IL-6及GATA-6甲基化抑制剂在动脉型肺动脉高压大鼠肺组织中的作用及意义*
廖剑雄, 罗杰, 刘琳, 张谦△, 刘维佳△
IL-6及GATA-6甲基化抑制剂在动脉型肺动脉高压大鼠肺组织中的作用及意义*
廖剑雄1, 罗杰2, 刘琳3, 张谦1△, 刘维佳3△
(1贵州省人民医院急诊内科,贵州 贵阳 550002,2贵州大学医学院,贵州 贵阳 550003,3贵州省人民医院呼吸与危重症医学科,贵州 贵阳 550002)
通过野百合碱(MCT)诱导的大鼠动脉型肺动脉高压(PAH)模型研究给予白细胞介素6(IL-6)及GATA结合蛋白6(GATA-6)甲基化抑制剂后大鼠肺组织中IL-6、GATA-6及GATA-6甲基化的变化及意义。将40只成年雄性SD大鼠随机分成对照组(按60 mg/kg单次腹腔内注射2∶8的无水乙醇生理盐水混合液)、MCT组(1% MCT按60 mg/kg单次腹腔内注射)、MCT+二甲基亚砜(DMSO)组(等量1% MCT腹腔内注射后每周3次腹部皮下注射1 mg/kg DMSO)、MCT+IL-6抗体(anti-IL-6)组(等量1% MCT腹腔内注射后每天腹部皮下注射40 μg/kg anti-IL-6)和MCT+5-氮杂-2'-脱氧胞苷(5-Aza)组(等量1% MCT腹腔内注射后每周3次皮下注射1 mg/kg 5-Aza),每组8只。于造模后第21天做右心室压力监测,计算右心室肥厚指数,肺组织HE染色观察肺病理改变,免疫荧光及Western blot检测肺组织中IL-6和GATA-6的表达,甲基化特异性PCR(MSP)检测肺组织中GATA-6甲基化程度。与对照组比较,MCT组、MCT+anti-IL-6组和MCT+5-Aza组右心室平均压力显著升高(<0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组右心室平均压力显著降低(<0.05)。与对照组比较,MCT组右心室肥厚指数显著增高(<0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组右心室肥厚指数显著减低(<0.05)。IL-6免疫荧光及Western blot结果显示,与对照组比较,MCT组和MCT+5-Aza组IL-6表达显著增多(<0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组IL-6表达显著减少(<0.05)。GATA-6免疫荧光及Western blot结果显示,与对照组比较,MCT组和MCT+5-Aza组GATA-6表达显著减少(<0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组GATA-6表达显著增多(<0.05)。MSP结果显示,对照组肺组织中GATA-6未发生明显甲基化;MCT组及MCT+DMSO组GATA-6呈高度甲基化;MCT+anti-IL-6组及MCT+5-Aza组GATA-6呈低度甲基化。在PAH大鼠肺组织中给予anti-IL-6后IL-6的表达显著减少,给予5-Aza后GATA-6的表达显著增多,而GATA-6甲基化程度显著降低。IL-6的高表达可能是导致GATA-6低表达的原因,其机制可能是IL-6诱导了GATA-6的甲基化。
动脉型肺动脉高压;白细胞介素6;GATA结合蛋白6;5-氮杂-2'-脱氧胞苷
动脉型肺动脉高压(pulmonary arterial hypertension, PAH)是一种进行性加重而不可治愈的慢性肺部疾病,因其导致患者的生活质量差、住院率及死亡率高而成为近年来研究的热点[1]。PAH的发病机制较为复杂,现有研究显示炎症反应及肺动脉平滑肌细胞(pulmonary artery smooth muscle cell, PASMC)异常增殖为主要发病机制[2-3]。之前我们在PAH动物模型的研究已经证实白细胞介素6(interleukin-6, IL-6)及GATA结合蛋白6(GATA-binding protein-6, GATA-6)在上述发病机制中可能起到作用,同时发现肺组织中IL-6与GATA-6表达呈显著负相关,而GTAT-6持续低表达可能与GATA-6甲基化有关[4-5]。
DNA甲基化是表观遗传学的一种化学修饰形式,会导致靶基因沉默而抑制其表达,这个过程中DNA甲基转移酶(DNA methyltransferases, DNMTs)作为关键酶起到决定性作用[6]。5-氮杂-2'-脱氧胞苷(5-aza-2'-deoxycytidine, 5-Aza)是一种被FDA批准DNMTs抑制剂,它通过催化DNMT1去甲基化依赖蛋白酶体的形成来降解DNMT1,从而实现靶基因的去甲基化,临床常用于治疗骨髓增生异常综合征[7]。为此本研究以野百合碱(monocrotaline, MCT)诱发大鼠PAH模型,并以5-Aza和IL-6抗体(anti-IL-6)为抑制剂,通过检测大鼠肺组织中IL-6和GATA-6表达及GATA-6甲基化程度,探讨三者在PAH发展中的作用及关系。
材料和方法
1 实验动物和主要试剂
健康成年清洁级雄性SD大鼠40只,体重250~300 g,第三军医大学动物实验室提供并饲养,本研究已通过贵州省人民医院伦理委员会审核(编号:2018-001),所有操作均遵守实验动物伦理规范。大鼠笼盒饲养,保持环境温度25 ℃,湿度60%~70%,自由进食、饮水。所有动物实验均在贵州医科大学动物实验中心进行,许可证号:SYXK(黔)2018-0001。
