Cytoreductive radical prostatectomy after chemohormonal therapy in patients with primary metastatic prostate cancer
Christ Bst , Thoms Amiel , Tois Murer ,Sophie Knipper , Luks Lunger , Roert Tuer ,Mrgitt Retz , Kthleen Herkommer , Mtthis Eier ,Gunhild von Amserg ,Mrkus Grefen ,Juergen Gschwend ,Thoms Steuer , Mtthis Heck ,
a Department of Urology, Technical University of Munich, Rechts der Isar Medical Center, Munich,Germanyb Martini-Klinik Prostate Cancer Center, Hamburg, Germany
KEYWORDS Metastatic hormonesensitive prostate cancer;Chemohormonal therapy;Cytoreductive radical prostatectomy;Feasibility;Prevent local complications;Continence rate
Abstract Objective: Cytoreductive radical prostatectomy (cRP) has been proposed as local treatment option in metastatic hormone-sensitive prostate cancer (mHSPC) to prevent local complications and potentially improve oncological outcomes.In this study, we examined the feasibility of a multimodal concept with primary chemohormonal therapy followed by cRP and analyzed prostate size reduction under systemic treatment, postoperative complication rates, as well as early postoperative continence.Methods: In this retrospective study,38 patients with mHSPC underwent cRP after primary chemohormonal therapy(3-monthly luteinising hormone-releasing hormone-analogue+six cycles3-weekly docetaxel 75 mg/m2)at two centers between September 2015 and December 2018.Results: Overall,10(26%)patients had high volume and 28(74%)patients had low volume disease at diagnosis, according to CHAARTED definition.Median prostate-specific antigen (PSA)decreased from 65 ng/mL(interquartile range[IQR]35.0-124.5 ng/mL)pre-chemotherapy to 1 ng/mL (IQR 0.3-1.7 ng/mL) post-chemotherapy.Prostate gland volume was significantly reduced by a median of 50%(IQR 29%-56%)under chemohormonal therapy(p=0.003).Postoperative histopathology showed seminal vesicle invasion in 33(87%)patients and negative surgical margins in 17(45%)patients.Severe complications(Grade 3 according to Clavien-Dindo)were observed in 4(11%)patients within 30 days.Continence was reached in 87%of patients after 1month and in 92%of patients after 6 months.Median time to castration-resistance from begin of chemohormonal therapy was 41.1 months and from cRP was 35.9 months.Postoperative PSAnadir ≤1 ng/mL versus >1 ng/mL was a significant predictor of time to castration-resistance after cRP(median not reached versus 5.3 months;p<0.0001).Conclusion: We observed a reduction of prostate volume under chemohormonal therapy going along with a low postoperative complication and high early continence rate.However,the oncologic benefit from cRP is still under evaluation.
1.Introduction
About 10% of patients diagnosed with prostate cancer present with metastatic disease at diagnosis or develop metastases after primary local therapy, so-called metastatic hormone-sensitive prostate cancer (mHSPC)[1].Chemohormonal therapy with androgen-deprivation therapy (ADT) and docetaxel was the first systemic treatment combination to show improved overall survival in this setting (CHAARTED and STAMPEDE trial) [2,3].However, it has been shown that lethal prostate cancer cells persist in the primary tumor of the prostate despite aggressive treatment with chemohormonal therapy [4].Moreover, it has been reported that new metastases will develop from both, metastases as well as from the primary tumor [5].Therefore, it has been hypothesized that patients may benefit from local treatment of the prostate even in a metastatic stage of the disease.In fact, the STAMPEDE trial [3] recently reported an overall survival benefit in a subgroup of patients with mHSPC undergoing local treatment of the prostate with radiation therapy.In the total patient cohort overall survival did not differ between the radiation arm and the control arm.However,in a preplanned subgroup analysis the authors stratified patients according to tumor volume.While patients with low tumor volume achieved longer overall survival, patients with high tumor volume (CHAARTED trial definition:Presence of visceral metastasis or presence of four or more bone metastases with at least one metastasis being localized outside of the pelvis or vertebral column) did not benefit from local radiation.However, local radiation of the prostate did not prevent local symptomatic complications resulting from prostate cancer progression of the primary tumor.
Even though data on randomized-controlled trials for cytoreductive radical prostatectomy(cRP)in primary mHSPC are not available yet,retrospective series reported that cRP leads to prolonged cancer-specific survival [6], as well as overall survival [7,8] and prevents local complications[9-11].
