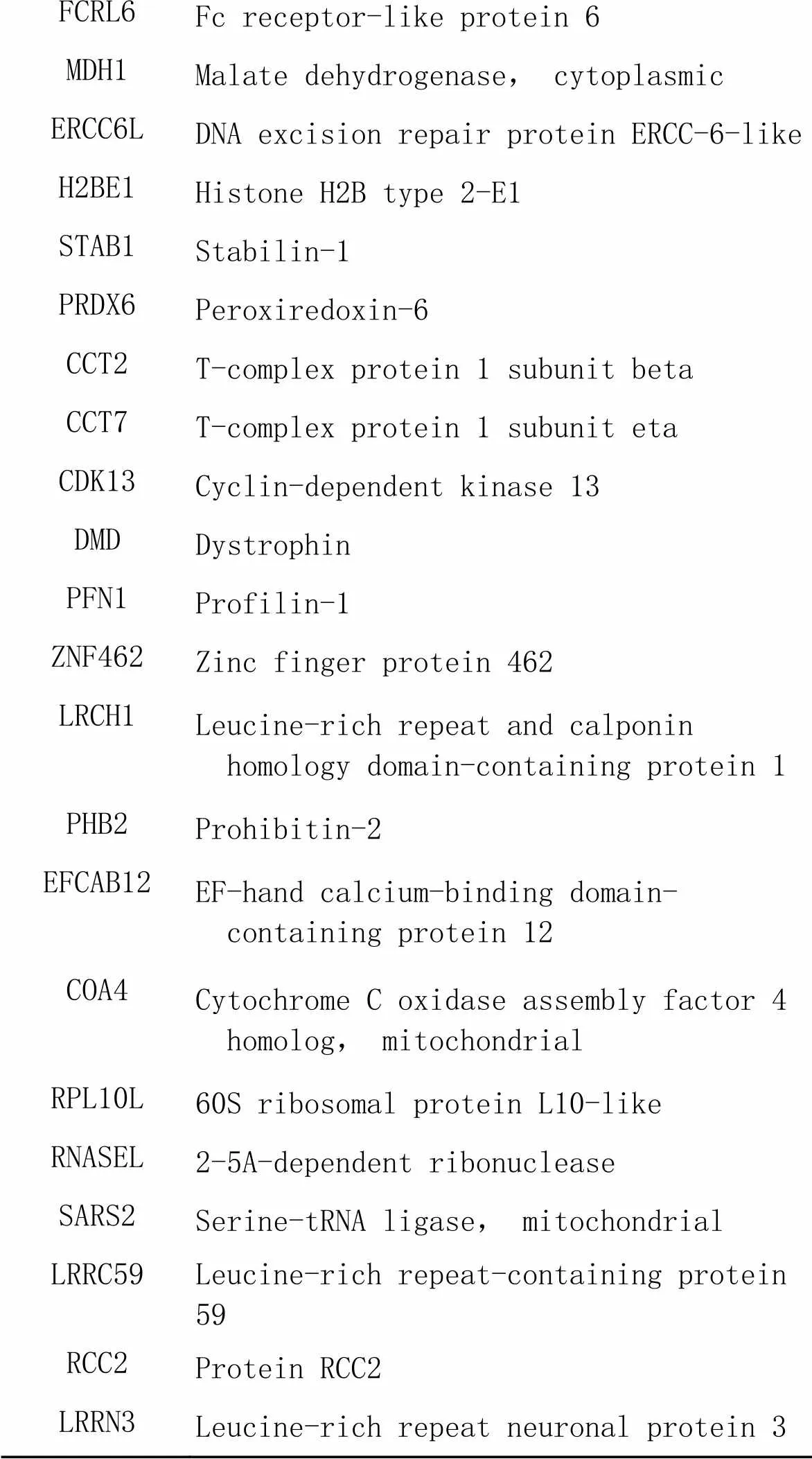
FCRL6Fc receptor-like protein 6 MDH1Malate dehydrogenase, cytoplasmic ERCC6LDNA excision repair protein ERCC-6-like H2BE1Histone H2B type 2-E1 STAB1Stabilin-1 PRDX6Peroxiredoxin-6 CCT2T-complex protein 1 subunit beta CCT7T-complex protein 1 subunit eta CDK13Cyclin-dependent kinase 13 DMDDystrophin PFN1Profilin-1 ZNF462Zinc finger protein 462 LRCH1Leucine-rich repeat and calponin homology domain-containing protein 1 PHB2Prohibitin-2 EFCAB12EF-hand calcium-binding domain-containing protein 12 COA4Cytochrome C oxidase assembly factor 4 homolog, mitochondrial RPL10L60S ribosomal protein L10-like RNASEL2-5A-dependent ribonuclease SARS2Serine-tRNA