Recent Insights into Signaling Responses to Cope Drought Stress in Rice
Muhammad Mahran Aslam, Muhammad Abdul Rehman Rashid, Mohammad Aquil Siddiqui, Muhammad Tahir Khan, Fozia Farhat, Shafquat Yasmeen, Imtiaz Ahmad Khan, Shameem Raja, Fatima Rasool, Mahboob Ali Sial, Zhao Yan
Review
Recent Insights into Signaling Responses to Cope Drought Stress in Rice
Muhammad Mahran Aslam1, Muhammad Abdul Rehman Rashid2,3, Mohammad Aquil Siddiqui1, Muhammad Tahir Khan1, Fozia Farhat4, Shafquat Yasmeen1, Imtiaz Ahmad Khan1, Shameem Raja4, Fatima Rasool5, Mahboob Ali Sial1, Zhao Yan6
()
Drought is one of the hot topics needing urgent attention in the current era of climate change.It massively disturbs the rice growth and productivity and isbecoming a serious threat.The drought avoidance strategies in rice include stomatal closure, cellular adaptation and changes in root development. Moreover, the endogenous plant hormones (abscisic acid and jasmonic acid) and reactive oxygen species have paramount importance in drought tolerance in rice. The drought tolerance induces modification in biochemical, molecular and physiological properties of plants.At the molecular level, expression of several transcription-factors is modulated which further determine the activation of drought responsive gene families. Mitogen activated protein kinases and Ca signaling pathways initiate an array of signaling cascade for mediating the gene expression in rice. Approaches, conventional breeding methods combined with modern emerging techniques such as genetic engineering, to improve rice drought tolerance were discussed. This review provided recent insights into major regulatory factors against drought stress, signaling mechanisms and molecular engineering strategies (including conventional transgenic and recent genome editing approaches) to induce drought tolerance in rice.
drought stress; rice; signaling response; regulatory factor
Rice (L.) is the staple food for more than 50% people on the globe, and 90% of the world’s rice is grown in Asia (Muthayya et al, 2014). It is cultivated as one of the major food cropsin south Asian region including Pakistan, China, Bangladesh, Indonesia, Laos and India (Hadiarto and Tran, 2011). The world population is expected to rise to 10 billion by 2050, and it requires 763 million tons of rice in 2020, while 852 million tons by 2035 (Ullah et al, 2017; Zhang et al, 2018).Rice being high delta crop consume 55–60 acre inch of water, which is 34%–43% of total irrigated water. Elliott et al (2014) showed that 1 kg rice production requires 1432 L of irrigated water. Water deficiency is a major problem, impacting the rice productivity especially in areas where rice is grown in rainfed conditions (Zhang et al, 2018). In the Asian region, there are 5 million hectares covered by upland rice and 34 million hectares by lowland rice, both repeatedly encounter drought stress, which ultimately reduce the rice yield (Serraj et al, 2011).
To mitigate this stress, alternative strategies should be applied, such as to develop drought resistance varieties, and increase modification in planting methods (Basu et al, 2016).The increasing demand of rice can only be met by using conventional breeding methods combined with modern emerging techniques such as genetic engineering. As an example, in Pakistan, a total of 2311 million hectares is dedicated to grow rice. The predominant rice growing areas (Gujranwala and Hafiz Abad cities of Pakistan) are often cultivated with two-line hybrid rice, including the thermal sensitive genetic male sterile lines as females and drought resistant donor lines as male to achieve maximum yield. Advanced genetic engineering approaches such as CRISPR/Cas9 can be employed in this regard (Ogata et al, 2020; Tabassum et al, 2021). This review introduces the morpho-physiological, biochemical and molecular responses of rice against drought stress, its signaling pathways, and strategies to induce rice drought tolerance.
Morpho-physiological responses of rice against drought stress
Water deficit conditions impair the normal growth pattern, disturb the water relation and reduce the water use efficiency. Plants display a variety of phenomena to withstand drought including uptake of water from deep and prolific root system, development of smaller and succulent leaves, increase in diffusive resistance, reduction of water loss through wilting of leaves and impeding the rate of transpiration (Farooq et al, 2012). Plants show a number of modifications in phenotypic architecture such as epidermal trichomes, smaller and denser stomata, higher ratio of palisade to spongy parenchyma thickness, and development of vascular bundle sheath and thicker cuticle on the epidermis (Silva et al, 2018). Drought stress forces the rice plant to modify its architecture (morpho-physiological changes). During evolution, several morphological and physiological strategies have evolved in rice to cope with the effects of drought stress, includingstomatal regulation, osmotic adjustment, photosynthetic response and root development(Fig. 1).
Stomatal regulation
Rice variety ‘IR64’ has defused and fewer stomata due to the over expression of rice epidermal patterning factor, which is a role player in determining stomatal density in rice. The transgenic variety, thus, having lower stomatal density transpires less water. Recently, a few metabolic mechanisms to improve the regulation of guard cells against drought stress have been reported. The introduction of light-activated synthetic K+ion channel (BLINK1) into guard cells has improved the synchronization of stomata with fluctuating light conditions (Gupta et al, 2020). The introduction of these mechanisms in rice can improve the water use efficiency by stomatal engineering and enhance the amount of fixed carbon per unit of water lost (Papanatsiou et al, 2019). Rice plants with lower stomatal density to an optimum level perform better during drought stress and conserve more water; this fact can be employed as a novel strategy against climate change which threatens the food security (Bertolino et al, 2019). Stomatal conductance plays a vital role in maintaining the temperature during heat stress and allows the assimilation of solar energy at the cellular level (Kartika et al, 2020). More than 90% of water transpiration occurs through the leaves, especially through stomata (An et al, 2019). Stomatal closure is a key factor to control the transpiration during drought stress in rice. Under drought stress, rice plants close their stomata, and hence there is a negative correlation between stomatal conductance and drought stress (Crawford et al, 2012; An et al, 2019).
