Efficacy and safety of desmopressin on frequency and urgency in female patients with overactive bladder and nocturia,current clinical features and outcomes: A systematic review
Br Brkt ,Knut Frnke Mtthis My ,Ulrih Guger ,Thoms-Alexner Vo¨geli
a Hospital Viersen, Department of Urology and Pediatric Urology, Viersen, Germany
b Hospital St.Elisabeth, Department of Urology, Straubing, Germany
c Institute for Medical Statistics, Berlin, Germany
d University Hospital RWTH Aachen, Department of Urology and Pediatric Urology, Aachen, Germany
KEYWORDS Overactive bladder;Nocturia;Desmopressin;Urinary urgency;Lower urinary tract
Abstract Objective: To evaluate the efficacy and safety of desmopressin on frequency and urgency in female patients with overactive bladder (OAB) and nocturia.Methods: A selective database search was conducted to validate the effectiveness of desmopressin in patients with OAB and nocturia.Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were utilised.The meta-analysis included 378 women (five studies) with OAB.The clinical outcomes and adverse events were analysed.Results: The treatment strategy of all the studies included can be divided into three categories: (1) The effect of desmopressin compared with baseline, (2) desmopressin compared with placebo, and (3) desmopressin and anticholinergic combination versus desmopressin monotherapy.There was a significant (50%) reduction in nocturia and urgency episodes after using desmopressin alone.Combined desmopressin and anticholinergic led to a decrease in the frequency of nocturia voids when only using anticholinergic (65% vs.33.2%).The time increased in the middle to the first nightly voids in the combination arm (65.11 min;p=0.045).The mean incidence (standard deviation) of leak-free episodes was higher under desmopressin than under placebo in the first 4 h (62% [35%] vs.48% [40%]) and in the first 8 h (55% [37%] vs.40% [41%]).The safety profile was comparable between treatments.Conclusion: Available data indicate that desmopressin is efficacious in significantly reducing nighttime urine production, episodes of nocturia, and urgency episodes.The affectivity of the combination therapy was very high with least side effects for the treatment of OAB/nocturnal polyuria.
1.Introduction
According to the International Continence Society(ICS),the overactive bladder(OAB)is described as a“urinary urgency,usually with urinary frequency and nocturia,with or without urgency urinary incontinence” symptom complex [1,2].Affected patients show a physical and psychological impairment of their quality of life.The National Overactive Bladder Evaluation Program was a nationwide, random sample, internet survey with a nested case-control followup study to evaluate the prevalence of OAB.The prevalence of overall OAB was 16.9% in women [3], 70% of them with OAB disrupt nocturia[4].Nocturia has been defined by the ICS as waking to pass urine during the main sleep period[5].In essence, nocturia is either nocturnal polyuria (NP),reduced bladder capacity with OAB, or a combination of both [5].Recently, the effects of nocturia on chronic sleep disorders have become increasingly recognised [6], both in terms of effects on the quality of life, and on long-term morbidity and mortality outcomes [7].Anticholinergics have traditionally been widely used to treat OAB.However,their effectiveness is known to be between 60%and 70%[8].In addition, they lack selectivity for the bladder and their effect on other organ systems can lead to side effects such as dry mouth, blurred vision, constipation, and dizziness,which limits their usefulness[9].In addition,treatment with anticholinergics does not reduce the frequency of nocturia,especially nocturia due to NP, which is the case for under 60%of patients with OAB[10,11].The antidiuretic hormone(ADH), arginine vasopressin (AVP), is a peptide hormone.It is produced in the hypothalamus and released from the posterior pituitary gland with neurophysin II and copeptin(CT-proAVP).As an “antidiuretic” hormone, it causes increased recovery of water from the primary urine, which concentrates the urine, reduces its volume and it binds to V2 receptors in the renal collecting duct and stimulates water reabsorption.Insufficient antidiuresis due to a lack of AVP secretion or resistance to its action at the kidney can lead to the development of clinical syndromes such as NP.Deficiencies in vasopressin secretion can often be corrected with desmopressin.It inhibits the massive water excretion.NP in the elderly is known to be caused by an age-related change and loss of circadian rhythm of AVP secretion [12].Nocturia and NP in older men with lower urinary tract symptoms (LUTS) might also result from bladder outlet obstruction and hypersensitive bladder[13].Women appear to be more sensitive to desmopressin than men.This has been attributed to the fact that the gene for the vasopressin V2 receptor is located on the X chromosome in a region with high probability of escape from inactivation [14,15].