ligase, mitochondrial LRRC59Leucine-rich repeat-containing protein 59 RCC2Protein RCC2 LRRN3Leucine-rich repeat neuronal protein 3
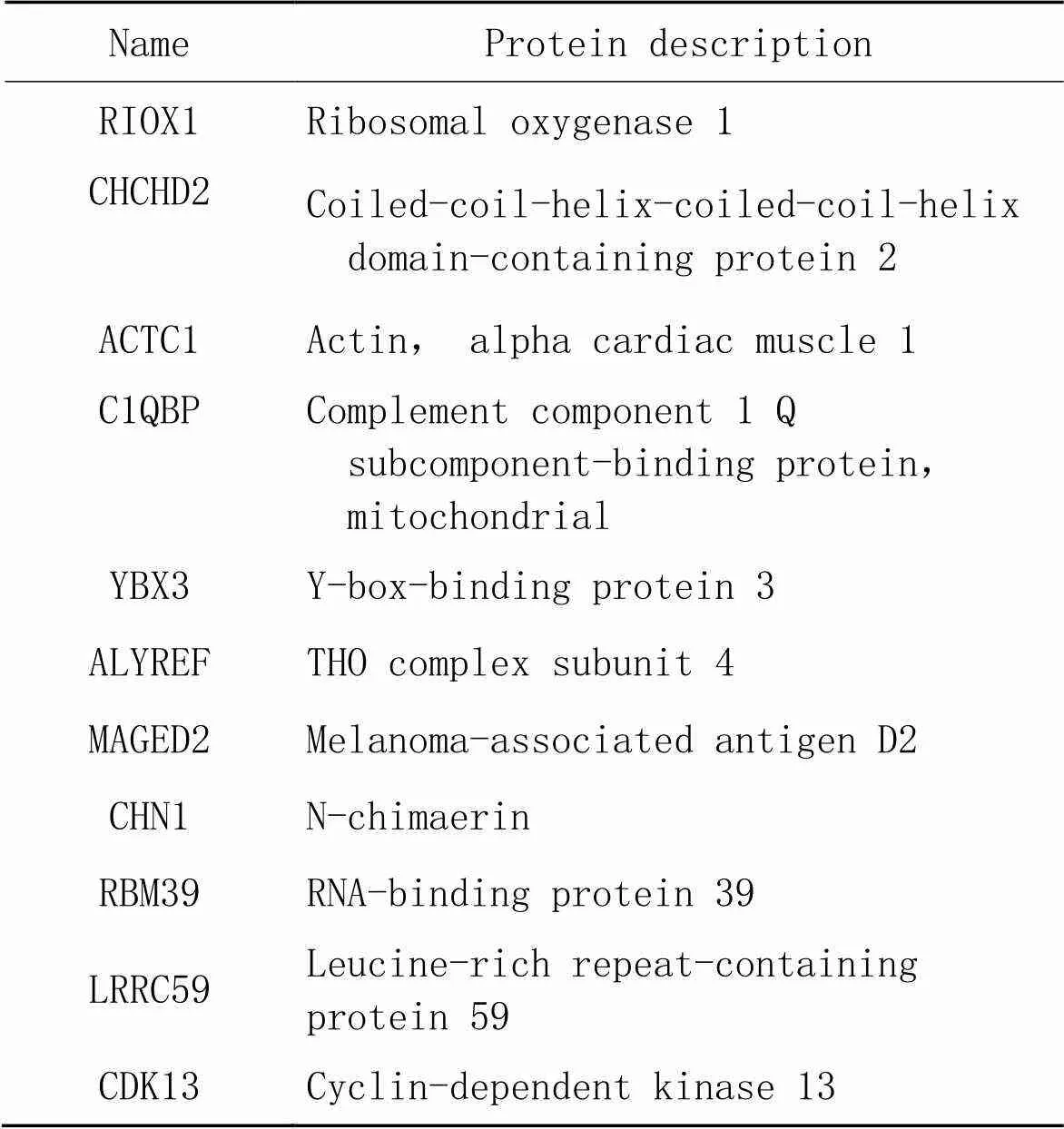
表3 生理状态组及氧化应激状态组共有差异表达蛋白
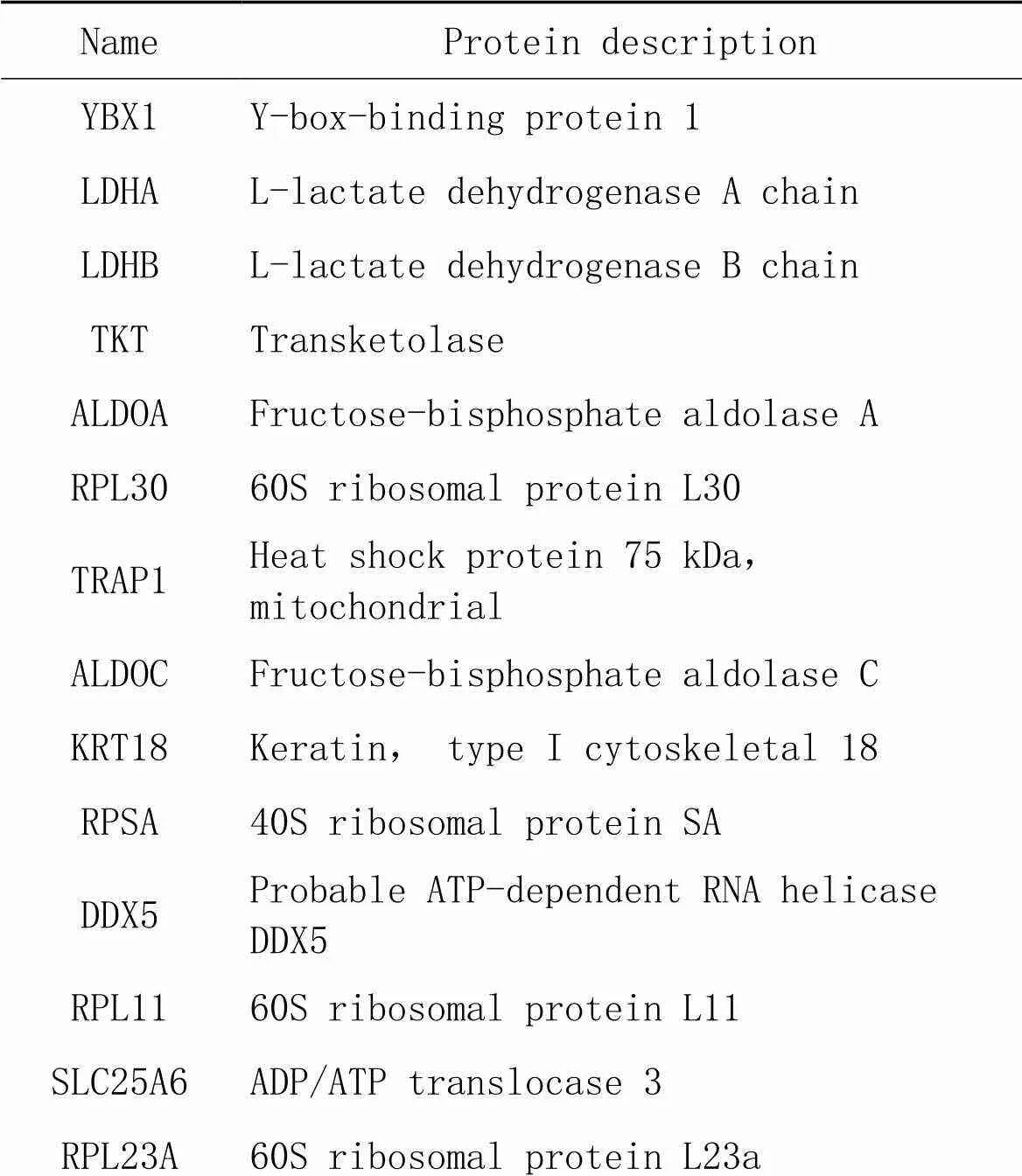
表4 氧化应激状态特有组差异表达蛋白
4 DEPs功能分类
为了挖掘CHCHD2通过哪些可能的信号通路途径来调控神经细胞对抗氧化应激损伤的作用,我们针对氧化应激状态下与CHCHD2互作的64个特有蛋白进行了广泛应用于基因或蛋白质功能注释与分析的GO分析。GO可分为三个子库,即生物学过程(biological process, BP)、细胞组分(cellular component, CC)和分子功能(molecular function, MF),旨在从不同角度描述和刻画蛋白所发挥的生物学作用。我们对这些DEPs在GO二级注释中的分布进行了统计和分析。首先,在BP层面,DEPs的功能主要集中在翻译(translation)、细胞黏附(cell-cell adhesion)、核糖体RNA加工(rRNA processing)、翻译起始(translational initiation)等。