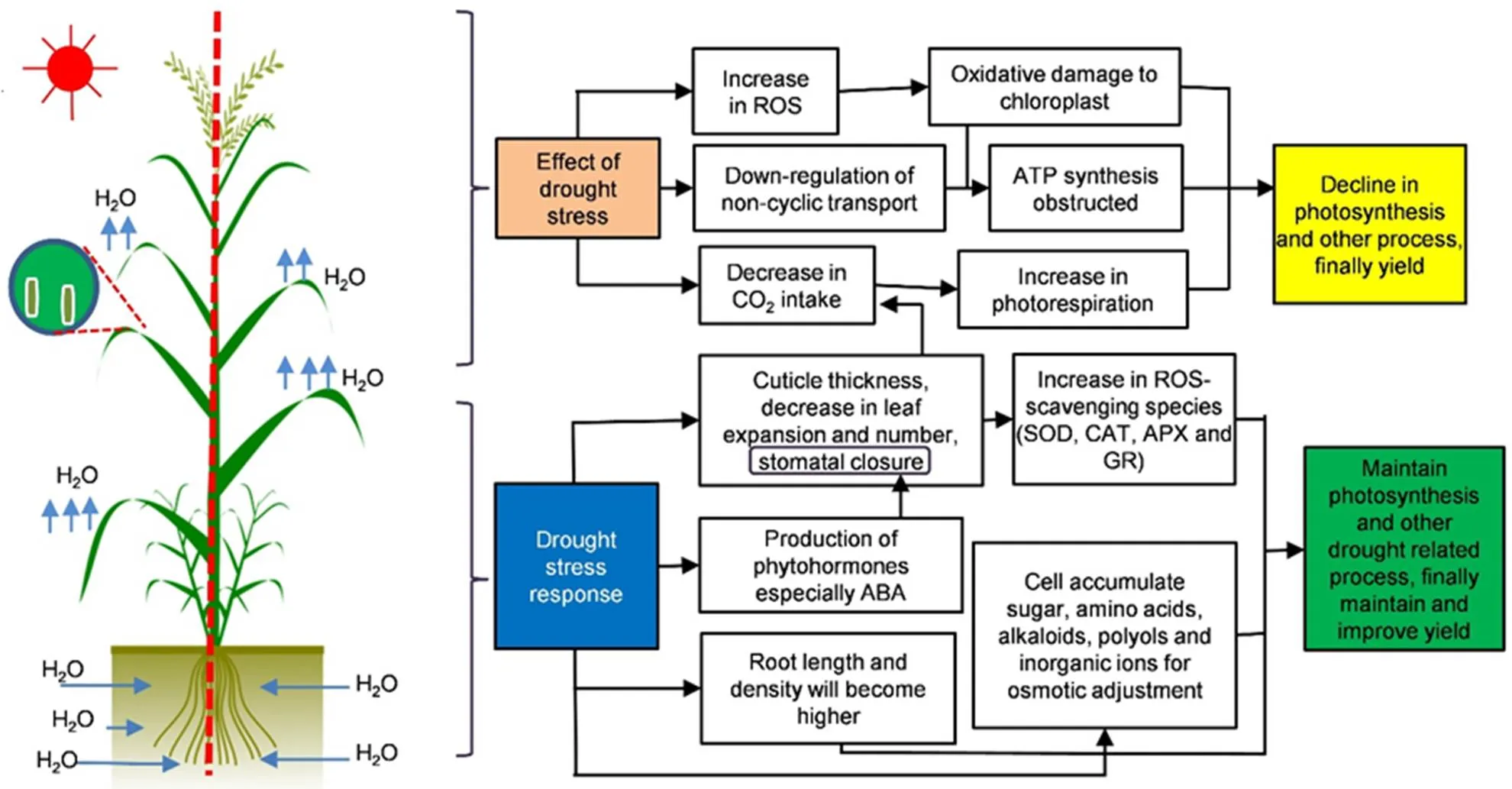
Fig. 1. Effects of drought and response in rice (Ullah et al, 2017).
ABA, Abscisic acid; APX, Ascorbate peroxidase; CAT, Catalase; GR, Glutathione reductase; ROS, Reactive oxygen species; SOD, Superoxide dismutase.
Root development
Rice breeders consider the variations in root traits to be a potential source to enhance crop productivity under drought conditions. However, the association of root traits with the plant development and understanding of functional traits of roots need to be dissected to promote productivity under drought stress. The functional root traits including coarse and fine roots, and root length, density, diameter and depth in soil in relation to available water are directly associated with drought stress(Henry et al, 2012). The seminal roots have the capability to conserve water in small xylem, and the later ones used for crop maturation eventually improve yield under drought conditions (Comas et al, 2013). Many studies have been conducted on the role of rice roots under drought stress, which has already advanced as compared to other cereal crops (Henry et al, 2012). Rice has a well-established and fibrous root system(Li et al, 2007; Rebouillat et al, 2009), and it shows the various morphological adaptations in response to drought stress, which can be the coordinated, cell division, elongation differentiation in root apex to increase the root length by reducing the branch angles and favoring the lateral roots toward patches of higher moisture (Gupta et al, 2020). The drought stress atthe early and middle stages promotes root growth, while longer duration under drought conditions leads to loss of root activity (Ding et al, 2018). The drought responsive ERF/AP2 transcription factor OsERF71 especially expresses in the root meristem, endodermis and pericycle (Lee et al, 2016), and its overexpression enhances the drought tolerance at the vegetative stage and the reproductive stage promoting grain yield by approximately 20% (Lee et al, 2016).