Several clinical trials have reported promising results that show an improvement in the symptoms of nocturnal voids and urgency in patients with OAB treated with desmopressin by decreasing renal urine production and increasing bladder filling time [16,17], to the extent that the use of desmopressin in the treatment of OAB patients leads to a reduction in nocturnal urine production.In turn,this is required to achieve functional bladder capacity between micturition periods, and a symptomatic improvement is thus achieved.The aim of this review is to evaluate efficacy and safety of desmopressin on frequency and urgency in female patients with OAB and NP in the literature.
2.Methods
2.1.Review criteria and data sources
A literature search of the “PubMed/PMC, Medline, the Cochrane Central Register of Controlled Trials (CENTRAL),Google Scholar, and Clinical Trials” databases was performed for clinical trials on the treatment of OAB with desmopressin, treatment of NP with desmopressin, and treatment of OAB in combination with anticholinergics.The systematic review was conducted according to the Cochrane Guidelines for Systematic Review and the Checklist Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) [18].
2.2.Study selection and quality evaluation
The search on the published studies was completed in March 2020 and included cohorts of women with OAB with nocturia who received therapy with desmopressin and anticholinergics.The following keywords were used in various combinations: “Desmopressin in nocturia”, “desmopressin in OAB”,“desmopressin”,and“OAB”.The search was limited to clinical trials, fully published articles, systematic reviews and original papers.Randomized controlled trials (RCTs), prospective and retrospective studies were selected.Reference lists of articles were also checked for relevant articles.In all the studies,OAB,nocturia was defined(according to the ICS)as waking to pass urine during the main sleep period.Reduced bladder capacity was defined as discordance between urine production and the bladder’s ability to store urine and diagnosed through the nocturnal bladder capacity index (NBCI).Inclusion criteria were women who had an OAB with nocturia and received therapy with desmopressin and possibly anticholinergics.Excluded from this analysis were studies that included a cohort of women with OAB without nocturia,women with nocturia without OAB,and women with multiple sclerosis.Furthermore, studies with mixed populations with female,male,and children were excluded.
The articles were first screened and selected based on their abstracts, and then examined in detail.To assess the bias risk, two reviewers (Franke K and Barakat B) independently identified all potential studies that met the inclusion criteria for a full review.The studies selected for inclusion were independently selected by the reviewers.Disagreements between the extracting authors were resolved by consensus or referred to the third author.
2.3.Data extraction and analysis
The following information was extracted from the studies that met the inclusion criteria (name of the first author,year of publication,study design,mean number of nocturnal voids duration of the first sleep period [FSP] before and after treatment,results and adverse effects).These details were then examined to determine the potential for conducting subgroup analyses.
2.4.Statistical analysis and data synthesis
The primary outcome of this analysis was the mean number of nocturnal voids and urgency in female patients with OAB and NP.We compared continuous variables with the nonparametric Kruskal-Wallis test and categorical variables with the Fisher test.All analyses were performed using the statistical analysis software “Comprehensive” version 2.0(Biostat, North Dean Street Englewood, NJ, USA).
2.4.1.Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.For this type of study, formal consent is not required.This article does not contain any studies with animals performed by any of the authors.
3.Results
3.1.Search results, study characteristics and outcomes
The search strategy is described in the PRISMA flow chart and the results of the literature search for the systematic review are shown in Fig.1.After duplication, 432 articles were screened for further analysis.The literature search retrieved 245 duplicated records.Of the 187 complete articles, 159 studies that did not meet the inclusion criteria were then excluded.Finally, five studies involving 378 women were included.
3.2.Characteristics of the studies included and methodological quality

Figure 1 Study selection flow chart, Preferred Reporting Items for Systematic Reviews and Meta-Analyses search strategy.OAB, overactive bladder; CENTRAL, the Cochrane Central Register of Controlled Trials.