值得注意的是,对过氧化氢反应(response to hydrogen peroxide)及负性调控ROS生物合成过程(negative regulation of reactive oxygen species biosynthetic process)等抗氧化应激重要的生物过程被激活,提示抗氧化应激相关蛋白可能代偿性地表达增高,以维持细胞功能正常进行(图4A);其次,在CC层面,DEPs主要在外泌体(extracellular exosome)、细胞质基质(cytosol)、细胞核(nucleus)和细胞质(cytoplasm)中发挥作用(图4B),表明CHCHD2可直接或间接与多部位的不同蛋白一起发挥抗氧化应激的功能;最后,在MF层面,DEPs主要功能集中于与蛋白、poly(A) RNA及ATP结合,而且还涉及氧化还原酶活性(oxidoreductase activity)及过氧化物还原酶的活性(peroxiredoxin activity)等分子功能(图4C),提示氧化应激状态下CHCHD2可通过与抗氧化相关蛋白结合而发挥分子功能。
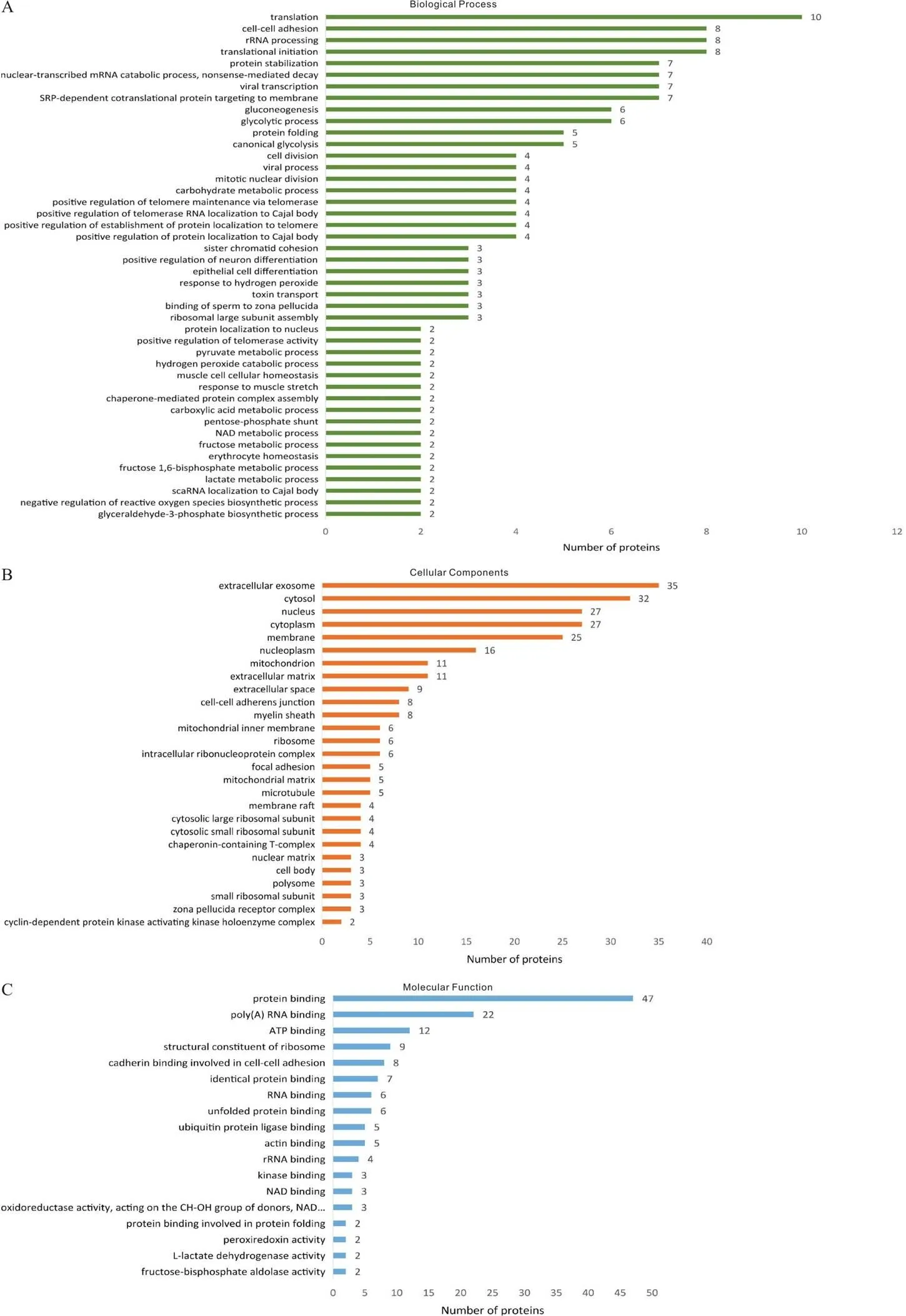
Figure 4.Functional classification of oxidative stress condition group-specific differentially expressed proteins by Gene Ontology (GO) analysis. A: biological process; B: cellular components; C: molecular function.