Photosynthesis
The drought stress causes stomatal closure which checks the CO2intake and reduces photosynthesis, affecting growth and yield (Xu et al, 2016). Decreased stomatal conductance may limit the CO2fixation and enhance water use efficiency under drought stress, and hence promote plant growth (Xu et al, 2016). However, in rice, the photosynthetic rate is greatly checked under drought conditions, which leads low crop productivity. Kusumi et al (2012) reported that the transpiration rate and photosynthesis in rice are affected under drought conditions. In the stress conditions, the mature leaves showed a decline up to 66% of net photosynthesis. Furthermore, the impact of combined drought and heat stress during the reproductive stage of rice has also been studied, and the synergistic effect of these stresses reduces fertile spikelet proportion and net photosynthesis, and limits the enzymatic activity of sucrose-starch conversion, which ultimately stops the filling and affects the ultimate grain weight of rice (Fahad et al, 2017; Mahrookashani et al, 2017).
Osmotic adjustment
Drought stress modifies the turgidity and osmotic balance of the crop plants (Saud et al, 2017). Plant adapts to drought stress through osmotic adjustments to diminish the plant damage. In response to drought conditions, the plant defense mechanisms activate accumulation of the osmolytes and osmoprotectants, which further regulate homoeostasis at the cellular level. Osmotic balance is negatively associated with the drought stress (Caine et al, 2019; Zafar et al, 2020).
Among osmoprotectants, organic substances such as sugars (e.g. trehalose, fructan etc.), polyols, alkaloids, mannitols, sorbitols, betaines, polyamines,-inonitol and prolines are directly involved in osmotic adjustment in response to drought stress. Moreover, certain inorganic compounds and glycine also take part in this response (Mehrotra et al, 2014; Zafar et al, 2020). The osmolytes assist cellular systems by providing protectionto integral proteins. Moreover, the higher concentration of oxidative and inorganic ions integrates the membrane to relieve the plant from drought stress (Gupta and Huang, 2014; Khan et al, 2018).
Plenty of rice transgenic lines for osmolytes have been developed and studied to enhance abiotic stress tolerance (Hasanuzzaman et al, 2019). The decisive roleof osmoprotectants (such as proline and glycinebetaine) has also been verified by exogenously applying of these substances to rice plants under drought stress. This leads to reduced damage (Hasanuzzaman et al, 2014; Caine et al, 2019). Furthermore, exogenous application of proline and glycinebetaine regulates the antioxidant-based plant defense mechanisms. These osmoprotectants also reduce the superoxide dismutase (SOD), glyoxalase II and glutathione S-transferase activities which increase during the drought stress (Hasanuzzaman et al, 2014).The role of osmoprotectants and osmolytes in rice drought tolerance has been exploited by various researchers to develop drought tolerant genotypes (Li et al, 2018).
Biochemical and molecular responses of rice against drought stress
Drought tolerance is a multigenic and extremely complicated phenomenon that involves several morphological, chemical, physical and molecular processes (Fig. 2). Many transcription factor (TF) families, such as WRKY, AP2 (APETALA2)/ERF (ethylene-responsive factor) and NAC (NAM, ATAF1/2 and CUC1/2), play important roles in plant- and/or tissue-specific regulation (Jiang et al, 2017; Ahmed et al, 2021).

Fig. 2. Various signaling pathways connectively enhanced drought tolerance in rice.
OPDA, Oxo-phytodienoic acid; ROS, Reactive oxygen species; JA, Jasmonic acid; JAZ, Jasmonate-zim domain; MYC2, Myelocytomatosis; MAPK, Mitogen-activated protein kinase; MAP2K, Mitogen-activated protein kinase kinase; MAP3K, Mitogen-activated protein kinase kinase kinase; ABA, Abscisic acid; PP2C, Protein phosphatase 2C; TF, Transcription factor; PYR, Pyrabactin resistance; PYL, PYR-like regulatory.
Abscisic acid (ABA)
ABA is synthesized in roots, and then transported to other parts of the plant, especially to stomata via vascular tissues, and causes closure of stomata in response to adverse conditions (Zhang et al, 1987; Kuromori et al, 2010). It is involved in numerous physiological processes which help the plant to sense stress conditions. ABA stimulates short and long term responses under osmotic conditions including high salinity and drought (Verma et al, 2016). It maintains water balance through stomatal closure (short term response) and sometimes under severe conditions stimulates the regulation of stress response genes (long term response).
Recent studies have highlighted the signal transduction and perception pathways in rice as ABA dependent pathway and ABA independent pathway (Haak et al, 2017). ABA dependent pathway plays an important role in the regulation and control of the expression of stress responsive elements during osmotic and drought stresses (Yamaguchi-Shinozaki and Shinozaki, 2006). The ABA-signaling works through receptors presenting in subcellular compartments such as the cytosol, plasma membrane, chloroplast envelope and nucleus (Kuromori et al, 2010). ABA content is low under normal circumstances in plants, restricting the activity of sucrose non-fermenting 1-related protein kinase 2 (SnRK2) by protein phosphatase 2C (PP2C) which limits the dephosphorylation. Under drought stress, ABA levels rise and restrict the activity of PP2C by binding the respective promotor Pyrabactin resistance (PYR)/PYR-like (PYL)/regulatory components of abscisic acid receptors (RCARs) and triggering the activity of, thus promoting the ABA dependent physiological and molecular response (Danquah et al, 2014; Mehrotra et al, 2014; Dong et al, 2015; Verma et al, 2016).