MCT(Lot#41616)和5-Aza(Lot#29075)均购于MCE;IL-6 Ⅰ抗(GR3190303-16)和GATA-6 Ⅰ抗(GR297606-4)均购于Abcam;山羊抗兔Ⅱ抗(ZF-0316)购于北京中杉金桥生物有限公司;一步法快速WB(HRP)试剂盒(兔,20330;鼠,30324)均购于康为世纪公司;PageRuler™ Plus Prestained Protein Ladder(00459134)购于Thermo;Methylation-Gold Kit[D5005S(10)]购于ZYMO;DNA提取试剂盒(DP304)购于天根生化科技(北京)有限公司。
2 方法
2.1实验动物分组、模型建立及检测时点确认大鼠自由饲养1周后于造模前1 d用混合溶剂(无水乙醇∶生理盐水=2∶8)将MCT溶解、稀释,配成1% MCT溶液37 ℃过夜;造模当天用二甲基亚砜(dimethyl sulfoxide, DMSO)将5-Aza溶解、稀释,配成1 g/L的5-Aza溶液。采用随机数字表法将大鼠分成5组,即对照组(于造模当天按60 mg/kg单次腹腔内注射2∶8的无水乙醇生理盐水混合液)、MCT组(于造模当天1% MCT按60 mg/kg单次腹腔内注射)、MCT+DMSO组(于造模当天等量1% MCT腹腔内注射后每周3次腹部皮下注射1 mg/kg DMSO,持续3周)、MCT+anti-IL-6组(于造模当天等量1% MCT腹腔内注射后每天腹部皮下注射40 μg/kg anti-IL-6,持续3周)和MCT+5-Aza组(于造模当天等量1% MCT腹腔内注射后每周3次皮下注射1 mg/kg 5-Aza,持续3周),每组8只。记造模当天为第1天,于第21天[8]将大鼠做检测。
2.2右心导管法测右心室压力于第21天分别取各组大鼠8只,手术前禁食8 h,10%水合氯醛(0.04 mL/kg)腹腔注射麻醉,将PE10管预充肝素盐水采用右心导管法经右侧颈外静脉插管至右心室,用BL-420S生物信号采集分析系统(成都泰盟软件有限公司生产)检测右心室压力,并计算右心室平均压力。
2.4称重法计算右心室肥厚指数各组大鼠测完右心室压力后均断头处死开胸后完整取出心脏,剪去左右心房及心耳,在肺动脉出口处剪开右心室游离壁组织(RV),其余则为左心室(LV)+室间隔组织(S),洗去血污,吸干水分,然后分别称重,按公式[右心肥厚指数=RV/(LV+S)]计算。
2.5肺组织HE染色病理学检测检测右心室压力后,取肺组织用生理盐水通过肺动脉冲洗剩余的血液,分离右侧第三叶肺组织放入4%多聚甲醛固定,常规脱水、石蜡包埋、切片、HE染色、封片。在数码三目摄像显微镜(BA400Digital,麦克奥迪实业集团有限公司)下对切片进行图像采集及分析。
2.6免疫荧光法检测肺组织IL-6和GATA-6表达检测右心室压力后,生理盐水冲洗干净肺组织,取右侧第三叶肺组织放入4%多聚甲醛固定、常规脱水、包埋、切片、脱蜡、抗原修复后进行如下操作:滴加新生牛血清封闭液室温孵育30 min;分别滴加GATA-6 Ⅰ抗(1∶200)和IL-6 Ⅰ抗(1∶100)4 ℃过夜;滴加罗丹明标记的Ⅱ抗(山羊抗兔原液),37 ℃孵育30 min;滴加DAPI复染,室温孵育10 min;抗荧光衰减封片剂封片和镜检;采用荧光扫描显微镜摄像系统对切片进行图像采集;应用Image-Pro Plus 6.0图像分析系统将绿色/红色荧光单色照片转换为黑白图片然后选取相同的黑色作为判断阳性的统一标准,计算累积光密度和面积,并求出面密度,使用3张图像的平均面密度再计算平均数,得出每例样本的平均面密度。
2.7Western blot检测肺组织IL-6和GATA-6蛋白表达称取适量肺组织,RIPA裂解液与PMSF体积比按99∶1混匀,充分裂解组织,冰上孵育20 min后,10 000 r/min离心20 min,收集上清。根据BCA蛋白试剂盒测蛋白浓度,垂直电泳后,转膜2 h,封闭20 min,IL-6和GATA-6 Ⅰ抗(1∶1 000)4 ℃过夜,HRP鼠IL-6 Ⅱ抗和HRP兔GATA-6 Ⅱ抗(1∶200)室温孵育2 h,IL-6的内参照为β-actin(1∶200),GATA-6的内参照为histone H3(1∶200),ECL发光液化学发光,胶片曝光。采用Gel-Pro Analyzer 4图像分析软件测定各条带的灰度值并定量分析。
2.8甲基化特异性PCR(methylation-specific PCR, MSP)法检测肺组织GATA-6甲基化程度将肺组织处理为细胞悬液,按组织基因组DNA提取试剂盒说明步骤提取基因组DNA,根据Methylation-Gold Kit试剂合步骤对基因组DNA进行重亚硫酸盐修饰。设置反应体系为:10× PCR Buffer 2.5 μL,2.5 mmol/L dNTPs 2.5 μL,10 μmol/L引物2.0 μL,基因组DNA 3.0 μL,5 U/μL Taq HS 0.1 μL,ddH2O 14.9 μL。设置反应条件为:95 ℃预变性5 min;95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸30 s,共45个循环;最后72 ℃末段延伸5 min。所有引物均交由武汉金开瑞生物工程有限公司设计合成(表1)。结果经2%琼脂凝胶电泳显像,并观察条带情况,照相记录。
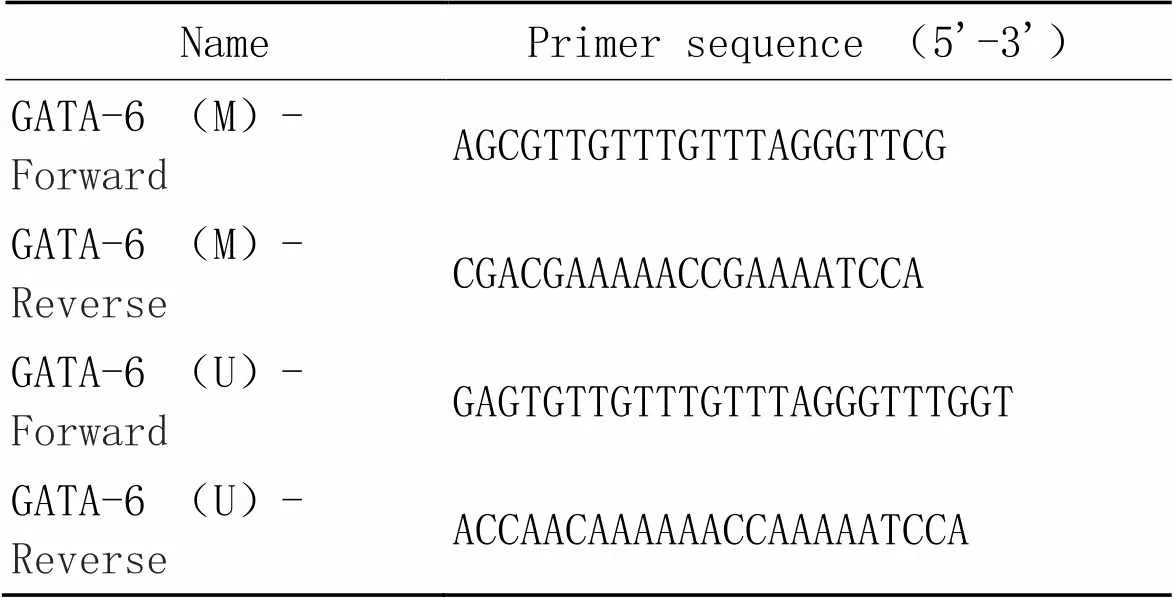
表1 甲基化特异性PCR的引物序列
M: methylated; U: unmethylated.