5 DEPs功能富集分析
我们对64个DEPs进行了GO分类和KEGG通路的富集分析,目的是挖掘DEPs是否在某些功能类型有显著性的富集趋势。对于富集分析(此处运用Fisher精确概率检验)得到的值通过气泡图方式展现了DEPs显著富集(<0.05)的功能节点和通路。气泡图中纵轴为功能节点或通路,横轴数值与圆圈颜色表示富集显著性[-log10(-value),值的负对数],圆圈大小表示功能分类或通路中DEPs个数。其中,GO富集中BP分析表明,DEPs主要参与糖酵解过程、翻译及翻译起始。此外,DEPs还参与了负性调控ROS生物合成过程和对过氧化氢的反应等抗氧化应激相关生物过程(图5A),其富集的主要功能蛋白为肿瘤坏死因子受体相关蛋白1(tumor necrosis factor receptor-associated protein 1, TRAP1)和热休克蛋白家族D成员1(heat shock protein family D member 1, HSPD1);MF显示,DEPs主要参与poly(A) RNA和蛋白的结合(图5B);CC显示,DEPs主要定位于外泌体、细胞质基质和细胞膜中(图5C)。在KEGG富集分析中,DEPs主要参与糖酵解/糖异生(glycolysis/gluconeogenesis)和磷酸戊糖途径(pentose phosphate pathway)等糖代谢过程(图5D)。根据富集分析我们推测在神经细胞发生氧化应激时,CHCHD2可能主要激活了糖代谢相关的抗氧化途径而发挥保护功能。首先,为了适应高ROS水平,细胞中葡萄糖代谢从有氧氧化转变为糖酵解,不仅可避免OXPHOS过程中ROS的进一步生成,且可以产生细胞代谢所需的能量。此外,细胞通过激活磷酸戊糖途径增加NADPH的生成,使细胞在高ROS水平下存活。最后,CHCHD2可能直接激活了与ROS生物合成过程的负调控以及对过氧化氢的响应等相关生物过程中的功能蛋白,从而直接发挥抗氧化应激功能。因此,参与CHCHD2对抗氧化应激损伤的DEPs具有多种分子功能,并参与多个生物学过程,表明CHCHD2保护神经细胞对抗氧化应激损伤是一个相对复杂的病理生理过程。
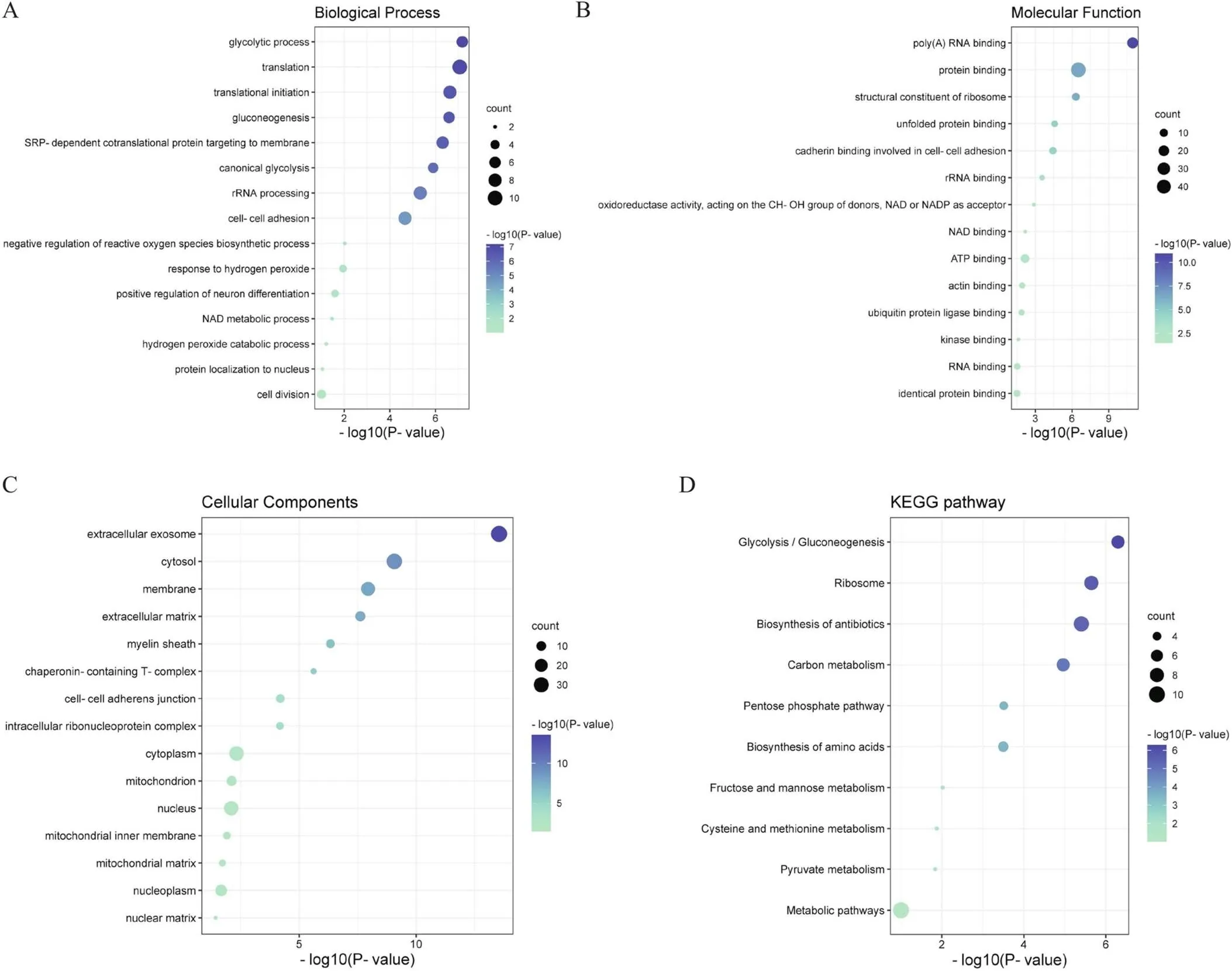
Figure 5.Functional enrichment analysis of oxidative stress condition group-specific differentially expressed proteins assessed by GO and KEGG pathway. A: GO biological process; B: GO molecular function; C: GO cellular components; D: KEGG pathway.