Kim et al (2014) observed an over-expression of an ABA induced receptor gene in transgenic rice plants which showed improved tolerance against drought stress. Histone mono-ubiquitination 2 () and short vegetative phase (SVP) have also been reported as a central regulator of ABA catabolism, and the regulatory pathway, involving SVP, CYP707A1/3 and AtBG1, plays a critical role in plant response to water deficit and plant drought resistance (Wang et al, 2018; Ma et al, 2019). Similarly, the potential candidate genein rice is also involved in drought tolerance during germination and post germination stages in over-expression lines (Lou et al, 2017). ABA signaling-mediated drought-induced reticulocalbin (RCN), in molecular pathway of drought-regulated flowering transition, displays early heading and makesthe flowers insensitive to drought stress (Wang Y et al, 2020). The transgenic lines of rice have smaller stomatal aperture and lower expression ofgene that enhances drought tolerance through modulating stomatal closure and changingH2O2concentration, ultimately conferring higher tolerance against drought stress via ABA dependent pathway (Hu et al, 2017).
Jasmonic acid (JA)
Jasmonates are phytohormones derived from linolenic acid. They play a significant role in defense mechanisms against biotic and abiotic stresses in plants. JA initiates the signaling pathways of various genes that influence plant growth and development (especially root growth), viable pollen production, fruit ripening, tendril coiling, and pathogen and pest resistance. Various genes have been reported in rice genome which are precursor of JA and participate in response to water deficit conditions in rice.
The exogenous application of JA has also been proposed to enhance the abiotic stress tolerance in rice (Dhakarey et al, 2017). JA plays a vital role in stomatal closure by conserving water for plant surviveduring water deficit and adverse environmental conditions, like ABA. JA signaling pathway is controlled by the jasmonate-zim domain (JAZ) repressor protein. JA,used as jasmonate-insensitive/jasmonate-zim (JAI3/JAZ)proteins, binds and suppresses the activity of respective transcription factors including myelocytomatosis (MYC2),which playsno any functions in the absence of JA. JA and its derivatives are synthesized during stress conditions which free the active MYC2 transcription factor by degrading the repressive protein that is involved in upregulation of stress responsive genes (Chini et al, 2007).
Reactive oxygen species (ROS)
ROS, also known as reactive O2intermediates or active O2species, are produced due to partial reduction of atmospheric oxygen. ROS are categorized into four basic forms: hydrogen peroxide (H2O2), superoxide radical (O2·̄), singlet oxygen (1O2) and the hydroxyl radical (-OH). These ROS species have oxidizing potential and a characteristic half-life. Unlike atmospheric oxygen, singlet oxygen and hydroxyl radical are very active and can oxidize various parts of cellular components such as RNA, DNA, lipids and proteins (Mittler, 2002). The excessive use of nitrogen fertilizer (especially NH4+) in rice causes a spontaneous ROS burst, resulting in carbon scarcity and low yield (Yang et al, 2020). The cell walls, cellular membranes, chloroplasts, nuclei and mitochondria are major ROS production sites. The drought stress aggravates the lipid peroxidation in rice leaves, resulting in the accumulation of ROS in less drought resistant varieties (Wang et al, 2010). The level ofROS production is enhanced under drought stress due to various reasons. If CO2fixation declines, the NADP+regeneration is limited in the Calvin cycle, ultimately reducing the activity of electron transport chain (You and Chan, 2015). In photosystem II, O2is reduced toO2·̄by donation of an electron during Mehler reaction and further converted into H2O2by SOD, and finally into water in the presence of ascorbate peroxidase (Heber, 2002). Drought stress enhances the activity of RuBP oxygenation due to the lower CO2presence. The elevation of ROS levels due to the Mehler reaction is more difficult than that due to the common photorespiration.
Photorespiration enhances the level of H2O2production by up to 70% during drought stress (Noctor et al, 2002). There are various mechanisms in the plant to monitor the levels of ROS and prevent excess accumulation. The antioxidant machinery enables the plant to survive under adverse conditions as demonstrated in Fig. 3. This machinery comprises of enzymatic and non-enzymatic molecules. The enzymes include ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase (DHAR), guaiacol peroxidase (GPX), monodehydroascorbate reductase (MDAR), catalase (CAT) and SOD. The non-enzymatic compounds are reduced glutathione (GSH), carotenoid, ascorbic acid, flavonoids, α-tocopherol and the osmolyte proline (Fig. 3). Both components of the anti-oxidative defense system work together to scavenge ROS (Das and Roychoudhury, 2014; Xu et al, 2016). Gibberellin-Insensitive Dwarf 1 (GID1) can enhance the ROS-scavenging ability to regulate the stomatal response and submergence tolerance through both the ABA and gibberellin (GA) signaling pathways in rice (Du et al, 2015). Moreover, drought stress enhances the activity of GR for oxidizing and reducing of glutathione at appropriate level (de Carvalho, 2008). Drought tolerance is directly associated with the capability of the plant to scavenge ROS. The drought tolerant genotypes have ability to protect the cellular components from damage by oxidative stress. The intensity and duration facilitate the understanding of the complexity of ROS production and scavenge mechanism in rice.Moreover, the process, through which the plants scavenge ROS and encounter the drought stress, needs to be further investigated. Regarding plant defenses against ROS, drought tolerance is an intricate phenomenon needing more explorations about biochemical, genetic and molecular aspects (You and Chan, 2015). In brief, drought resistance is significantly associated with the activities of glutathione, malondialdehyde, superoxide free radicals (O2T) contents and antioxidative enzymes in rice. It directly correlates with oxidation capacity in rice (Wang et al, 2010).