3 统计学处理
应用SPSS 20.0软件包进行统计分析。计量资料采用均数±标准差(mean±SD)表示。组间比较采用单因素方差分析,多重比较均采用LSD法。以<0.05为差异有统计学意义。
结果
1 各组大鼠右心室平均压力变化
与对照组比较,MCT、MCT+anti-IL-6和MCT+5-Aza组右心室平均压力显著升高(<0.05);MCT+DMSO组与MCT组比较无显著差异(>0.05);与MCT组比较,MCT+anti-IL-6和MCT+5-Aza组右心室平均压力显著降低(<0.05),见图1。
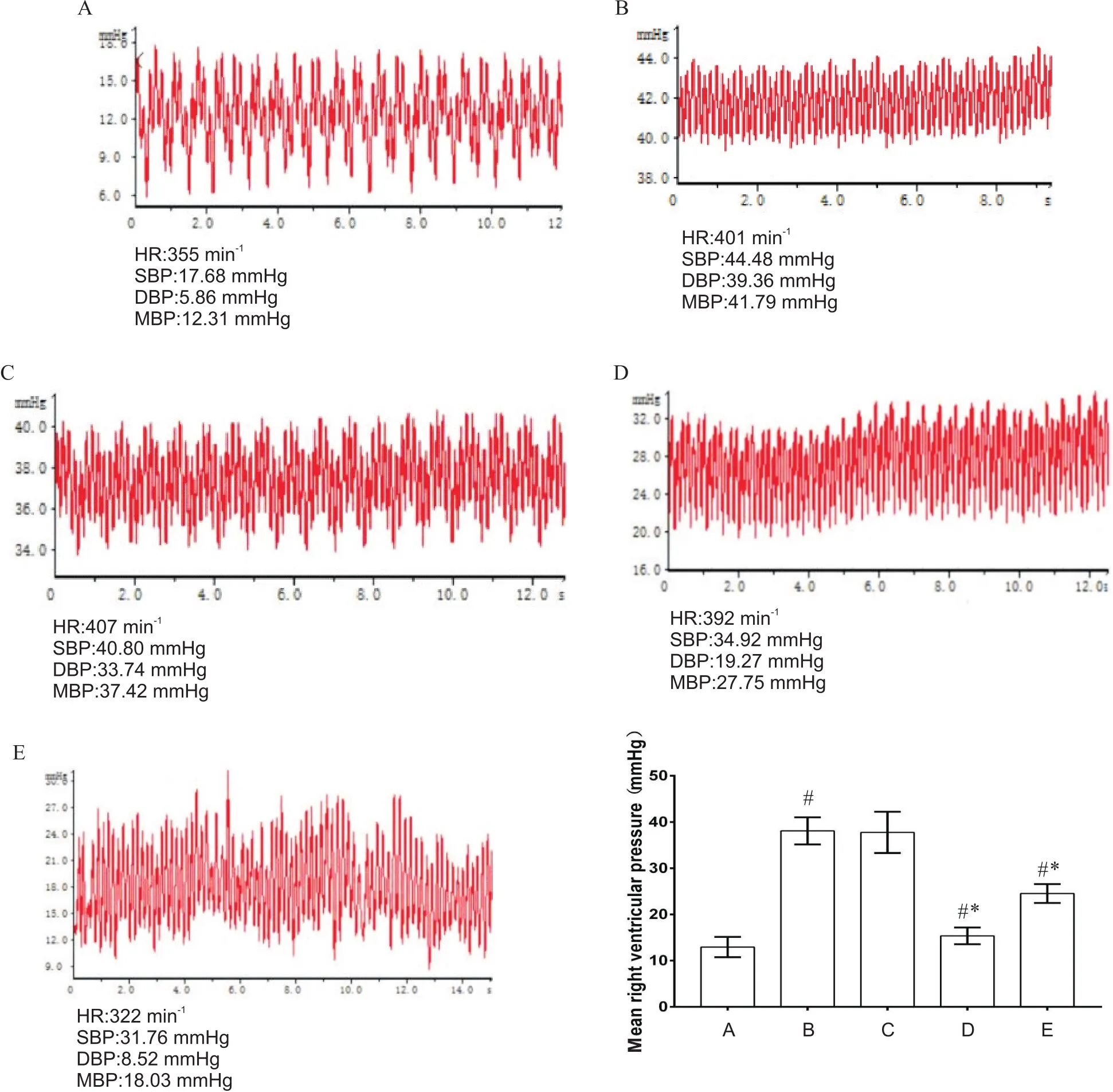
Figure 1.Comparison of mean right ventricular pressure in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group. Mean±SD. n=8. #P<0.05 vs A; *P<0.05 vs B.
2 各组大鼠右心室肥厚指数变化
与对照组比较,MCT组右心室肥厚指数显著增高(<0.05),MCT+anti-IL-6组和MCT+5-Aza组未见显著差异(>0.05);MCT+DMSO组与MCT组比较无显著差异(>0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组右心室肥厚指数显著减低(<0.05),见图2。
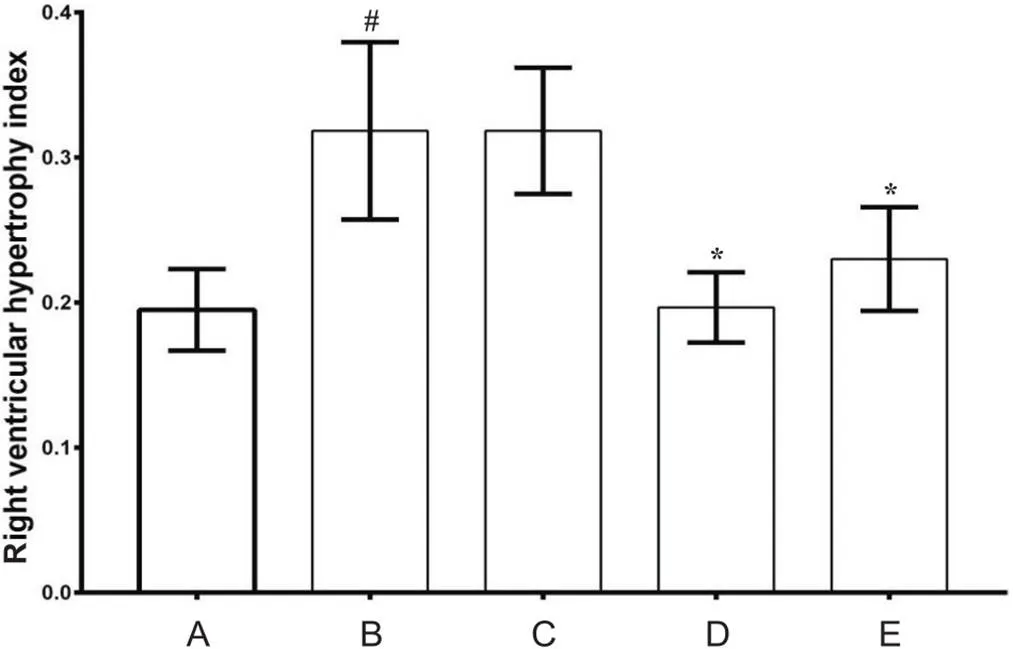
Figure 2.Comparison of right ventricular hypertrophy index in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group. Mean±SD. n=8. #P<0.05 vs A; *P<0.05 vs B.