The main aim was to evaluate the efficacy of desmopressin,with or without anticholinergics, in extending the time for first nocturia episodes and reduction of urgency episodes.Further aims were to evaluate the efficacy of desmopressin in reducing the average number of micturition, urgency,and urgency urinary incontinence episodes.Three RCTs[17,21,22] and two prospective studies [19,20] were included(Table 1).Three of them were randomised double blind studies with cross-over design.Important parameters,such as the duration of the follow-up,were described in all the studies (Table 1).All five studies identified in this review were evaluated with low bias potential.The rank of the hierarchies of the studies, according to the probability of bias, was Level IIb.Clinical symptoms after treatment were assessed by means of a questionnaire (ICIQ-OABqol,UDI-6, IIQ-7).
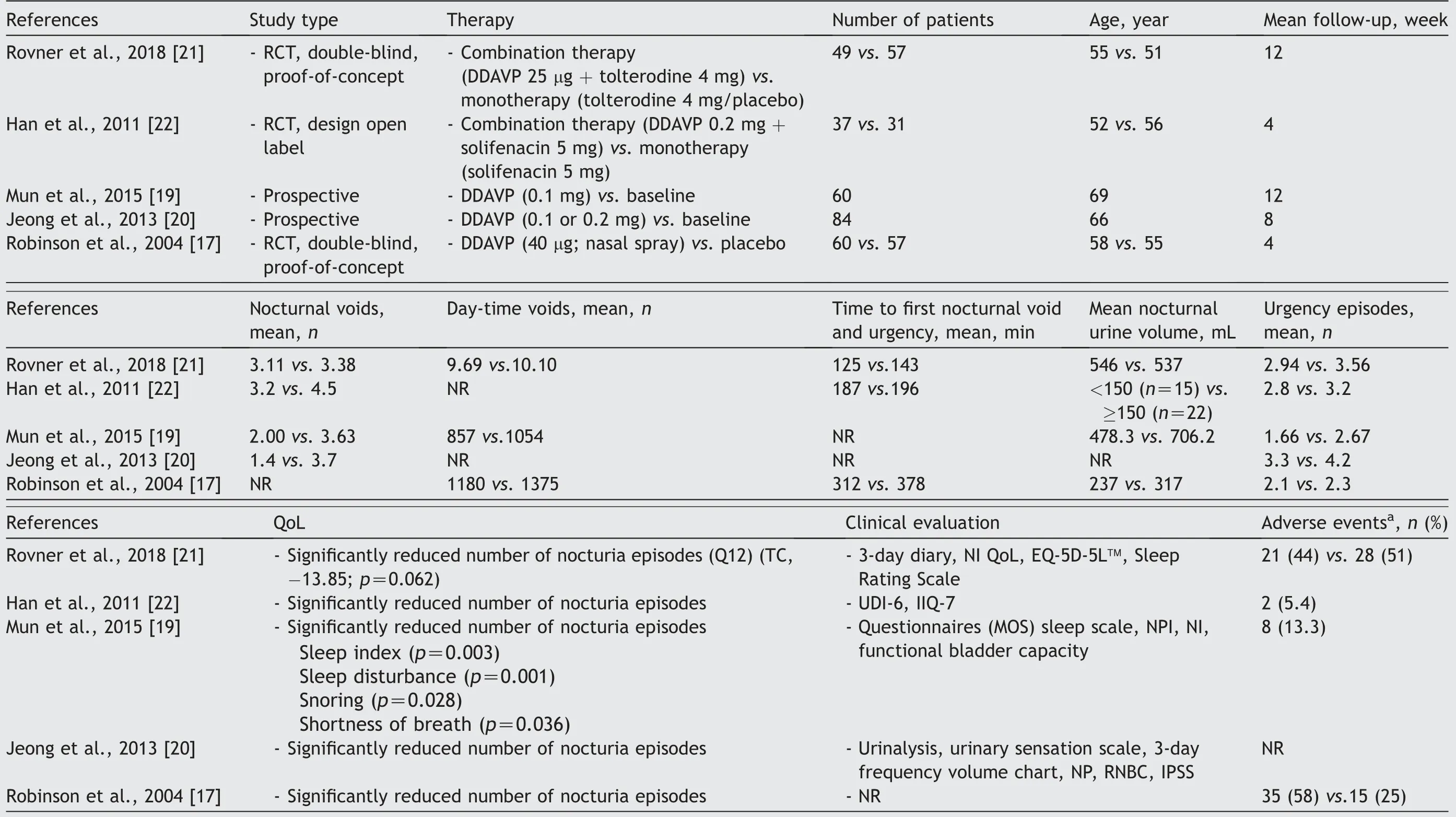
Table 1 Demographic characteristics, therapeutic effects, and adverse effects of desmopressin therapy in the comparative studies.