2.Methods
Based on these data,we pursued in a series of 38 patients,a multimodal concept offering cRP to patients with primary mHSPC who responded well to primary chemohormonal therapy.In this retrospective analysis, we aimed to demonstrate the feasibility of cRP after chemohormonal therapy with prostate size reduction under chemohormonal therapy, postoperative complication rates according to Clavien-Dindo [12], as well as early postoperative continence assessing the number of pads used per day.
All patients presented with newly diagnosed mHSPC and received primary chemohormonal therapy followed by cRP (35 open surgeries and three robotic) between September 2015 and December 2018 at two centers(Department of Urology at Technical University of Munich[n=26] and Martini Klinik in Hamburg [n=12], Germany).
The initial diagnosis of mHSPC was confirmed by transrectal biopsy of the prostate gland.All patients received chemohormonal therapy (3-monthly luteinising hormonereleasing hormone-analogue continuously + six cycles 3-weekly docetaxel 75 mg/m2) [3].Subsequently, cRP was offered to patients at the discretion of the treating urologist based on prostate-specific antigen (PSA) reduction >50% and at least partial response on imaging according to Response Evaluation Criteria in Solid Tumors.Staging procedures at initial diagnosis and response assessment after chemohormonal therapy included computed tomography (CT) and bone scan or prostate specific-membrane antigen-ligand positron emission tomography (PSMA PET).
Castration-resistance was defined as rise in PSA resulting in a 50% increase over the nadir and PSA >2 ng/mL under systemic therapy with testosterone-levels <50 ng/dL according to current European Association of Urology guidelines [13].
Time to castration-resistance and associated 95% confidence intervals (CIs) with log-rank statistics were calculated with the use of the Kaplan-Meier method.Data analyses were performed using SPSS version 25.0(IBM Corp,Armonk, NY, USA).
3.Results
Patient characteristics of a total of 38 included patients are shown in Table 1.At initial diagnosis 28 (74%)patients presented with low volume disease and 10 (26%)patients with high volume disease.The median time from the end of chemotherapy to surgery was 61 days (interquartile range[IQR]47-82 days).Chemohormonal therapyled to a significant median prostate volume reduction of 50% (IQR 29%-56%) from a median of 50 mL pre-chemotherapy to 25 mL post-chemotherapy(p=0.003).Median PSA decreased from 65.0 (35.0-124.5) ng/mL pre-chemotherapy to 1 (0.3-1.7) ng/mL post-chemotherapy.