3 各组大鼠肺组织HE染色光镜下病理改变
对照组各级动脉壁结构清晰,厚度正常,血管周围无明显炎症细胞浸润,肺泡内无明显巨噬细胞浸润;MCT组血管周围炎症细胞呈环状浸润,形成血管袖套(黑色箭头),平滑肌增生肥厚,管壁肌型化(红色箭头),组织小范围可见肺泡性水肿,大量肺泡内充满嗜酸性蛋白样物质(绿色箭头),并伴较多炎性细胞渗出(黄色箭头);MCT+DMSO组血管周围炎症细胞呈环状浸润,血管壁结构疏松,层次不清(红色箭头),组织可见肺泡性水肿,大量肺泡内充满嗜酸性蛋白样物质(绿色箭头),并伴较多炎症细胞渗出(黄色箭头);MCT+anti-IL-6组动脉管壁结构清晰,血管周围无炎症细胞浸润,少量动脉壁明显增厚,平滑肌增生肥厚,管壁肌型化(黑色箭头),管壁周围间隙增宽,伴蛋白样液渗出(红色箭头),少量肺泡内可见泡沫状巨噬细胞(黄色箭头);MCT+5-Aza组大量血管周围炎症细胞呈环状浸润,形成血管袖套(黑色箭头),局部肺泡内较多泡沫状巨噬细胞聚集(黄色箭头),见图3。
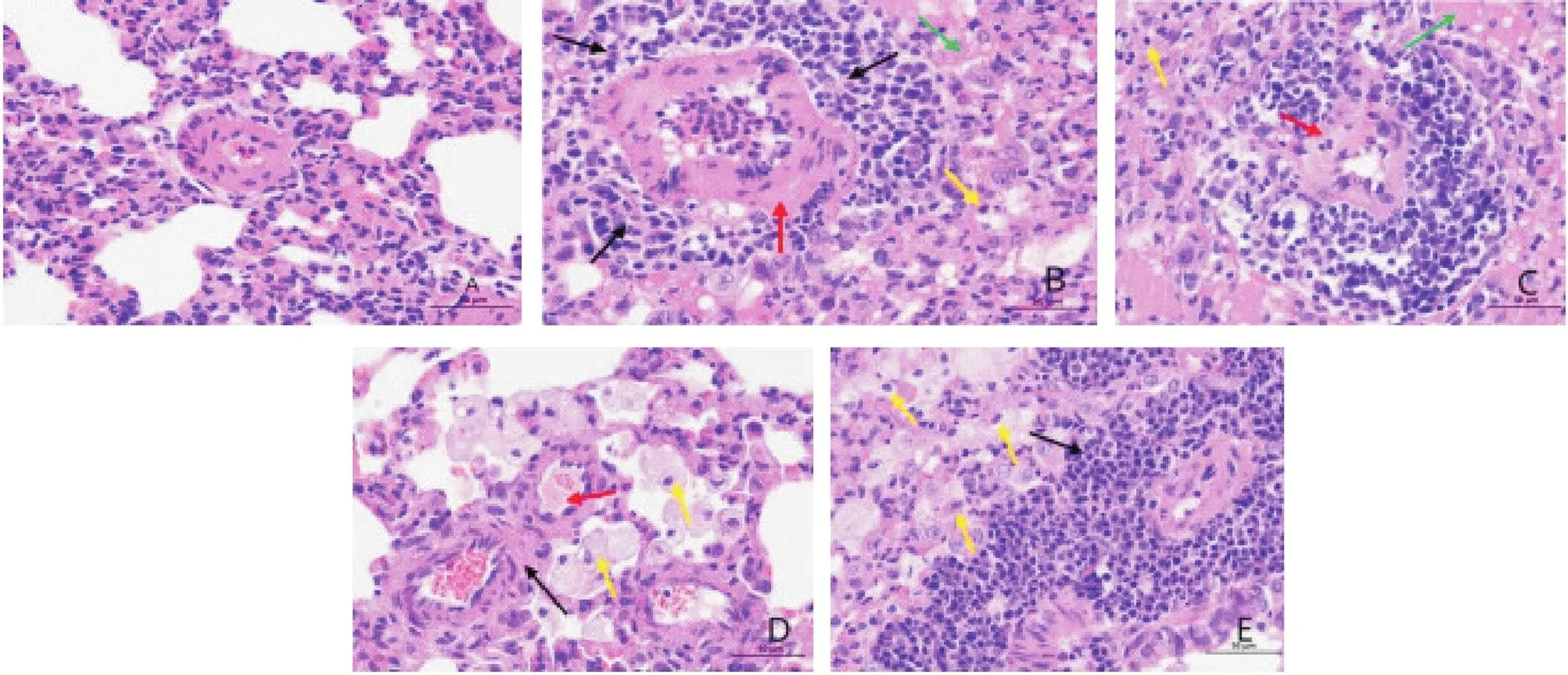
Figure 3.staining of Lung tissue in the mice of each group (HE staining, ×400). A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group.