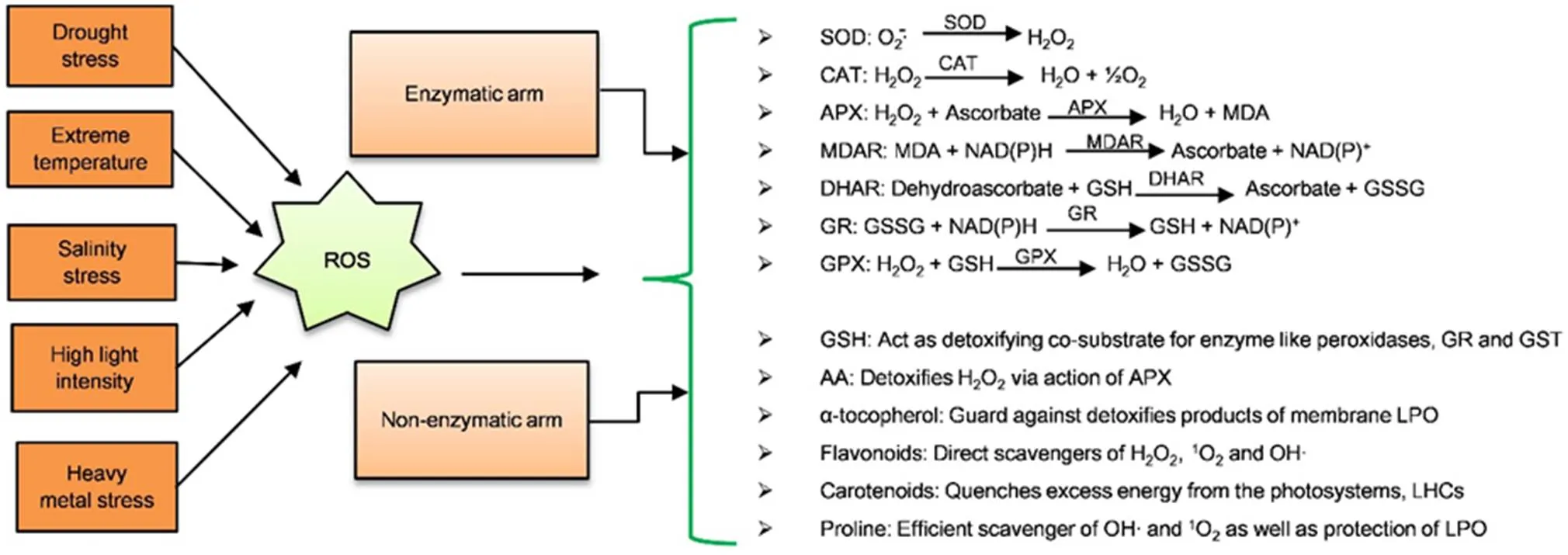
Fig. 3. ROS scavenging machinery having two arms: Enzymatic arm and non-enzymatic arm (Ullah et al, 2017).
Enzymatic arm contains various enzymes which convert ROS into other substances. Likewise, non-enzymatic arm contains other substances which scavenge ROS.
ROS, Reactive oxygen species; SOD, Superoxide dismutase; CAT, Catalase; APX, Ascorbate peroxidase; MDAR, Monodehydroascorbate reductase; MDA, Monodehydroascobate; NAD(P)H, Reduced nicotinamide adenine dinucleotide phosphate; DHAR, Dehydroascorbate reductase; GSH, Reduced glutathione; GSSG, Glutathione disulfide; GR, Glutathione reductase; GPX, Guaiacol peroxidase; GST, Glutathione S-transferase; AA, Ascorbic acid; LPO, Lactoperoxidas; LHC, Light harvesting complex.
Signaling pathways in response to drought stress in rice
Various signaling pathway responses encounter the drought stress in rice. The major pathways are discussed below.
Mitogen-activated protein kinase (MAPK)signaling pathway
The well-maintained pathways like MAPK exist in plants, yeasts and animals (Gustin et al, 1998). Phosphorylation cascades in MAPK protein usually contain three successively acting protein [a MAPK, a MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK)] and linkage of these three proteins with the diversity of extracellular stimuli. Despite insufficient knowledge about classical three-component module compositions and functions in rice, many MAPKs have been identified to be involved in transduction of signal against abiotic stress (Zhou et al, 2017). Salt, cold and sugar malnourishments persuade the expression of, which show expression at different development stages (Zhou et al, 2017)., a MAPK gene, is induced when manifold biotic and abiotic stresses like pathogen infection, drought, low temperature, salinity and ABA occur (Xiong et al, 2018).can inversely play roles in controlling abiotic stress tolerance. Overexpression ofin plants is linked with kinase activity and further with improved stress tolerance. Moreover, rice lines having suppressed expression ofshow less resistance against abiotic stress but augmented resistance against fungal and bacterial diseases (Bai and Matton, 2018; Quan et al, 2018).
Expression analysis shows that MAPKKK, ortholog of, possibly links with development and defense related response in rice (Ku et al, 2018). The MAPK kinaseexpression pattern is parallel tounder response to abiotic stress. BothandmRNA levels are also induced in abiotic stress. Yeast two-hybrid analysis shows that OsMAP1 interacts with OsMEK1. The-pathway may be involved in stress signaling (Lazar et al, 2014). The activation of numeroushas also been observed in stress induced by heavy metals like zinc, cadmium and copper (Gu and Lin, 2010). In, H2O2can precisely induce MAPKKK and ANP1, which cause activation ofand(Danquah et al, 2014; Ullah et al, 2017; Zhou et al, 2017). In rice roots, Zn-induced activation of 40- and 42-kDa MAPK is blocked by treatment with a ROS scavenger, suggesting that the second messenger ROS may mediate a Zn-triggered MAPK pathway (Bray et al, 2000; Liu and He, 2017; Quan et al, 2018).