6 蛋白互作网络分析
为进一步分析DEPs间的相互作用,我们进行了基于STRING数据库的蛋白互作网络分析。具体而言,即将筛选得到的DEPs,通过与STRING 11.0蛋白网络互作数据库比对后,选择confidence score > 0.4(medium confidence)的互作关系,构建蛋白质-蛋白质互作网络并使用cytoscape 3.9.1软件绘制蛋白互作网络图。图6显示了生理状态DEPs、氧化应激状态DEPs及氧化应激状态特有DEPs的互作网络分析。其中,在氧化应激特有DEPs中Y盒结合蛋白1(Y-box-binding protein 1, YBX1)、含TCP1分子伴侣亚基6A(chaperonin containing TCP1 subunit 6A, CCT6A)和细胞色素C氧化酶装配因子4同源物(cytochrome C oxidase assembly factor 4 homolog, COA4)与CHCHD2有直接互作关系(图6C),表明这3个蛋白很可能特异性地参与了CHCHD2响应外源性氧化应激损伤的过程。
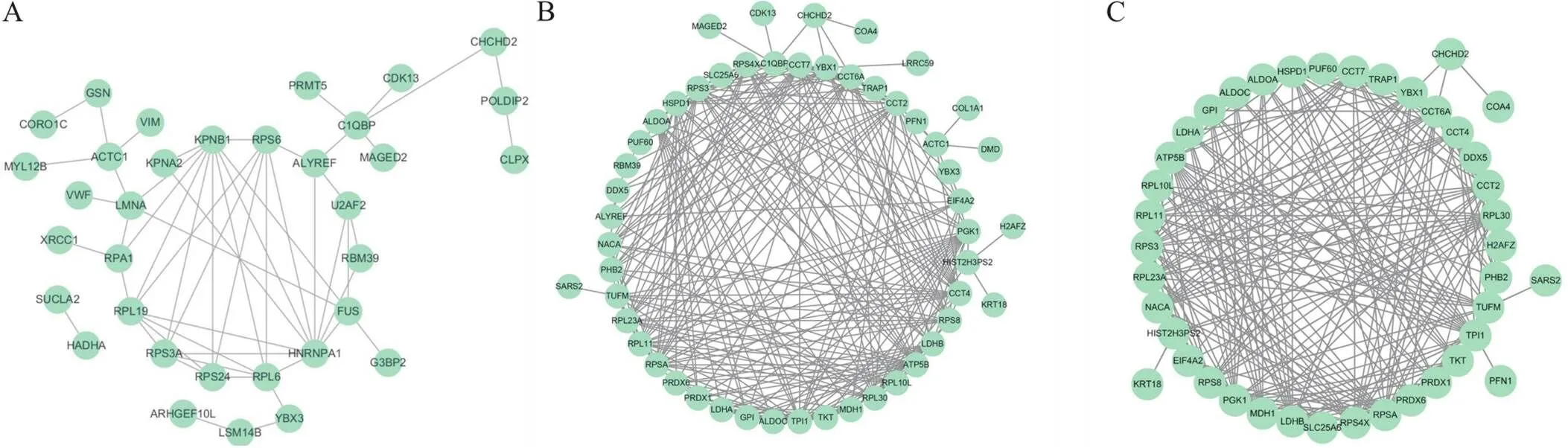
Figure 6.Interaction network analysis of differentially expressed proteins (DEPs) using STRING software. A: protein-protein interaction network (PPIN) analysis of DEGs in physiological condition group; B: PPIN analysis of DEGs in oxidative stress condition group; C: PPIN analysis of DEGs specially detected in oxidative stress condition group. In the network, nodes are proteins, and lines represent functional associations between proteins. The resulting networks were constructed with confidence scores higher than 4.
讨论
氧化应激是由于细胞和组织中ROS产生和消除间不平衡,进而导致ROS累积的一种现象。TBHP是一种ROS诱导剂,可刺激ROS过量产生,产生丙二醛,降低谷胱甘肽水平,诱导细胞凋亡,是一种常用的外源性氧化应激损伤诱导剂。大量证据表明,氧化应激在神经退行性疾病的发生和发展中可能发挥不同程度的重要作用[6]。因此,在本研究中,我们采用THBP诱导神经母细瘤SH-SY5Y细胞损伤来模拟神经退行性疾病中氧化应激损伤的病理状态,并着重挖掘抗氧化应激相关蛋白CHCHD2的互作蛋白。
CHCHD2是“CHCH”蛋白质家族成员之一,定位于线粒体和细胞核[3],其突变最先发现与PD相关[7],近年来被众多学者关注。CHCHD2与其他核编码的线粒体蛋白共同维持OXPHOS过程的稳定,而的敲除降低了复合体I和IV的活性[8],并增加了细胞内的ROS水平[3]。CHCHD2与Bcl-xL及细胞色素C共同定位于MICS1(线粒体形态和嵴结构),被认为是一种凋亡抑制因子[5, 9]。然而,CHCHD2在神经细胞中抗氧化应激功能是如何发挥作用的,目前仍不明确。为探索这一问题,我们采用CoIP-MS的方法对SH-SY5Y细胞中与CHCHD2相结合的蛋白进行了生物信息学分析。
将生理状态组与氧化应激状态组取交集得到11个蛋白(表3),其中的补体C1q结合蛋白(complement C1q binding protein, C1QBP)已经被Wei等[10]验证与CHCHD2存在蛋白互作关系,且其生物学功能与ROS的生成及氧化应激过程密切相关。C1QBP又称作p32、gC1qR或HABP-1,对OXPHOS起关键作用,被独立鉴定为人类mRNA剪接因子SF2的一个亚基[11-12]。已有研究表明,p32的主要功能之一是通过调节线粒体蛋白的翻译来维持线粒体的功能[13-14]。除了作为线粒体功能的调节器外,p32还与定位于细胞表面、细胞核、细胞质或细胞外空间的各种蛋白相互作用[15]。绵羊成肌细胞中基因的敲除有效抑制了成肌细胞的分化和增殖,并促进了细胞凋亡;的干扰也改变了绵羊成肌细胞的能量代谢,使其由OXPHOS转变为糖酵解,并激活了AMPK磷酸化[16]。另有报道称,p32与C1q球状头部的结合抑制了经典途径的补体激活[17]。如前所述,我们推测C1QBP可能与CHCHD2介导的抗氧化应激过程高度相关。
在针对氧化应激状态特有DEPs的GO富集分析中,我们发现CHCHD2激活了负性调控ROS生物合成过程,主要富集TRAP1与HSPD1两个蛋白。TRAP1又称热休克蛋白75(heat shock protein 75, HSP75),主要存在于线粒体中。作为线粒体分子伴侣,TRAP1支持蛋白质折叠,并有助于维持线粒体的完整性。TRAP1是一种细胞调节器,在线粒体生物能量学,氧化还原稳态,氧化应激诱导的细胞死亡、细胞凋亡和内质网未折叠蛋白反应中均发挥作用[18]。TRAP1对线粒体功能障碍有保护作用,TRAP1过表达可减少ROS的产生和积累,从而降低氧化应激损伤[19-21]。而沉默导致氧化应激敏感性增加,细胞总ROS水平与TRAP1表达水平呈负相关[19-20, 22-24]。先前的几项研究表明,在Warburg效应的基础上,TRAP1可诱导有氧糖酵解上调而减少ROS的产生,促进ROS清除剂NADPH的增多,从而发挥抗氧化、抗凋亡的功能[25-28]。多项研究表明,TRAP1与神经退行性疾病的发病密切相关,尤其在PD中。Fitzgerald等[29]报道了一例迟发性PD患者外显子2中存在纯合p.Arg47Ter单核苷酸交换(R47X),导致转运序列提前出现终止密码子和截短体。Cechetto等[30]及Masgras等[31]的研究表明,TRAP1在神经元中的特异性表达似乎足以抑制神经退化和肌肉退化,并逆转缺失或突变果蝇的呼吸缺陷。在表达α-synuclein[A53T]突变蛋白的果蝇中,敲减进一步加剧了多巴胺能神经元的丢失,而TRAP1的过表达可拮抗α-synuclein[A53T]突变蛋白在大鼠原代神经元和SH-SY5Y人神经细胞诱导的线粒体应激性损伤,表明TRAP1具有对抗氧化应激损伤的神经保护作用[32]。这些结果表明,TRAP1在神经退行性疾病及抗氧化应激过程中发挥重要作用。