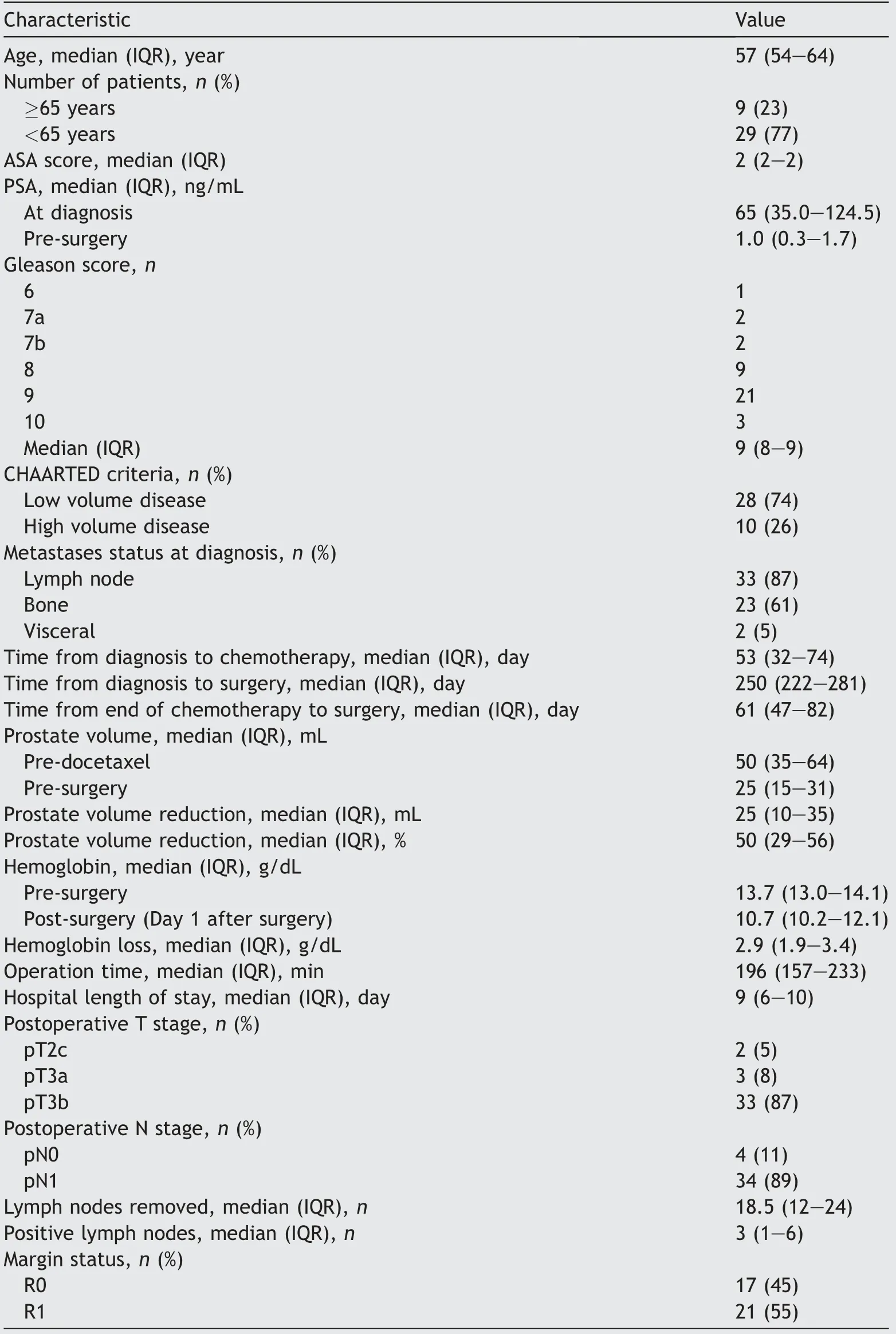
Table 1 Descriptive characteristics of 38 patients treated with cytoreductive radical prostatectomy after chemohormonal therapy.
Median time to castration-resistance from begin of chemohormonal therapy was 41.1 months (95% CI not available[NA])(Fig.1).Median follow-up time after surgery was 22.6 months (range 5.7-48.6 months).During this time, 14 of 38 patients (37%) developed a rising PSA above 2 ng/mL postoperatively.
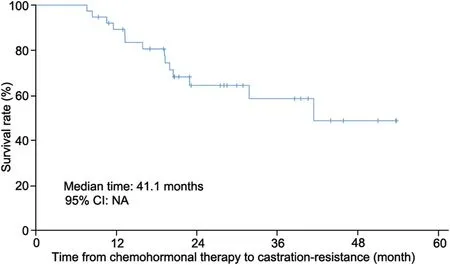
Figure 1 Kaplan-Meier curve showing the time from begin of chemohormonal therapy to development of castration resistance.NA, not available; CI, confidence interval.
Median time from cRP to castrations-resistance was 35.9 months(95%CI NA)(Fig.2).Preoperative PSA-nadir ≤1 ng/mL versus >1 ng/mL was not associated with time to castrationresistance (p=0.9).However, postoperative PSA-nadir was a significant predictor of time to castration-resistance after cRP.In patients with PSA-nadir values ≤1 ng/mL after cRP, the time to castration-resistance was significantly longer than that in patients with PSA-nadir values >1 ng/mL(95%CI 0.0-12.3;p<0.0001) (Fig.3).
After cRP, histopathologic work-up showed seminal vesicle invasion in 33 (87%) patients; out of those 27 (71%)men had bilateral infiltration, while a negative surgical margin could be achieved in 17(45%)patients.Overall 37 of 38(97%)patients had histopathologic positive lymph nodes.
Within 30 days postoperatively most patients (33 [87%])did not develop any complications.One patient developed an asymptomatic lymphocele(Grade I according to Clavien-Dindo).Severe complications (Grade 3 according to Clavien-Dindo) were observed in 4 (11%) patients within 30 days.Two (5%) patients with lymphoceles required percutaneous drainage, out of which 1 (3%) patient received a laparoscopic marsupialization.Moreover, 1 (3%) patient needed a percutaneous nephrostomy with antegrade ureteral stent insertion.The stent could be removed later without further urinary obstruction.

Figure 2 Kaplan-Meier curve showing the time from cytoreductive prostatectomy to development of castration resistance.CI, confidence interval; NA, not available.