3.3.Desmopressin dose
The desmopressin dose ranged from the lowest dose(25 μg)to the highest dose (0.4 mg) before going to sleep[17,19-22].The study included the following periods:Screening (1 week), dose titration (1-3 weeks), and treatment (4 weeks).Rovner et al.[21] used in their work the lowest therapeutically dose of desmopressin.Patients in studies with combinations therapy received desmopressin 25 μg + tolterodine 4 mg or desmopressin 0.2 mg + solifenacin 5 mg or monotherapy with anticholinergic approximately 1 h prior to bedtime [21,22].In the studies with desmopressin alone, the titration dose (0.1,0.2 or 0.4 mg) was increased weekly based on the number of nocturnal nocturia and extent of symptoms and side effects [17,19,20].If the patients tolerated desmopressin well, they received the maximum dose instead of the low dose.For side effects of desmopressin, a low dose was given.In other trials, a fixed dose of desmopressin was given, and the evaluation of symptoms was carried out at fixed intervals [16,17].
3.4.Effect of desmopressin alone on OAB
We recruited two studies with 144 women who had experienced more than two episodes of nocturia[19,20](Table 1).Jeong et al.[20] recruited in their work 70.2% with OAB,7.1% with reduced nocturnal bladder capacity, and 22.6%with mixed type of urgency.In this work, all patients suffered from OAB with nocturia and NP [20].All the studies included reported a number of nighttime episodes(nocturnal voids per night).After treatment with desmopressin, a reduction in nocturia of more than 50% from the baseline was considered effective.The number of nocturnal voids per night after treatment with desmopressin was significantly reduced when compared to the baseline(standard deviation [SD] -1.3232; 95% confidence interval[CI]: [-1.94, -0.71]; p=0.02) (Fig.2).
The significant reduction in the number of nocturnal episodes in two studies was achieved with a low dose of desmopressin 0.1 mg [19,20].After desmopressin treatment,significant reductions in nocturnal urine volume (p=0.01),nocturnal polyuria index (p=0.01) and nocturia index(p=0.01) were also observed [19].Both studies also presented a reduction in the mean number of urgency episodes by more than 50%compared to the baseline.The number of nocturnal urgency episodes after treatment with desmopressin was significantly reduced when compared to the baseline (SD -1.03; 95% CI: [-1.56, -0.51]; p=0.04)(Fig.3).Sleep quality was evaluated in the study by Mun et al.[19] using the sleep indexes questionnaire.The analysis showed a significant subjective difference between the groups regarding sleep index (p=0.003), sleep disturbance(p=0.001), snoring (p=0.028) and shortness of breath(p=0.036)[16].These aspects show a clear effect on quality of life.
3.5.Effect of desmopressin vs.placebo on OAB
In a multi-centre randomised cross-over exploratory study,the efficacy of desmopressin in the treatment of OAB with urge incontinence was investigated [17] (Table 1).All the women complained of severe urinary incontinence.Of this 15 (25%) had predominantly stress incontinence; 13 (22%)predominantly urge incontinence; 32 (53%) mixed incontinence.The average leak volume per episode of incontinence was 28.3(9.0-96.0)mL.The mean incidence(SD)of leak-free episodes was higher under desmopressin than under placebo in the first 4 h (62% [35%] vs.48% [40%]) and in the first 8 h(55%[37%]vs.40%[41%]).After desmopressin administration, 36% of patients had no leakage.The mean time(SD)from dosage to the first incontinence episode was longer under desmopressin,at 6.3(2.5)h versus 5.2(3.3)h,and thus the probability of remaining dry in the first 4 h was 1.72 times higher under desmopressin than under placebo.