HSPD1又称作HSP60,主要在炎症相关过程中发挥病理生理功能。已有研究发现,HSP60在活化的小胶质细胞中高度表达,当其在细胞外释放时会诱导神经炎症发生并导致神经元细胞死亡[33],并于与多种神经退行性疾病的病理过程密切相关[34]。HSP60可以通过MyD88依赖途径与小胶质细胞表面的TLR4相互作用,并通过小胶质细胞LOX-1诱导促炎因子的产生,介导神经炎症[35-36]。鞘内注射HSP60通过激活小胶质细胞的TLR4/MyD88信号通路,导致神经退行性变和脱髓鞘[37]。HSP60可诱导小胶质细胞中ROS的生成,而基因的下调可降低体内和体外IL-1β的产生[38]。在肾透明细胞癌细胞中,敲除激活了NRF2介导的氧化应激反应,从而增加谷胱甘肽的产生,进一步抑制了快速增殖细胞中产生的ROS[39]。从AD患者分离的淋巴细胞中发现HSP60表达水平升高[40-41]。在小鼠模型中,Aβ-HSP60肽结合疫苗可诱导脑组织淀粉样蛋白降低且伴有大脑炎症反应的显著减少[42]。在PD患者的路易小体中,HSP60、HSP70及HSP90被发现与α-synuclein相互作用[43]。在体内和体外PD模型中,6-羟基多巴胺作用于多巴胺能神经元后,退化神经元释放HSP60以激活小胶质细胞,从而导致细胞中HSP60的表达水平逐渐降低[44]。因此,通过抑制HSP60的表达和释放来抑制神经炎症及伴随的氧化应激可能是一种适用于神经退行性疾病的治疗机制。如前所述,我们推测TRAP1和HSPD1可能与神经细胞中CHCHD2对抗氧化应激损伤及炎症反应过程密切相关,是我们需要关注的重点候选蛋白。
此外,通过比较氧化应激状态组与生理状态组的蛋白互作网络分析(图6),我们发现YBX1、CCT6A和COA4蛋白与CHCHD2有直接相互作用关系,并在氧化应激状态组中特异表达,且YBX1也已经被证实与CHCHD2存在直接相互作用[10]。YBX1是DNA和RNA结合蛋白家族中的成员之一,具有1个保守的冷休克结构域。YBX1参与多种DNA及RNA的相关过程,包括DNA修复、转录调控、前体mRNA剪接、mRNA包装以及翻译调控。在细胞水平上,YBX1可促进细胞增殖、抗凋亡、促进细胞分化、在多种应激情况下作为核转录因子发挥保护作用[45]。在肾细胞癌细胞中YBX1显著促进了细胞黏附,迁移和侵袭。YBX1通过Kindlin-2调节RCC细胞凋亡和ROS的产生[46]。人类端粒酶逆转录酶通过招募YBX1共定位于启动子P2区域来激活其启动子,从而上调NRF2的表达,促进大肠癌增殖和迁移[47]。外源性hsa-miR-760通过靶向编码区并与YBX1蛋白相互作用,有效地上调了Hmox1的表达,降低了ROS水平,并使PM2.5诱导的支气管上皮细胞免于凋亡[48]。CCT6A是CCT6的一个亚基,在多种癌症中起着至关重要的作用[49]。CCT6A可能通过转化生长因子β信号通路增强淋巴结转移,进而加速非小细胞肺癌的进展[49]。高表达的CCT6A通过维持细胞周期素D的表达以及加速第一个GAP期向合成期(G1~S)的转变,促进肝癌细胞的增殖[50]。此外,CCT6A已被鉴定为内源性细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)1/2信号复合物的特异成分[51],且具有丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)/ERK的磷酸化位点[52-53]。同时,CCT6A和MAPK信号通路在基质金属蛋白酶3依赖的轴突生长调控和神经元迁移中起关键作用[54]。COA4又称作CHCHD8,是CHCHD2的同源蛋白,COA4的缺失会导致COX的组装缺陷,尤其是COX1的缺失以及COX2和COX3的快速降解[55]。在本研究中,我们发现氧化应激状态下YBX1、CCT6A和COA4在CHCHD2过表达的SH-SY5Y神经细胞中特异性表达,提示这3个蛋白除已报道的功能外,还可能参与CHCHD2的抗氧化应激过程和神经保护作用,也是我们后续需要关注的候选蛋白。
综上所述,本研究首次证实CHCHD2具有保护SH-SY5Y神经细胞对抗外源性氧化应激损伤的作用;并且通过CoIP-MS的方法,我们共筛选出64个氧化应激状态下与CHCHD2发生互作的特有候选蛋白,并进一步通过生物信息学分析挖掘出与其抗氧化应激过程密切相关的2个蛋白(TRAP1和HSPD1)及另外3个可与其直接作用的蛋白(YBX1、CCT6A和COA4)作为后续重点关注及验证的候选蛋白。这些蛋白多与细胞对抗氧化应激、抗凋亡、细胞增殖和迁移等有一定的关系,我们推测这些蛋白在CHCHD2执行信号传递中发挥协同作用,但是它们与CHCHD2是否存在实际的互作关系,以及通过何种信号通路发挥功能,需要未来通过后续的实验进行进一步验证。最后,本研究结果为进一步深入探索CHCHD2抗氧化应激作用中的生物过程及分子机制奠定基础,为开发CHCHD2作为抗氧化应激损伤治疗靶点来治疗神经退行性疾病提供了新思路。
[1] Singh A, Kukreti R, Saso L, et al. Oxidative stress: a key modulator in neurodegenerative diseases[J]. Molecules, 2019, 24(8):1583.
[2] Kee TR, Espinoza Gonzalez P, Wehinger JL, et al. Mitochondrial CHCHD2: disease-associated mutations, physiological functions, and current animal models[J]. Front Aging Neurosci, 2021, 13:660843.
[3] Aras S, Bai M, Lee I, et al. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism[J]. Mitochondrion, 2015, 20:43-51.
[4] Liu Y, Zhang Y. CHCHD2 connects mitochondrial metabolism to apoptosis[J]. Mol Cell Oncol, 2015, 2(4):e1004964.
[5] Meng H, Yamashita C, Shiba-Fukushima K, et al. Loss of Parkinson's disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c[J]. Nat Commun, 2017, 8:15500.