4 各组大鼠肺组织IL-6表达
Western blot和免疫荧光结果均显示,与对照组比较,MCT组和MCT+5-Aza组IL-6的表达显著增高(<0.05),MCT+anti-IL-6组无显著差异(>0.05);MCT+DMSO组与MCT组比较无显著差异(>0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组IL-6的表达显著减低(<0.05),见图4、5。
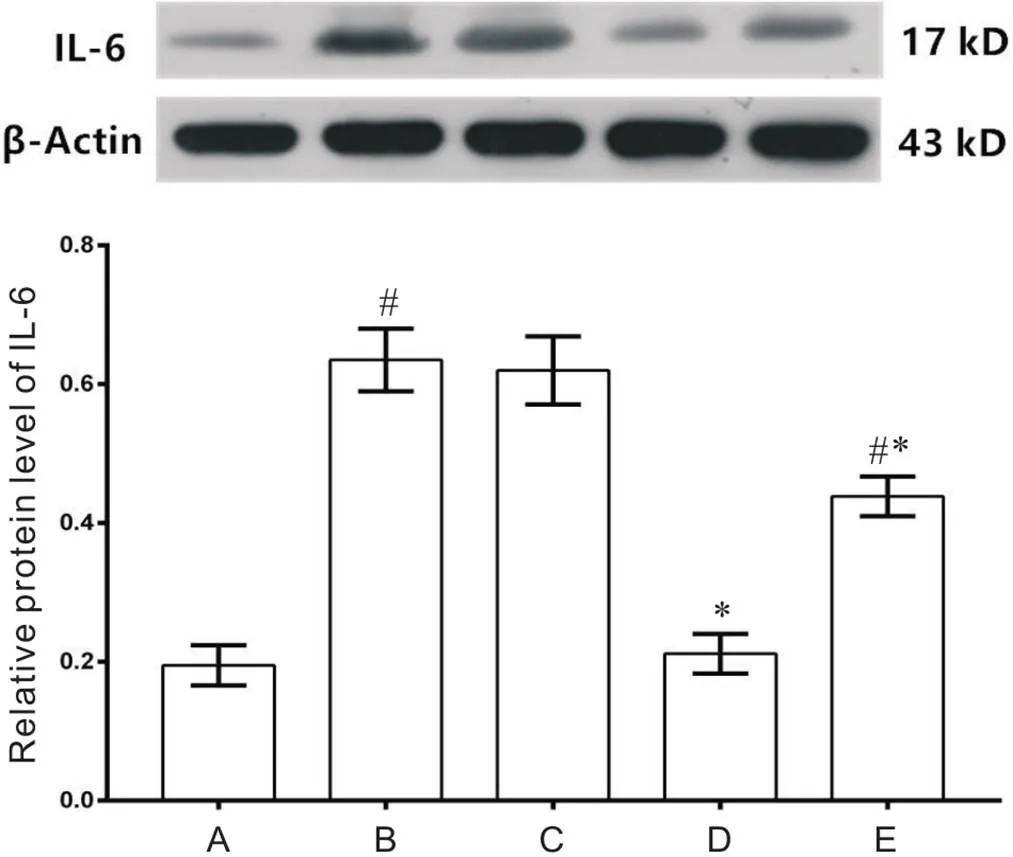
Figure 4.Western blot detection of IL-6 protein in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group. Mean±SD. n=8. #P<0.05 vs A; *P<0.05 vs B.
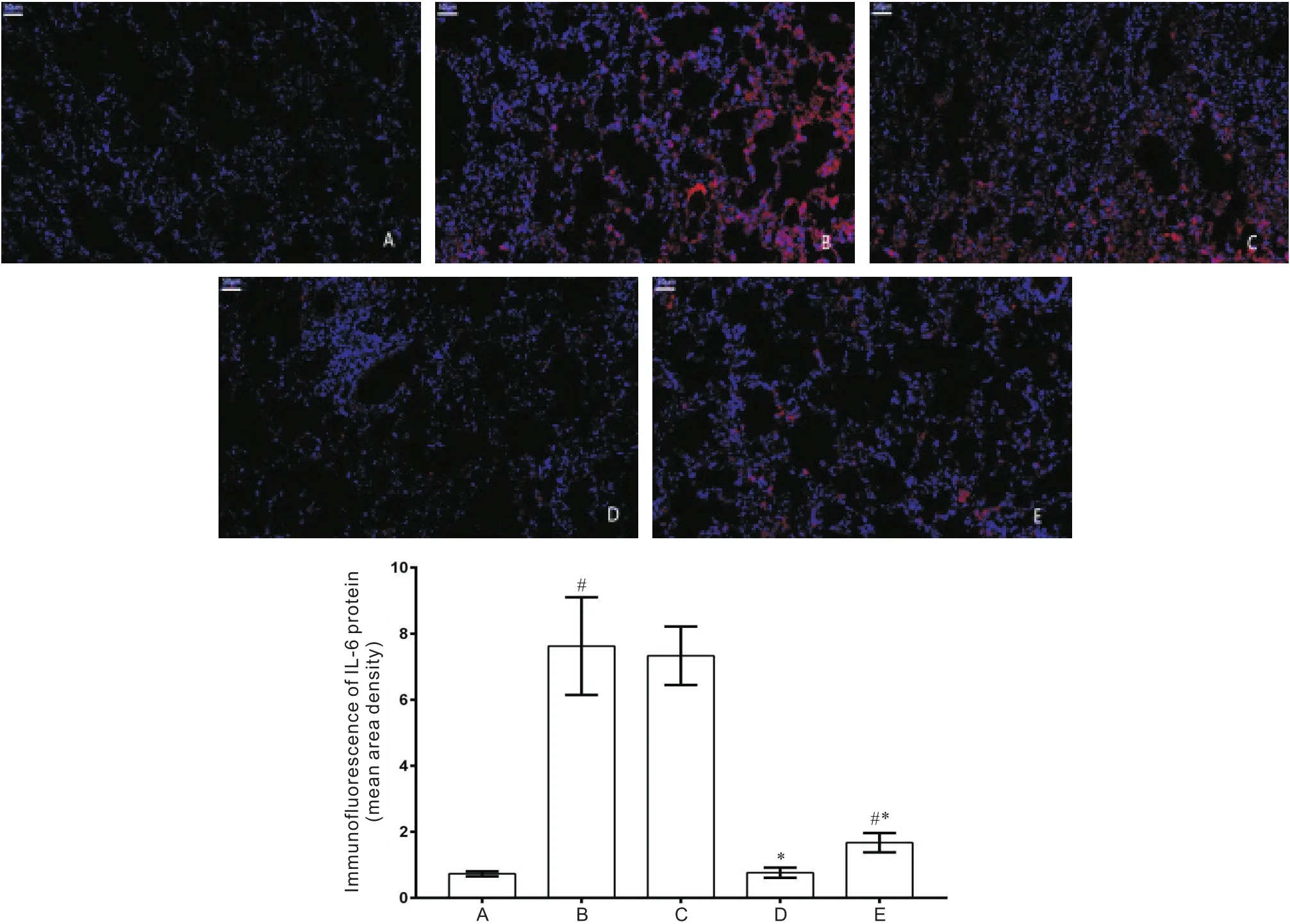
Figure 5.Immunofluorescence of IL-6 protein in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group.
5 各组大鼠肺组织GATA-6表达的免疫荧光结果
Western blot和免疫荧光结果均显示,与对照组比较,MCT组和MCT+5-Aza组GATA-6的表达显著减低(<0.05),MCT+anti-IL-6组无显著差异(>0.05);MCT+DMSO组与MCT组比较无显著差异(>0.05);与MCT组比较,MCT+anti-IL-6组和MCT+5-Aza组GATA-6的表达显著增高(<0.05),见图6、7。

Figure 6.Western blot detection of GATA-6 protein in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group. Mean±SD. n=8. #P<0.05 vs A; *P<0.05 vs B.

Figure 7.Immunofluorescence comparison GATA-6 of protein in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group. Mean±SD. n=8. #P<0.05 vs A; *P<0.05 vs B.