3.6.Effect of desmopressin combined with anticholinergic on OAB
In our analysis, we included two randomised double-blind studies that investigated the effect of desmopressin in combination with anticholinergic drugs in the treatment of OAB [21,22] (Table 1).The patients included with ≥2 nocturia received either a combination of desmopressin/anticholinergic or a monotherapy anticholinergic/placebo.There was a trend towards a reduction in mean nocturnal void with combination therapy when compared to monotherapy (SD 0.35; 95% CI: [0.05, 0.66]; p=0.76) (Fig.4).Additionally, analysis by Rovner et al.[21] showed a reduction in mean nocturnal void volume in combinationtherapy versus monotherapy (-166.0 mL; p=0.034).The mean time of first nocturnal voiding increased in the combination arm(65.11 min;p=0.045).A significant advantage of combination therapy over monotherapy was shown.Significantly more patients achieved responder status using combination therapy(odds ratio 4.21;95% CI[1.38, 12.82];p=0.0114) [21].This led to an improvement in quality of life with a mean improvement of Nocturia Index Diary©>10 points [21].

Figure 2 Meta-analysis for mean number of nocturnal voids of desmopressin alone on overactive bladder.SD,standard deviation;SMD, standardized mean difference; CI, confidence interval.
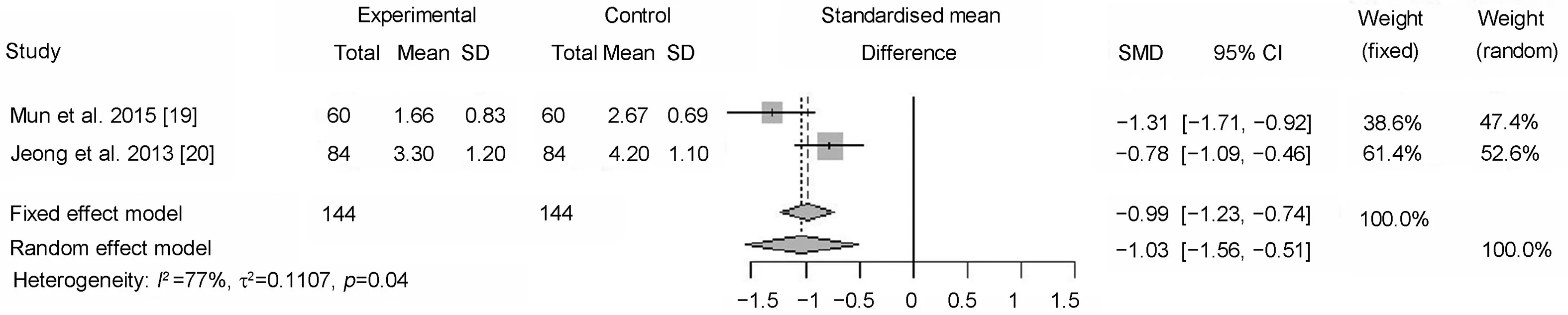
Figure 3 Meta-analysis for mean number of urgency episodes of desmopressin alone on overactive bladder.SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.

Figure 4 Meta-analysis of desmopressin combined with anticholinergic on overactive bladder.SMD, standardised mean difference; CI, confidence interval; TE, estimate of treatment effect; seTE, standard error of treatment estimate.
Rovner et al.[21] concluded that combination therapy with desmopressin and anticholinergics is more effective than anticholinergics alone for the treatment of OAB/NP,but the authors emphasised that a further prospective validation study of combination therapy is necessary.