[6] Jurcau A. Insights into the pathogenesis of neurodegenerative diseases: focus on mitochondrial dysfunction and oxidative stress[J]. Int J Mol Sci, 2021, 22(21):11847.
[7] Funayama M, Ohe K, Amo T, et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson's disease: a genome-wide linkage and sequencing study[J]. Lancet Neurol, 2015, 14(3):274-282.
[8] Baughman JM, Nilsson R, Gohil VM, et al. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis[J]. PLoS Genet, 2009, 5(8):e1000590.
[9] Liu Y, Clegg HV, Leslie PL, et al. CHCHD2 inhibits apoptosis by interacting with Bcl-xL to regulate Bax activation[J]. Cell Death Differ, 2015, 22(6):1035-1046.
[10] Wei Y, Vellanki RN, Coyaud É, et al. CHCHD2 is coamplified with EGFR in NSCLC and regulates mitochondrial function and cell migration[J]. Mol Cancer Res, 2015, 13(7):1119-1129.
[11] Krainer AR, Mayeda A, Kozak D, et al. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators[J]. Cell, 1991, 66(2):383-394.
[12] Honoré B, Madsen P, Rasmussen HH, et al. Cloning and expression of a cDNA covering the complete coding region of the P32 subunit of human pre-mRNA splicing factor SF2[J]. Gene, 1993, 134(2):283-287.
[13] Yagi M, Uchiumi T, Takazaki S, et al. p32/gC1qR is indispensable for fetal development and mitochondrial translation: importance of its RNA-binding ability[J]. Nucleic Acids Res, 2012, 40(19):9717-9737.
[14] Hillman GA, Henry MF. The yeast protein Mam33 functions in the assembly of the mitochondrial ribosome[J]. J Biol Chem, 2019, 294(25):9813-9829.
[15] Saha P, Datta K. Multi-functional, multicompartmental hyaluronan-binding protein 1 (HABP1/p32/gC1qR): implication in cancer progression and metastasis[J]. Oncotarget, 2018, 9(12):10784-10807.
[16] Ma J, Ren C, Yang H, et al. The expression pattern ofin sheep muscle and its role in differentiation, cell proliferation, and apoptosis of myoblasts[J]. Int J Mol Sci, 2019, 20(20):5161.
[17] Ghebrehiwet B, Lim BL, Peerschke EI, et al. Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular "heads" of C1q[J]. J Exp Med, 1994, 179(6):1809-1821.
[18] D'souza M, Datta K. Evidence for naturally occurring hyaluronic acid binding protein in rat liver[J]. Biochem Int, 1985, 10(1):43-51.
[19] Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis[J]. J Biol Chem, 2007, 282(28):20553-20560.
[20] Im CN, Lee JS, Zheng Y, et al. Iron chelation study in a normal human hepatocyte cell line suggests that tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates production of reactive oxygen species[J]. J Cell Biochem, 2007, 100(2):474-486.
[21] Zhang P, Lu Y, Yu D, et al. TRAP1 provides protection against myocardial ischemia-reperfusion injury by ameliorating mitochondrial dysfunction[J]. Cell Physiol Biochem, 2015, 36(5):2072-2082.
[22] Matassa DS, Amoroso MR, Agliarulo I, et al. Translational control in the stress adaptive response of cancer cells: a novel role for the heat shock protein TRAP1[J]. Cell Death Dis, 2013, 4(10):e851.
[23] Yoshida S, Tsutsumi S, Muhlebach G, et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis[J]. Proc Natl Acad Sci U S A, 2013, 110(17):E1604-E1612.
[24] Montesano Gesualdi N, Chirico G, Pirozzi G, et al. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis[J]. Stress, 2007, 10(4):342-350.
[25] Rasola A, Neckers L, Picard D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells[J]. Trends Cell Biol, 2014, 24(8):455-463.
[26] Sciacovelli M, Guzzo G, Morello V, et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase[J]. Cell Metab, 2013, 17(6):988-999.
[27] Chae YC, Caino MC, Lisanti S, et al. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s[J]. Cancer Cell, 2012, 22(3):331-344.
[28] Dekker FA, Rüdiger SGD. The mitochondrial Hsp90 TRAP1 and Alzheimer's disease[J]. Front Mol Biosci, 2021, 8:697913.
[29] Fitzgerald JC, Zimprich A, Carvajal Berrio DA, et al. Metformin reverses TRAP1 mutation-associated alterations in mitochondrial function in Parkinson's disease[J]. Brain, 2017, 140(9):2444-2459.
[30] Cechetto JD, Gupta RS. Immunoelectron microscopy provides evidence that tumor necrosis factor receptor-associated protein 1 (TRAP-1) is a mitochondrial protein which also localizes at specific extramitochondrial sites[J]. Exp Cell Res, 2000, 260(1):30-39.
[31] Masgras I, Laquatra C, Cannino G, et al. The molecular chaperone TRAP1 in cancer: from the basics of biology to pharmacological targeting[J]. Semin Cancer Biol, 2021, 76:45-53.
[32] Butler EK, Voigt A, Lutz AK, et al. The mitochondrial chaperone protein TRAP1 mitigates α-synuclein toxicity[J]. PLoS Genet, 2012, 8(2):e1002488.
[33] Sun Y, Zheng J, Xu Y, et al. Paraquat-induced inflammatory response of microglia through HSP60/TLR4 signaling[J]. Hum Exp Toxicol, 2018, 37(11):1161-1168.
[34] Alberti G, Paladino L, Vitale AM, et al. Functions and therapeutic potential of extracellular Hsp60, Hsp70, and Hsp90 in neuroinflammatory disorders[J]. Appl Sci, 2021, 11(2):736.
[35] Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS[J]. J Neurosci, 2008, 28(10):2320-2331.
[36] Zhang D, Sun L, Zhu H, et al. Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity[J]. Neurochem Int, 2012, 61(7):1021-1035.
[37] Rosenberger K, Dembny P, Derkow K, et al. Intrathecal heat shock protein 60 mediates neurodegeneration and demyelination in the CNS through a TLR4- and MyD88-dependent pathway[J]. Mol Neurodegener, 2015, 10:5.
[38] Swaroop S, Mahadevan A, Shankar SK, et al. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway[J]. J Neuroinflammation, 2018, 15(1):177.
[39] Teng R, Liu Z, Tang H, et al. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells[J]. Redox Biol, 2019, 24:101218.
[40] Cappello F, Angileri F, de Macario EC, et al. Chaperonopathies and chaperonotherapy. Hsp60 as therapeutic target in cancer: potential benefits and risks[J]. Curr Pharm Des, 2013, 19(3):452-457.