Calcium signaling pathway
Calcium (Ca), as a second messenger, shows significant roles in a variety of signal transduction pathways. Numerous classes of Ca-sensing proteins with calcineurin B-like (CBL) proteins, calmodulin (CaM) and Ca-dependent protein kinases (CDPKs) have been characterized in plants (Shi et al, 2018). Ca2+activates the CDPKs and downstreams targets of Ca signaling in plants (Schulz et al, 2013). CDPKs contribute in stress signaling transduction pathways through either stimulus- dependent activation or directed functional target protein phosphorylation (Franz et al, 2011; Shi et al, 2018).
Approximately 34 CDPK genes have been identified by genome-wide analysis in(Mittal et al, 2017). Among these CDPKs, some are involved in different responses to abiotic stress and others in signaling of ABA. Loss-of-function inandmutants exhibits low tolerance against drought stress, and ABA-insensitive phenotypes for stomatal movement, seed germination and seedling growth. CPK4 and CPK11 phosphorylate two ABA-responsive transcription factors namely ABF1 and ABF4 to facilitate ABA signaling pathway (Zhou et al, 2016; Shi et al, 2018).overexpressing plants show enhanced tolerance to salt and drought stresses (Das and Roychoudhury, 2014).andalso take part in regulating ABA stomatal signaling and ion channel of guard cells./mutants show partial impairment of ABA-induced stomatal signaling (Mori et al, 2006). CPK6 stimulates the slow anion channel (SLAC1), and CPK3 triggers SLAC1 and its guard cell homolog (SLAH3). These initiations are totally Ca-dependent and controlled by the ABA signaling component phosphatase ABI1 (Zhang et al, 2016). CPK32 phosphorylates the ABA-responsive transcription factor ABF4, and the overexpression ofin plants improves sensitivity to ABA during seed germination as a result of up-regulated expressions of genes which are controlled by ABF4 (Wei et al, 2014). Mutant analysis indicates thatplays an important role in drought stress tolerance. Furthermore, CPK10, on interaction with heat shock protein 1 (HSP1), plays an important part in ABA- and Ca2+-mediated regulation of stomatal movement (Wei et al, 2014; Zhou et al, 2017).
Strategies to induce drought tolerance in rice
Reference to climate change scenario and ever-increasing demands of food, novel approaches of increasing crop vigor and tolerance against environmental stresses, especially drought, need to be utilized. There is an urgent need to develop rice genotypes, withlow water requiring, high yielding and good quality. For developing promising rice genotypes and making large screening program practical, approaches like marker-assisted breeding, QTL mapping, transgenic approach and genome editing can be used. Moreover, exogenous applications of various minerals, osmoprotectants and growth regulators for enhancing the drought tolerance can also be employed. Strenuous efforts are required for developing crop genotypes capable of maintaining yields under extreme environmental conditions.
Exogenous application of substances
Various plant regulators and osmoprotectants have been discovered and applied exogenenously to increase the tolerance of rice plant against drought. Foliar application of various hormones and omsoprotectants, such as polyamine, proline, ABA, GA3, glycinebetaine and salicylic acid,can mitigate the effects of stress by enhancingthe stomatic adjustment of the plants which improves the turgar pressure and increases the antioxident accumulation for ROS detoxification(Ullah et al, 2017). Moreover, such approaches help the plants in maintaining the intergrity of enzymes, macromolecules and membrane structures in response to drought stress (Ullah et al, 2017).
Foliar application of glycinebetaine and kinetin at the flowering stage decreases the adverse effects of drought on rice by elevating the level of proline and soluble sugar in leaves and panicles (Din et al, 2015). The non-mutants as compared to the mutants for drought and salinity tolerence are reported to negatively affect on stomata regulation by changing H2O2homeostasis. Similarly, the exogeneous application of GA increases the stomatal conductance, xylem vessel size, net photosynthsis rate, leaf growth and transpiration rate in rice (Borah and Baruah, 2016). Moreover, the exogeneous applications of JA and MeJAs (methyl jasmonates) are also proposed to augment the drought tolerance in rice. Nevertheless, the overall impact of these hormones depends on the genotype, and the concentration of enzyme used which needs to be optimized depending upon numerous conditions (Ahmad et al, 2016).
Marker-assisted selection (MAS) based on drought-related QTLs/genes
Breeders are facing various challenges to improve the drought resistance trait in rice. The conventional breeding methodologies are unable to achieve the goals because of epistatic interaction in resistance contributing genes (Khan et al, 2018). DNA markers have been used to elaborate the drought tolerance in rice under the agro-ecosystems. It is reported that the substantial portion which involves in improving the abiotic resistance is explained by one or two QTLs derived from wild progenitor. However, small portion of variability is explained by QTLs for abiotic resistance under various environmental conditions(Gao et al, 2015).