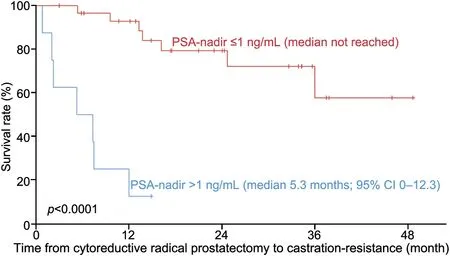
Figure 3 Kaplan-Meier curve showing the time from cytoreductive prostatectomy to development of castration resistance stratified by postoperative PSA-nadir ≤1 ng/mL versus >1 ng/mL.CI, confidence interval.
Looking at the first year postoperatively, 29 (76%) patients did not develop any complications.Severe complications (Grade 3) were observed in 7 (18%) patients.Two more patients had to be treated with laparoscopic marsupialization for lymphoceles (3 months and 4 months after surgery) and one patient suffered from a stricture of the vesico-urethral anastomosis, which was treated by internal urethrotomy, 11 months after surgery.Grade ≥4 complications were not observed.As a limitation, in four patients we could only collect follow-up data up to 6 months after surgery.
Data on postoperative continence after 1, 6 and 12 months were available for 31 (82%), 25 (66%) and 26 (68%)patients, respectively.Continence, defined as usage of up to one pad for safety,was reached in 27 of 31(87%)patients after 1 month, 23 of 25 (92%) patients after 6 months, and 23 of 26 (88%) after 1 year (Table 2).
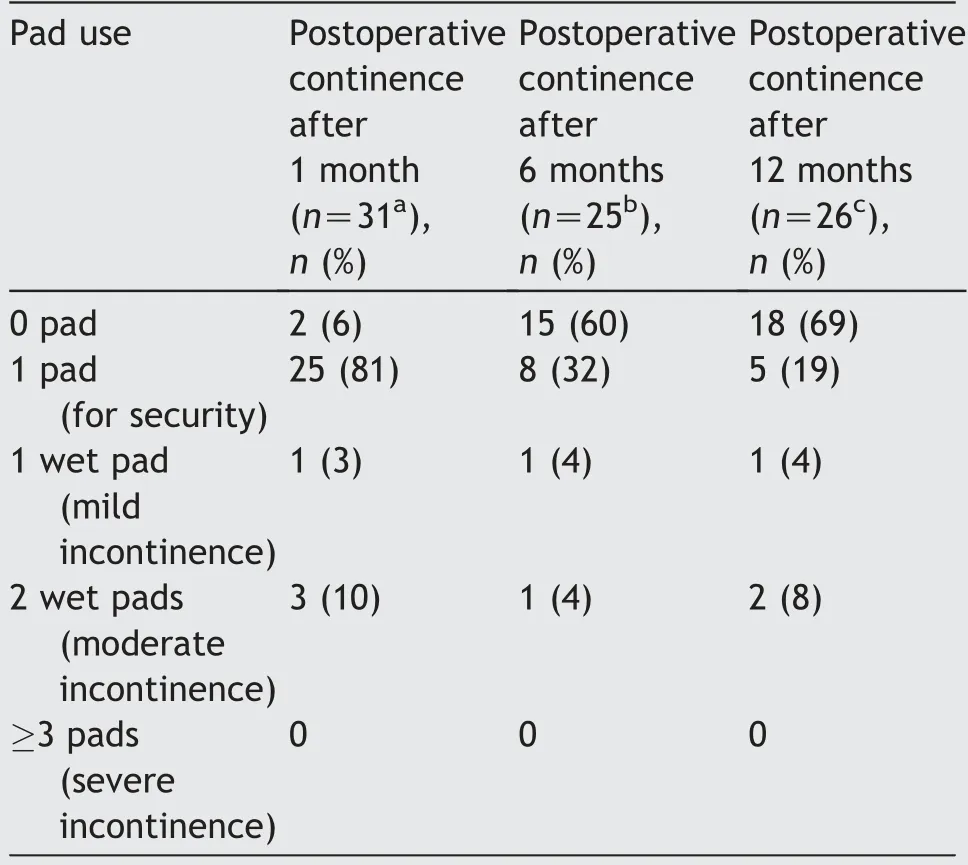
Table 2 Continence and pad use.