6 各组大鼠肺组织GATA-6甲基化结果
对照组大鼠肺组织中GATA-6未发生明显甲基化;MCT组及MCT+DMSO组大鼠肺组织中GATA-6呈高度甲基化;MCT+anti-IL-6组及MCT+5-Aza组肺组织中GATA-6呈低甲基化,与MCT组比较甲基化程度明显减弱,见图8。
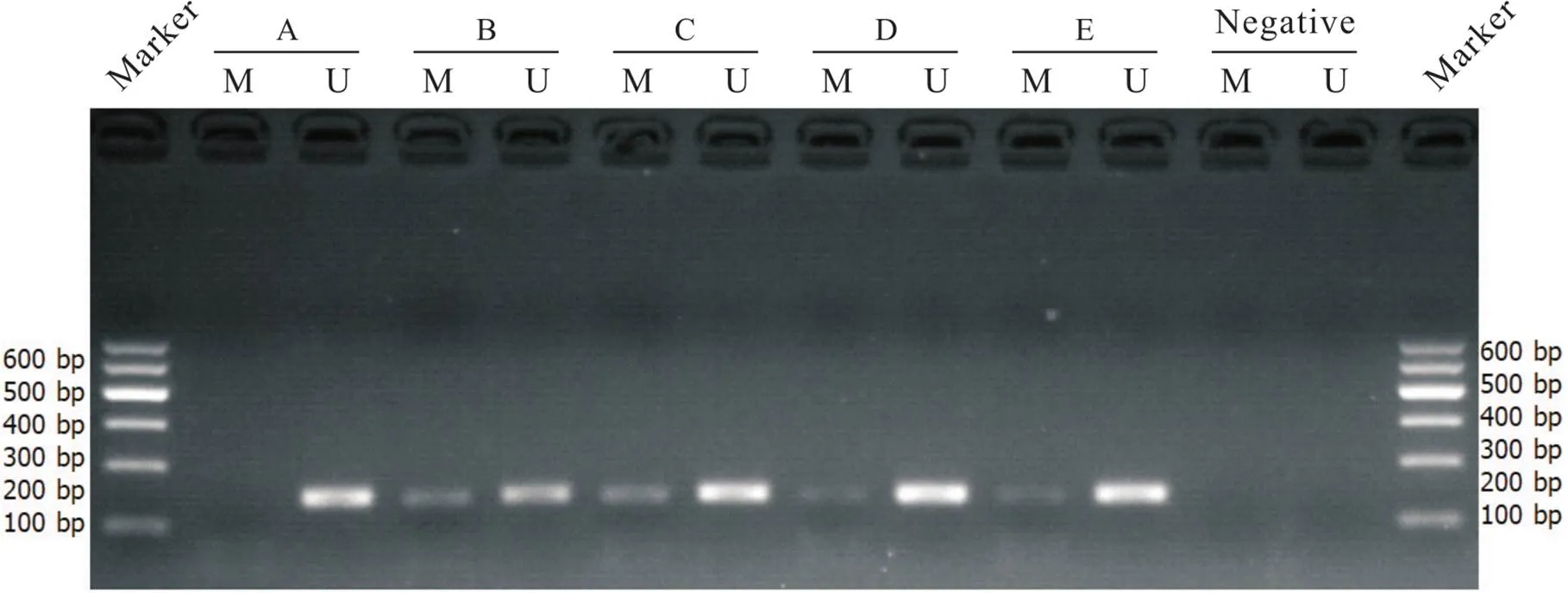
Figure 8.GATA-6 MSP electrophoretogram in the mice of each group. A: control group; B: MCT group; C: MCT+DMSO group; D: MCT+anti-IL-6 group; E: MCT+5-Aza group; M: methylated; U: unmethylated.
讨论
IL-6作为PAH发病过程中最重要的因子之一已被国内外大多学者认可,一方面IL-6通过激活信号转导及转录激活因子3(signal transducer and activator of transcription 3, STAT3)在Y705位点的磷酸化,导致细胞内DNA结合、二聚体形成、核易位来增强细胞收缩能力,延长细胞存活,从而促进PASMC增殖[9];另一方面IL-6可以干预骨形态发生蛋白(BMP)信号通路中BMP9的活性使得内皮细胞表型异常转化,从而导致内皮-间质转化(EndMT)过程中EndMT信号延长,其结果是细胞内促炎症反应信号持续加强(如TGF-β1、p-Smad2/3等)、细胞缺氧加重及凋亡加速,最终导致PASMC异常增殖[10-11]。一份来自美国国立卫生研究院肺动脉高压生物库(PAHB)和科罗拉多州儿童医院(CHC)共236位PAH患者的血清队列研究显示IL-6与患者的死亡率、肺移植的成功率、姑息性手术时机显著相关,此后该研究还通过对患者平均肺动脉压力、肺动脉楔压、右心室输出功率、左心输出量等血流动力学指标进行分析推测IL-6作为PAH的早期标记物能更早的预警PAH导致的心力衰竭,其敏感性甚至高于N末端B型利钠肽前体(NT-proBNP)[12-13]。同时Hernández-Sánchez等[14]的临床研究显示给予IL-6受体抑制剂后能有效降低肺动脉压力并改善PAH患者的生活质量,提示IL-6也是目前治疗PAH潜在研究靶点。我们的研究结果显示在PAH模型建立成功后MCT组肺组织HE染色有大量炎症细胞及肺水肿表现,且肺组织中IL-6的Western blot及免疫荧光结果显示与对照组比较显著增高,再次证实IL-6在PAH形成中发挥着重要作用,同时我们给予MCT+anti-IL-6组大鼠IL-6抑制剂后发现MCT+anti-IL-6组大鼠右心室平均压力、右心室肥厚指数、肺组织中IL-6的Western blot及免疫荧光表达较MCT组均显著降低,HE染色也显示给予IL-6抑制剂后肺组织炎症反应及肺水肿均明显好转,其结果与上述文献报道一致,也提示IL-6在PAH的发展中所涉及的机制可能是成为治疗PAH潜在靶点。
研究证实GATA-6作为锌指结构转录因子其高表达可以维持正常血管平滑肌细胞的稳定,但低表达则促进了VSMC的损伤,机制可能为损伤导致了GATA-6 的沉默,而GATA-6的沉默可通过内皮细胞-GATA-6-PDGF(血小板衍生的生长因子)-B通路诱导VSMC表型转换的失调来实现VSMC的损伤,所以推测GATA-6可能是发生VSMC异常增殖的主要靶点[15]。最新报道显示BMPR2突变可以引起PASMC增殖异常[16],但抑制人类直系同源物(TWIST1)则可逆转这一现象,主要原因为TWIST1的抑制可以促进GATA-6合成所依赖的结合蛋白释放,而GATA-6的大量合成则直接导致了BMPR2的表达受限(GATA-6被证实为BMPR2启动子区下游靶点)从而抑制了PASMC的异常增殖[17]。从Lentjes等[18]的综述中我们发现GATA-6作为胚体内胚层的代表基因可以促进心血管上皮细胞的分化及发育,并且能防止该类细胞的过分化,此后一项胚胎干细胞的研究显示GATA-6的沉默主要是GATA-6启动子区发生了甲基化的结果,而5-Aza作为去甲基化剂可以逆转GATA-6的沉默,恢复GATA-6的功能[19]。我们的研究在PAH模型建立成功后MCT组大鼠肺组织中Western blot及免疫荧光结果显示GATA-6表达较对照组均显著降低,而GATA-6的MSP结果提示对照组大鼠并未出现明显甲基化,而MCT组大鼠出现了高度的甲基化,提示PAH发生过程中GATA-6的低表达可能是因为GATA-6甲基化所致,在此基础上我们给予5-Aza处理MCT+5-Aza组大鼠发现该组大鼠右心室平均压力及右心室肥厚指数较MCT组显著降低,再次对MCT+5-Aza组肺组织进行Western Blot及免疫荧光检测发现GATA-6的表达较MCT组显著增高,MCT+5-Aza组GATA-6的MSP结果也显示甲基化程度较MCT组显著减弱,而且MCT+5-Aza组HE染色结果显示肺组织中炎症反应及肺水肿情况与MCT组比较明显改善,提示大鼠PAH有大幅度好转,与上述文献报道结果相似,再次证实GATA-6在PAH的发展中可能起到不可忽略的作用。
最后我们在PAH模型建立成功后给予IL-6抑制剂处理MCT+anti-IL-6组大鼠发现,与MCT组比较MCT+anti-IL-6组大鼠右心室平均压力及右心室肥厚指数显著降低(Western Blot及免疫荧光结果显示肺组织中IL-6的表达显著减低,而GATA-6的表达显著增加,HE染色结果也显示MCT+anti-IL-6组大鼠肺组织炎症反应与肺水肿较MCT组大幅度好转,同时GATA-6的MSP结果显示MCT+anti-IL-6组大鼠GATA-6呈低甲基化,再将MCT+anti-IL-6组大鼠右心室肥厚指数、肺组织中IL-6及GATA-6的表达与对照组比较甚至发现无统计学意义,而这些结果与MCT组大鼠肺组织检测结果恰恰相反。查阅相关文献我们发现关于IL-6诱导靶基因甲基化导致靶基因沉默的研究屡见不鲜,例如:(1)在肠预激综合征(IBS)的研究中发现IL-6增加了组蛋白H3赖氨酸9(H3K9)的甲基化,使得肠上皮细胞紧密连接蛋白(TJ)表达下调,导致细胞通透性增加而产生内脏痛觉过敏[20]:(2)脓毒症的相关研究显示对单核细胞中与脓毒症发病相关的全基因组进行甲基化分析发现随着IL-6水平的增加出现了2492个CpG位点高甲基化及909个CpG位点低甲基化[21];(3)在骨骼肌疾病的研究中发现IL-6通过增加DNMT的表达间接性的导致微小RNA142-3p(miR142-3p)启动子的高度甲基化,从而促进胶质母细胞瘤的生长[22]。所以结合本次研究结果,我们有理由怀疑PAH的形成中GATA-6的高度甲基化可能是高表达的IL-6诱导的,而该机制可能在PAH的发展过程中起到作用。