3.7.Clinical outcomes and adverse events
The reported adverse side effect of desmopressin therapy in elderly patients was hyponatremia [23].Four studies reported no significant treatment-related complications or clinically significant hyponatremia by using low-dose desmopressin [17,19-21] (Table 1).Rovner et al.[21] demonstrated only 1 (0.94%) case of clinically significant hyponatremia by using low-dose desmopressin.Although desmopressin in included studies is effective in decreasing nocturnal voids in elderly patients and safe by using low dose, the reported safety in other studies in patients older than 65 years is a great concern.Elderly patients (>65 years)with a baseline serum sodium level below the normal range are at high risk (75%) of developing significant hyponatremia [24].Long-term desmopressin treatment gradually decreased the natrium concentration and induced significant hyponatremia in patients who did not have initial hyponatremia [25-27].
However, adverse effects such as headaches, dizziness,nausea, oliguria, diarrhoea, oliguria, and incontinence were also described [23].A total of eight patients (13.3%)were observed to have mild dizziness, mild facial swelling,and fatigue.The randomised studies [21,22] included evaluated safety and adverse effects in combination therapy by evaluating vital/laboratory parameters and clinical/physical examination.In a safety analysis,Rovner et al.[21]found no safety concerns with the simultaneous use of tolterodine and desmopressin in women with OAB and nocturia.Here, side effects in the combination therapy group occurred in ≥5% of patients.In particular,anticholinergic side effects associated with tolterodine,such as a dry mouth and headache, are known to be more common in a combination therapy group than one with monotherapy.Han et al.[22]reported in their analysis that no significant changes in serum sodium or body weight were observed in combination therapy.However,adverse events such as headaches and diarrhoea occurred in 5.4% of patients.
4.Discussion
A reduced ability of the bladder to store urine leads to nocturia.This is caused by decreased nocturnal bladder capacity, more irritable symptoms, and comorbid conditions,such as OAB.Nocturia in women was,for many years,primarily attributed to OAB and mainly treated with anticholinergics alone.However, recently the introduction of the β3-adrenergic receptors agonist (Mirabegron) for the treatment of OAB symptoms improved bladder storage capacity without impairing the voiding phase of the micturition cycle [28].Three studies were meta-analysed to compare the efficacy and safety of β3-adrenergic receptors agonist with tolterodine in the reduction of nocturia episodes[26,29,30].Although in these studies no statistically significant difference was found between the treatment arms,two other studies demonstrated a significant reduction of nocturia episodes with β3-AR agonist when compared with a placebo [31,32].Understanding its underlying causes, risk factors, and consequences is essential in formulating the most suitable management strategy.Nocturia and incontinence are also important symptoms of a sleep disorder.Due to the multifactorial genesis of OAB and nocturia, the therapy requires a multimodal concept.Desmopressin is the synthetic analogue of the antidiuretic hormone AVP.It has been available clinically for >30 years in a variety of formulations.Therefore, the rationale for use of desmopressin in the treatment of adults with OAB syndrome is that,through a decrease in urine production at night, it will increase the time taken to reach functional bladder capacity between daytime voids, reducing frequency and urgency,and benefiting adults suffering from OAB.
The effectiveness and safety of desmopressin in patients with nocturia has been studied numerous times.A doubleblind, placebo-controlled study in men with nocturia revealed 34% of the desmopressin group had significant fewer nocturnal voids compared to the baseline [33].Additionally, the duration of the FSP also increased in the desmopressin group, which significantly improved the quality of sleep versus placebo [34].
From our reading of the literature, we found a renewed interest in the use of desmopressin for the treatment of female OAB.The treatment strategy of the studies included can be divided into three categories.The studies which examined the effect of desmopressin versus placebo on OAB were the first to explore the concept of desmopressin as a strategy for management of OAB syndrome in women,and the results were very encouraging.Robinson et al.[17]reported 40 μg intranasal desmopressin to 64 women with urinary incontinence lasting over 10 days.Results showed that patients taking desmopressin had a significantly higher mean incidence of periods without leakage during the first 4 h and 8 h after taking desmopressin versus placebo.They did not report the effect on other OAB symptoms, such as frequency and urgency.