QTLs and genes related with drought tolerance mechanisms have been identified by fine mapped and characterized as mendelian factors (Dixit et al, 2014). Ramegowda et al (2014) reported a drought-inducible receptor-like cytoplasmic kinase GUDK is required for grain yield under drought and well-watered conditions. Loss-of-functionmutant lines exhibit sensitivity to salinity, osmotic stress and ABA treatments at the seedling stage, and a reduction in photosynthesis and plant biomass under controlled drought stress at the vegetative stage. In rice, the() gene family contain seven members. Wang et al (2014)found that-overexpression rice shows drought tolerance by decreased water loss from leaves and enhanced ROS-scavenging activity under drought conditions. Wu et al (2019) characterized a new gene(), a member of group E of bZIP transcription factor family in rice, which plays a positive role in drought tolerance and provides valuable targets for breeding drought-tolerant rice genotypes. The potential genes linked to DNA markers can be used in MAS and molecular breeding of drought-tolerant rice to enhance yield.
Transgenic approaches
The transgenic lines are more tolerant against drought. OsERF71 is a localized nuclear protein involved in reducing the water loss, and also participates to regulate several proline and ABA response biosynthesis genes during drought stress in rice. The drought- inducible, a group Ib member of the rice ERF family with four conserved motifs, CMI-1, -2, -3 and -4. Whenis overexpressed in an root- specific (ROX) or whole-body (OX) manner, transgenicROXplants exhibit higher grain yield thanOXand non-transgenic plants under field-drought conditions (Jung et al, 2017). Hence, it is evident that the transgenic approaches making use of such proteins can play a role in developing water stress tolerant rice genotypes. OsSKIPa, a rice homolog of human Ski-interacting protein (SKIP), can complement the lethal defect of the knockout mutant of SKIP homolog in yeast and positively modulate cell viability and stress tolerance of rice. Transgenic rice overexpressingexhibits significantly improved growth performance in the medium containing stress agents (ABA, salt or mannitol) and drought resistance at both the seedling and reproductive stages. The-overexpressing rice shows significantly increased ROS-scavenging ability and transcript levels of many stress-related genes, includingand rice homologs of,and, under drought stress conditions (Hou et al, 2009).
Photosynthesis-related gene expression and senescence- related degradation process are reduced in drought tolerance lines as compared to drought sensitive lines. These comprehensive expression data facilitate the researchers to construct marker transcript for selection of drought tolerance lines from the range of rice germplasm through integrated analysis of gene expression and stress tolerance (Huang et al, 2018; Sahebi et al, 2018). These transcript markers show a significant correlation between tolerance and expression levels under drought stress in rice germplasm. The genes are involved in production of cytosolic fructose- 1,6-bisphosphatase that catalyzes the regulation in C-metabolism is also considered to be important in drought tolerance in rice. Several studies reported that drought responsive genes overlap with those that expressed during panicle and seed development (Todaka et al, 2015).
CRISPR/Cpf1 and CRISPR/Cas9 systems and theirapplication in rice genome
In the recent era, the emergence and advancement in modern genome editing technology curtailed hurdle of conventional breeding for crop improvement. This technology gives us the opportunity to modify a desirable gene with the help of engineered site- specific nucleases (SSNs) on the specific location in the genome (Zaman et al, 2019). The genome editing tools open the new era and extend the research to enhance the yield potential and develop new stress tolerant genotypes. The SSNs such as TALENs (transcriptional activator-like effector nucleases) and CRISPR (clustered regularly interspaced short palindromic repeats)-associated endonuclease Cas9 (CRISPR/Cas9) break the targeted DNA repaired through natural repair mechanism including HR (homologous recombination) and NHEJ (non-homologous end joining). Homologous recombination pathway is precise gene replacement or gene knock in via exchange of homologous sequence while the non-homologous end joining is less error prone pathway (Baltes et al, 2014; Mishra et al, 2018).
The genes related with drought tolerance mechanisms, including transcriptional factors (OsNAC006) and ABA receptors (OsPYL), are the positive regulators and can be targets for genome editing in rice (Biswal et al, 2019). Meanwhile, some negative regulators, such as drought hypersensitive gene (),and, and the gain in function mutant ofas a promoter can also be candidate for genome editing to impart the drought resistance. Wang B et al (2020)discovered thatis constitutively expressed in rice, and regulated by H2O2, cold, heat, ABA, indole-3-acetic acid (IAA), GA, NaCl and polyethylene glycol (PEG) 6000 treatments. Furthermore, knockout ofusing the CRISPR/Cas9 system results in drought and heat sensitivity. Usman et al (2020) reported thatis mutagenized through CRISPR/Cas9 in rice. Themutants show an increase in grain yield under both drought and well watered field conditions. Most of the different expressed proteins related to circadian clock rhythm, drought response, and reactive oxygen species are upregulated in the mutant plants.
The submergence tolerance gene () gives an opportunity to induce the higher tolerance by creation of alleles using the base editing approaches (Fukao and Bailey-Serres, 2008). A mutant allele of drought and salt tolerance (DST) gene has been created by CRISPR/Cas9. The recessive mutant of the genehas broader leaves with reduced stomatal density. It clearly enhances the water retention in leaves during stress period. Meanwhile, the loss-of-function mutant ofis involved in down-regulation of,andstomatal development genes (Santosh et al, 2020).
Future perspectives
In current situation, drought is a major constraint in rice production. The situation is expected to even worsen in future as water availability is reducing over time. To meet the global needs of food, it is imperative to develop drought tolerance rice varieties. Physiological factors like JA, ABA and antioxidant defense mechanisms are crucial to plant’s survive under drought. Further research is underway to uncover potential regulatory mechanisms and novel genes involved in the response to drought stress in rice. Exploitation of genetic resources from drought stress adapted extremophiles is also being investigated. The elucidation of such genetic resources has become relatively easy because of progress in functional genomics. Recent advancements in expression profiling and molecular biology have opened new vistas of research which need to be exploited for developing tolerance against abiotic stress in rice. Therefore, currently available options of marker-assisted breeding,transgenic rice and genome editing should be employed at a rapid pace. These techniques can significantly contribute in enhancing drought tolerance in rice in coming years. Such rice cultivars will not only feed the world, but also the use of limited water resource towards rice production will diminish offering a sustainable future to this crop. Nevertheless, intensive efforts are required to dissect the complex traits of drought tolerance through a system biology approach to interrelate the plant genetics, physiology, root architecture, osmotic adjustments, stomatal conductance, field performance, and stress tolerance.