综上所述,在PAH大鼠肺组织中给予IL-6抗体后IL-6的表达显著减低,给予5-Aza后GATA-6的表达显著增高,而GATA-6甲基化程度显著减低;同时IL-6的高表达可能是导致GATA-6低表达的原因,其机制可能为IL-6诱导了GATA-6的甲基化,而该机制是否在PAH的发展中起到作用有望本研究的体内实验进一步明确。
[1] Gonzalez-Garcia MC, Fatehi F, Varnfield M, et al. Use of eHealth in the management of pulmonary arterial hypertension: review of the literature[J]. BMJ Health Care Inform, 2020, 27(3):e100176.
[2] Li TZ, Li SQ, Feng YL, et al. Combination of dichloroacetate and atorvastatin regulates excessive proliferation and oxidative stress in pulmonary arterial hypertension development via p38 signaling[J]. Oxid Med Cell Longev, 2020, 2020:6973636.
[3]张婷, 蔡海鉴, 杨林, 等. 积雪草苷通过抑制NF-κB/p38通路减轻小鼠低氧性肺动脉高压[J]. 中国病理生理杂志, 2019, 35(9):1600-1607.
Zhang T, Cai HJ, Yang L, et al. Asiaticoside attenuates hypoxic pulmonary hypertension in mice by inhibiting NF-κB/p38 signaling pathway[J]. Chin J Pathophysiol, 2019, 35(9):1600-1607.
[4]廖剑雄. 肺动脉高压大鼠肺组织中IL-6、GATA-6表达水平及GATA-6甲基化程度的意义[D]. 遵义: 遵义医科大学, 2020.
Liao JX. The significance of IL-6, GATA-6 and GATA-6 methylation in pulmonary tissues of rats with pulmonary arterial hypertension[D]. Zunyi: Zunyi Medical University, 2020.
[5]廖剑雄, 张一驰, 王炳今, 等. IL-6与GATA-6在肺动脉高压大鼠肺组织中的表达及意义[J]. 遵义医科大学学报, 2019, 42(5):534-538.
Liao JX, Zhang YC, Wang BJ, et al. Expression and significance of IL-6 and GATA-6 in pulmonary hypertension rats[J]. J Zunyi Med Univ, 2019, 42(5):534-538.
[6] Dahlet T, Argüeso LA, Ai AH, et al. Genome-wide analysis in the mouse embryo reveals the importance of DNA methylation for transcription integrity[J]. Nat Commun, 2020, 11:3153.
[7] Chen JY, Wu LX, Xu H, et al. 5-Aza-CdR regulates RASSF1A by inhibiting DNMT1 to affect colon cancer cell proliferation, migration and apoptosis[J]. Cancer Manag Res, 2019, 11:9517-9528.
[8] Guihaire J, Bogaard HJ, Flécher E, et al. Experimental models of right heart failure: a window for translational research in pulmonary hypertension[J]. Semin Respir Crit Care Med, 2013, 34(5):689-699.
[9] Kurahara LH, Hiraishi K, Yamamura A, et al. Eicosapentaenoic acid ameliorates pulmonary hypertension via inhibition of tyrosine kinase Fyn[J]. J Mol Cell Cardiol, 2020, 148:50-62.
[10] 张静静, 王岗, 李满祥, 等. 内皮-间充质转化在肺动脉高压发病机制中的研究进展[J]. 中国病理生理杂志, 2018, 34(9):1724-1728.
Zhang JJ, Wang L, Li MX, et al. Pathogenesis of endothelial-mesenchymal transition in pulmonary arterial hypertension[J]. Chin J Pathophysiol, 2018, 34(9):1724-1728.