Jeong et al.[20] reported about significantly reduced mean number of nocturia episodes compared to the baseline (SD -1.32; 95% CI [-1.94, -0.71]; p<0.001).Furthermore,a significantly lower number of urgency episodes was observed in women whose nocturia was reduced by more than half when compared with that of the baseline (SD-1.03; 95% Cl: [-1.56, -0.51]; p=0.04).Additionally,Mun et al.[19] investigated the impact of desmopressin treatment on sleep quality using the subjective medical outcomes study sleep scale.They found that desmopressin therapy significantly improved sleep quality, sleep index,sleep disturbance, snoring, and shortness of breath significantly, with a decreased number of nocturia episodes on the medical outcomes study sleep scale.
Other authors examined the effect of desmopressin combined with anticholinergic on OAB.Recently, in two RCT double-blind placebo-controlled studies that evaluated the efficacy of desmopressin combined with anticholinergic in the treatment of female patients with OAB, desmopressin induced a significant increase in the time to the first urgency episode, with a significant reduction in the mean numbers of urgency episodes when compared to the placebo, and subjective improvement was observed in frequency and urgency as well as overall QoL [21,22].There was a trend towards a reduction in mean nocturnal void with combination therapy compared to monotherapy (SD 0.35; 95% Cl: [0.05, 0.66], p=0.76).Safety assessment in the combination strategies demonstrated no safety concerns with concomitant use of tolterodine/solifenacin and desmopressin in women with OAB and nocturia, which is consistent with clinical experience of this combination therapy for the treatment of incontinence [21,22].Indeed,in all the studies include for the role of desmopressin in the management of female OAB syndrome, the incidence of hyponatremia and more specifically of clinically relevant hyponatremia was not significant.
Our analysis is the first review to examine the effect of desmopressin combined with anticholinergic on OAB.Desmopressin administration achieved a significant reduction in nocturia episodes and urgency episodes [19-22], which in most trials leads to improvement in sleep and quality of life.Studies conducted on females with OAB syndrome have confirmed that desmopressin is effective in at least postponing the development of storage symptoms,and useful in reducing frequency and urgency for the management of OAB, particularly in combination with other treatments.
Although the results of this study might indicate an influence of OAB syndrome on the treatment outcome of desmopressin in women with nocturia, this study had some limitations.For instance, the number of studies included in our review was relatively small.All these studies evaluated different treatment management and different parameters.There was often a lack of standardised desmopressin doses and standardised questionnaires for evaluation after therapy.Furthermore, it remains unclear to what extent patients with other causes of NP were excluded from clinical trials.Further evaluation is thus necessary in a large number of women with nocturia and other voiding dysfunctions.
5.Conclusion
Desmopressin treatment shows a significant improvement in the number of nocturia episodes and urgency episodes.Desmopressin improves quality of sleep by prolonging the period of sleep until the first void and is shown to have an acceptable safety profile in patients with overactive bladder syndrome.The affectivity of the combination therapy was very high with least side effects for the treatment of OAB/NP.
Author contributions
Study concept and design:Bara Barakat,Thomas-Alexander Vo¨geli.
Data acquisition: Bara Barakat, Matthias May.
Data analysis: Bara Barakat, Ulrich Gauger.
Drafting of manuscript: Bara Barakat, Knut Franke,Thomas-Alexander Vo¨geli.
Critical revision of the manuscript: Thomas-Alexander Vo¨geli, Matthias May.
Administrative, technical, or material support: Bara Barakat.
Supervision: Thomas-Alexander Vo¨geli.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2022年1期
Asian Journal of Urology2022年1期
- Asian Journal of Urology的其它文章
- Chromophobe renal cell carcinoma: Novel molecular insights and clinicopathologic updates
- The role of preoperative dutasteride in reducing bleeding during transurethral resection of the prostate: A systematic review and meta-analysis of randomized controlled trials
- The impact of the coronavirus disease 2019 pandemic on elective urological procedures in Australia
- Efficacy of a combination of dutasteride,tadalafil,and solifenacin in the treatment of previously unsuccessful patients
- Associations between IL-1RN variable number of tandem repeat, IL-1β(-511)and IL-1β (+3954) gene polymorphisms and urolithiasis in Uighur children of China
- Ultrasound heterogeneity as an indicator of testicular salvage in testicular torsion: A single center experience