4.Discussion
To our knowledge, this is the first study investigating a multimodal concept with chemohormonal therapy followedby cRP in mHSPC.In our study, systemic therapy led to a significant prostate volume reduction potentially alleviating subsequent surgery with a low rate of postoperative complications and good early continence.The observed complication and continence rates are comparable to those observed in patients undergoing radical prostatectomy for localized prostate cancer [14,15].This is demonstrated in data with a large contemporary prostatectomy series with curative intention from our institution, where we analyzed the complication rate 3 months postoperatively.It showed similar data with 20.6% at least having one Clavien-Dindo complication; out of them, 7% had severe complications[14].Also the current data concerning the continence rate are similar to a recently published prostatectomy series of high risk and very high-risk prostate cancer patients from our institution.Continence rates after standard radical prostatectomy were 69% at 3 months and 82% after 1 year[16].Thus, sequential therapy with downsizing of the prostate and the tumor by previous systemic therapy may be beneficial.
A multimodal concept with primary systemic chemohormonal therapy followed by local therapy is also described in the randomized phase-III trial STAMPEDE [3].Here, a subgroup of 183 (18%) patients received chemohormonal therapy followed by radiation of the prostate.In this trial docetaxel was permitted in addition to ADT after its approval in the UK.The study showed an overall survival benefit in patients with low volume disease.However,prevention of local complications could not be shown for local radiation therapy.
In contrast, a survival benefit, as well as prevention of local complications has been shown for the concept of cRP,although only in retrospective studies and without investigating multimodal treatment strategies[6-8,10].However,prospective randomized-controlled trials are still ongoing and therefore, have to be awaited to validate the therapeutic role of cRP in mHSPC.Moreover,it’s unclear whether patients undergoing cRP should be selected based on low tumor volume as indicated for radiation therapy in the STAMPEDE trial.
In the current series of multimodal treatment with chemohormonal therapy followed by cRP, about one quarter of patients had high volume and three quarters had low volume disease.While the CHAARTED trial only showed a survival benefit from chemohormonal therapy for patients with high volume disease [17], latest data from the STAMPEDE trial suggested that chemohormonal therapy improved overall survival in newly diagnosed mHSPC regardless of tumor volume [18].The difference may arise from different inclusion criteria in both trials with mainly including de novo metastatic disease in the STAMPEDE trial and in contrast a high rate of patients with tumor recurrence following local therapy in the CHAARTED trial, which may represent biologically different tumors and were unequally distributed between patients with high and low volume disease [19].Thus, our approach offering primary chemohormonal therapy in de novo metastatic HSPC independent of tumor volume is supported by the STAMPEDE trial.
Meanwhile, several other systemic therapies have shown survival benefits in mHSPC including the androgenbiosynthesis-inhibitor abiraterone (LATITUDE and STAMPEDE trial) [3,20], as well as the antiandrogens enzalutamide(ARCHES and ENZAMET trial)[21,22]and apalutamide(TITAN trial) [23],which could also be combined with local therapy in a multimodal concept followed by local treatment.
Limitations of our case series are retrospective design,small number of patients, and missing long-term oncologic outcome.
5.Conclusion
In summary, cRP after chemohormonal therapy seems feasible in selected patients with no major complications and good postoperative continence rates.Future trials will have to clarify the oncological benefit of this concept.
Author contributions
Study concept and design: Matthias Heck.
Data acquisition: Christa Babst, Thomas Amiel, Sophie Knipper, Kathleen Herkommer.
Data analysis: Christa Babst, Lukas Lunger, Matthias Heck.Drafting of manuscript: Christa Babst, Thomas Amiel,Matthias Heck.
Critical revision of the manuscript: Tobias Maurer, Sophie Knipper, Lukas Lunger, Robert Tauber, Margitta Retz,Kathleen Herkommer,Matthias Eiber,Gunhild von Amsberg,
Markus Graefen, Juergen Gschwend, Thomas Steuber.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2022年1期
Asian Journal of Urology2022年1期
- Asian Journal of Urology的其它文章
- Chromophobe renal cell carcinoma: Novel molecular insights and clinicopathologic updates
- The role of preoperative dutasteride in reducing bleeding during transurethral resection of the prostate: A systematic review and meta-analysis of randomized controlled trials
- Efficacy and safety of desmopressin on frequency and urgency in female patients with overactive bladder and nocturia,current clinical features and outcomes: A systematic review
- The impact of the coronavirus disease 2019 pandemic on elective urological procedures in Australia
- Efficacy of a combination of dutasteride,tadalafil,and solifenacin in the treatment of previously unsuccessful patients
- Associations between IL-1RN variable number of tandem repeat, IL-1β(-511)and IL-1β (+3954) gene polymorphisms and urolithiasis in Uighur children of China