[41] Cappello F, Conway de Macario E, Marino Gammazza A, et al. Hsp60 and human aging: Les liaisons dangereuses[J]. Front Biosci (Landmark Ed), 2013, 18:626-637.
[42] Nemirovsky A, Fisher Y, Baron R, et al. Amyloid beta-HSP60 peptide conjugate vaccine treats a mouse model of Alzheimer's disease[J]. Vaccine, 2011, 29(23):4043-4050.
[43] Pemberton S, Melki R. The interaction of Hsc70 protein with fibrillar α-synuclein and its therapeutic potential in Parkinson's disease[J]. Commun Integr Biol, 2012, 5(1):94-95.
[44] Feng M, Zhang L, Liu Z, et al. The expression and release of Hsp60 in 6-OHDA induced in vivo and in vitro models of Parkinson's disease[J]. Neurochem Res, 2013, 38(10):2180-2189.
[45] Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation[J]. Wiley Interdiscip Rev RNA, 2014, 5(1):95-110.
[46] Cui Q, Wang C, Liu S, et al. YBX1 knockdown induces renal cell carcinoma cell apoptosis via Kindlin-2[J]. Cell Cycle, 2021, 20(22):2413-2427.
[47] Gong C, Yang H, Wang S, et al. hTERT promotes CRC proliferation and migration by recruiting YBX1 to increase NRF2 expression[J]. Front Cell Dev Biol, 2021, 9:658101.
[48] Xu L, Zhao Q, Li D, et al. MicroRNA-760 resists ambient PM2.5-induced apoptosis in human bronchial epithelial cells through elevating heme-oxygenase 1 expression[J]. Environ Pollut, 2021, 284:117213.
[49] Zhang T, Shi W, Tian K, et al. Chaperonin containing T-complex polypeptide 1 subunit 6A correlates with lymph node metastasis, abnormal carcinoembryonic antigen and poor survival profiles in non-small cell lung carcinoma[J]. World J Surg Oncol, 2020, 18(1):156.
[50] Zeng G, Wang J, Huang Y, et al. Overexpressing CCT6A contributes to cancer cell growth by affecting the G1-to-S phase transition and predicts a negative prognosis in hepatocellular carcinoma[J]. Onco Targets Ther, 2019, 12:10427-10439.
[51] Von Kriegsheim A, Baiocchi D, Birtwistle M, et al. Cell fate decisions are specified by the dynamic ERK interactome[J]. Nat Cell Biol, 2009, 11(12):1458-1464.
[52] Xiang B, Chatti K, Qiu H, et al. Brk is coamplified with ErbB2 to promote proliferation in breast cancer[J]. Proc Natl Acad Sci U S A, 2008, 105(34):12463-12468.
[53] Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation[J]. Pharmacol Res, 2012, 66(2):105-143.
[54] Van Hove I, Verslegers M, Hu TT, et al. A proteomic approach to understand MMP-3-driven developmental processes in the postnatal cerebellum: chaperonin CCT6A and MAP kinase as contributing factors[J]. Dev Neurobiol, 2015, 75(9):1033-1048.
[55] Bode M, Longen S, Morgan B, et al. Inaccurately assembled cytochrome c oxidase can lead to oxidative stress-induced growth arrest[J]. Antioxid Redox Signal, 2013, 18(13):1597-612.
Identification and preliminary functional analysis of CHCHD2 interacting proteins by CoIP-MS
LIU Xuan-zhuo, WANG Ying-ying, FAN Xin-man, WANG Fang, XU An-ding, XU Xiao-hong△
(,,510630,)
To screen the proteins interacting with coiled-coil-helix-coiled-coil-helix domain-containing 2 (CHCHD2) under oxidative stress, and to explore the underlying mechanism of CHCHD2 protecting against oxidative stress-induced neuronal damage.The control plasmid and the CHCHD2 overexpression plasmid containing Flag tags were transfected into human neuroblastoma cell line SH-SY5Y using Lipofectamine 2000. After treatment with 100 μmol/L tert-butyl hydroperoxide (TBHP) or ddH2O for 24 h, the CHCHD2-binding proteins in different groups were enriched by co-immunoprecipitation (CoIP). The proteins were concentrated by SDS-PAGE, and the bands in the gel were cut for further enzymolysis and analysis by liquid chromatography with tandem mass spectrometry (LC-MS/MS). The CHCHD2-interacting proteins were identified via database searching and bioinformatic analysis.(1) CHCHD2 played a role in protecting SH-SY5Y cells against THBP-induced oxidative stress injury. (2) The results of CoIP-MS showed that a total of 64 CHCHD2-interacting proteins were specifically detected under oxidative stress condition rather than physiological condition. (3) The results of GO function annotation and KEGG pathway enrichment showed that the differentially expressed proteins (DEPs) specifically detected in oxidative stress group mainly existed in the exosome and cytosol. These DEPs participated in biological processes including protein translation and initiation, protein and/or ploy(A) RNA binding, and glucose metabolism. (4) The DEPs were also involved in anti-oxidative stress-related biological processes including negative regulation of reactive oxygen species biosynthetic process, and response to hydrogen peroxide. Tumor necrosis factor receptor-associated protein 1 (TRAP1) and heat shock protein family D member 1 (HSPD1) were two important candidate proteins. (5) The results of protein-protein interaction network analysis showed that Y-box-binding protein 1 (YBX1), chaperonin containing TCP1 subunit 6A (CCT6A) and cytochrome C oxidase assembly factor 4 homolog (COA4) directly interacted with CHCHD2 under oxidative stress condition rather than physiological condition, and were also candidate proteins for further validation.The CHCHD2-interacting proteins under physiological condition and oxidative stress condition were successfully identified by CoIP-MS, and two proteins (TRAP1 and HSPD1) closely related to the anti-oxidative stress process, as well as three proteins (YBX1, CCT6A and COA4) directly interacted with CHCHD2 under oxidative stress condition were identified as top-candidate proteins. These findings support the necessity for further exploration of the molecular mechanism and biological process of CHCHD2 in its anti-oxidative stress effect.
CHCHD2 protein; Oxidative stress; Co-immunoprecipitation; Mass spectrometry; Neurodegenerative diseases
R741.02; R363
A
10.3969/j.issn.1000-4718.2022.03.010
1000-4718(2022)03-0457-14
2021-12-27
2022-02-21
[基金项目]国家自然科学基金资助项目(No. 81901295)
Tel: 020-38688071; E-mail: xiaohong_xu86@163.com
(责任编辑:卢萍,罗森)