[11] 张聪聪, 张晶晶, 武垣伶, 等. TGF-β/Smads通路参与EndoMT在HHPH中的作用及益气温阳活血化痰方的干预效应[J]. 中国病理生理杂志, 2018, 34(3):507-514.
Zhang CC, Zhang JJ, Wu HL, et al. Role of EndoMT in HHPH based on TGF-β/Smads signaling pathway and regulated by Yiqi-Wenyang-Huoxue-Huatan formula[J]. Chin J Pathophysiol, 2018, 34(3):507-514.
[12] Chen JY, Griffiths M,Yang J, et al. Elevated interleukin-6 levels predict clinical worsening in pediatric pulmonary arterial hypertension[J]. J Pediatr, 2020, 223:164-169.e1.
[13] Simpson CE, Chen JY, Damico RL, et al. Cellular sources of interleukin-6 and associations with clinical phenotypes and outcomes in pulmonary arterial hypertension[J]. Eur Respir J, 2020, 55(4):1971061.
[14] Hernández-Sánchez J, Harlow L, Church C, et al. Clinical trial protocol for TRANSFORM-UK: a therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension[J]. Pulm Circ, 2018, 8(1):2045893217735820.
[15] Zhuang T, Liu J, Chen XL, et al. Cell-specific effects of GATA (GATA zinc finger transcription factor family)-6 in vascular smooth muscle and endothelial cells on vascular injury neointimal formation[J]. Arterioscler Thromb Vasc Biol, 2019, 39(5):888-901.
[16] Happé C, Kurakula K, Sun XQ, et al. The BMP receptor 2 in pulmonary arterial hypertension: when and where the animal model matches the patient[J]. Cells, 2020, 9(6):1422.
[17] Fan Y, Gu X, Zhang J, et al. TWIST1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of GATA-6 and BMPR2[J]. Am J Respir Crit Care Med, 2020, 202(9):1283-1296.
[18] Lentjes MH, Niessen HEC, Akiyama Y, et al. The emerging role of GATA transcription factors in development and disease[J]. Expert Rev Mol Med, 2016, 18:e3.
[19] Diomede F, Zini N, Pizzicannella J, et al. 5-Aza exposure improves reprogramming process through embryoid body formation in human gingival stem cells[J]. Front Genet, 2018, 9:419.
[20] Wiley JW, Zong Y, Zheng G, et al. Histone H3K9 methylation regulates chronic stress and IL-6-induced colon epithelial permeability and visceral pain[J]. Neurogastroenterol Motil, 2020, 32(12):e13941.
[21] Lorente-Sorolla C, Garcia-Gomez A, Català-Moll F, et al. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis[J]. Genome Med, 2019, 11:66.
[22] Moresi V, Adamo S, Berghella L. The JAK/STAT pathway in skeletal muscle pathophysiology[J]. Front Physiol, 2019, 10:500.
(责任编辑:卢萍,罗森)
Role and significance of IL-6 and GATA-6 methylation inhibitors in lung tissue of rats with pulmonary arterial hypertension
LIAO Jian-xiong1, LUO Jie2, LIU Lin3, ZHANG Qian1△, LIU Wei-jia3△
(1,,550002,;2,,550003,;3,,550002,)
To study the changes and significance of interleukin-6 (IL-6), GATA-binding protein-6 (GATA-6) and GATA-6 methylation in monocrotaline (MCT)-induced rat pulmonary arterial hypertension (PAH) model.Forty adult male SD rats were randomly divided into control group, MCT group, MCT+dimethyl sulfoxide (DMSO) group, MCT+IL-6 antibody (anti-IL-6) group and MCT+5-aza-2'-deoxycytidine (5-Aza) group, with 8 rats in each group. The rats in control group
a single intraperitoneal injection of 2∶8 anhydrous ethanol and physiological saline mixture at 60 mg/kg. The rats in MCT group received a single intraperitoneal injection of 1% MCT at 60 mg/kg. The rats in MCT+DMSO group were intraperitoneally injected with the same amount of 1% MCT and subcutaneously injected DMSO at 1 mg/kg into the abdomen 3 times a week. The rats in MCT+anti-IL-6 group were intraperitoneally injected with the same amount of 1% MCT and then intraperitoneally injected with anti-IL-6 at 40 μg/kg every day. The rats in MCT+5-Aza group were intraperitoneally injected with the same amount of 1% MCT and then injected subcutaneously with 5-Aza at 1 mg/kg 3 times a week. On the 21st day after modeling, the rats in each group were monitored for right ventricular pressure and right ventricular hypertrophy index. Lung tissue HE staining was performed to observe lung pathological changes. The IL-6 and GATA-6 expression in lung tissues was detected by immunofluorescence and Western blot. The GATA-6 methylation in lung tissues was detected by methylation-specific PCR (MSP).Compared with control group, the average right ventricular pressure in MCT, MCT+anti-IL-6 and MCT+5-Aza groups was significantly increased (<0.05). Compared with MCT group, the average pressure of right ventricle in MCT+anti-IL-6 and MCT+5-Aza groups was significantly decreased (<0.05). Compared with control group, the right ventricular hypertrophy index in MCT group was significantly increased (<0.05). Compared with MCT group, the right ventricular hypertrophy index in MCT+anti-IL-6 and MCT+5-Aza groups was significantly decreased (<0.05). Immunofluorescence and Western blot results showed that the expression of IL-6 in MCT and MCT+5-Aza groups was significantly increased compared with control group (<0.05), while that in MCT+anti-IL-6 and MCT+5-Aza groups was significantly reduced compared with MCT group (<0.05). The expression of GATA-6 in MCT and MCT+5-Aza groups was significantly reduced compared with control group (<0.05), while that in MCT+anti-IL-6 and MCT+5-Aza groups was significantly increased compared with MCT group (<0.05). The MSP results showed that GATA-6 in lung tissues was highly methylated in MCT and MCT+DMSO groups, hypomethylated in MCT+anti-IL-6 and MCT+5-Aza groups, and unmethylated in control group.In the lung tissue of rats with PAH, the IL-6 expression is significantly reduced after anti-IL-6 treatment, the GATA-6 expression is significantly increased after 5-Aza treatment, and the degree of GATA-6 methylation is significantly reduced. The high expression of IL-6 may be a result of low GATA-6 expression, which is caused by GATA-6 methylation.
Pulmonary arterial hypertension; Interleukin-6; GATA-binding protein-6; 5-Aza-2'-deoxycytidine
R543.1; R363.2
A
10.3969/j.issn.1000-4718.2022.03.017
1000-4718(2022)03-0517-09
2021-08-26
2021-12-24
[基金项目]贵州省科技计划项目(黔科合基础[2018]1408号)
刘维佳 Tel: 18984383552; E-mail: weijia902@126.com; 张谦 Tel: 18985008119; E-mail: zhangqian800@126.com