Ahmad P, Rasool S, Gul A, Sheikh S A, Akram N A, Ashraf M, Kazi A M, Gucel S. 2016. Jasmonates: Multifunctional roles in stress tolerance., 7: 813.
Ahmed S, Rashid M A R, Zafar S A, Azhar M T, Waqas M, Uzair M, Rana I A, Azeem F, Chung G, Ali Z, Atif R M. 2021. Genome- wide investigation and expression analysis of APETALA-2 transcription factor subfamily reveals its evolution, expansion and regulatory role in abiotic stress responses inrice (L. ssp.)., 113(1): 1029‒1043.
An J, Li Q X, Yang J J, Zhang G Q, Zhao Z X, Wu Y Z, Wang Y, Wang W. 2019. Wheat F-box protein TaFBA1 positively regulates plant drought tolerance but negatively regulates stomatal closure., 10: 1242.
Bai F W, Matton D P. 2018. The Arabidopsis Mitogen-Activated Protein Kinase Kinase Kinase 20 (MKKK20) C-terminal domain interacts with MKK3 and harbors a typical DEF mammalian MAP kinase docking site., 13(8): e1503498.
Baltes N J, Gil-Humanes J, Cermak T, Atkins P A, Voytas D F. 2014. DNA replicons for plant genome engineering., 26(1): 151‒163.
Basu S, Ramegowda V, Kumar A, Pereira A. 2016. Plant adaptation to drought stress., 5: 1554.
Bertolino L T, Caine R S, Gray J E. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world., 10: 225.
Biswal A K, Mangrauthia S K, Reddy M R, Yugandhar P. 2019. CRISPR mediated genome engineering to develop climate smart rice: Challenges and opportunities., 96: 100‒106.
Borah L, Baruah K K. 2016. Effects of foliar application of plant growth hormone on methane emission from tropical rice paddy., 233: 75‒84.
Bray E A, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stresses.: Gruissem W, Buchannan B, Jones R. Biochemistry and Molecular Biology of Plants. Rockville, MD, USA: American Society of Plant Physiologists: 1158‒1249.
Caine R S, Yin X J, Sloan J, Harrison E L, Mohammed U, Fulton T, Biswal A K, Dionora J, Chater C C, Coe R A, Bandyopadhyay A, Murchie E H, Swarup R, Quick W P, Gray J E. 2019. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions., 221(1): 371‒384.
Chini A, Fonseca S, Fernández G, Adie B, Chico J M, Lorenzo O, García-Casado G, López-Vidriero I, Lozano F M, Ponce M R, Micol J L, Solano R. 2007. The JAZ family of repressors is the missing link in jasmonate signalling., 448: 666‒671.
Comas L H, Becker S R, Cruz V M V, Byrne P F, Dierig D A. 2013. Root traits contributing to plant productivity under drought., 4: 442.
Crawford A J, McLachlan D H, Hetherington A M, Franklin K A. 2012. High temperature exposure increases plant cooling capacity., 22(10): R396‒R397.
Danquah A, de Zelicourt A, Colcombet J, Hirt H. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses., 32(1): 40‒52.
Das K, Roychoudhury A. 2014. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants., 2: 53.
de Carvalho M H C. 2008. Drought stress and reactive oxygen species: Production, scavenging and signaling., 3(3): 156‒165.
Dhakarey R, Raorane M L, Treumann A, Peethambaran P K, Schendel R R, Sahi V P, Hause B, Bunzel M, Henry A, Kohli A, Riemann M. 2017. Physiological and proteomic analysis of the rice mutantsuggests a negative regulatory role of jasmonic acid in drought tolerance., 8: 1903.
Din J U, Khan S U, Khan A, Naveed S. 2015. Effect of exogenously applied kinetin and glycinebetaine on metabolic and yield attributes of rice (L.) under drought stress., 27(1): 75‒81.
Ding W N, Wu J, Ye J, Zheng W J, Wang S S, Zhu X N, Zhou J Q, Pan Z C, Zhang B T, Zhu S H. 2018. A Pelota-like gene regulates root development and defence responses in rice., 122(3): 359‒371.
Dixit S, Huang B E, Sta Cruz M T, Maturan P T, Ontoy J C E, Kumar A. 2014. QTLs for tolerance of drought and breeding for tolerance of abiotic and biotic stress: An integrated approach., 9(10): e109574.
Dong T, Park Y, Hwang I. 2015. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling., 58: 29‒48.
Du H, Chang Y, Huang F, Xiong L Z. 2015. GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice., 57(11): 954‒968.
Elliott J, Deryng D, Müller C, Frieler K, Konzmann M, Gerten D, Glotter M, Flörke M, Wada Y, Best N, Eisner S, Fekete B M, Folberth C, Foster I, Gosling S N, Haddeland I, Khabarov N, Ludwig F, Masaki Y, Olin S, Rosenzweig C, Ruane A C, Satoh Y, Schmid E, Stacke T, Tang Q H, Wisser D. 2014. Constraints and potentials of future irrigation water availability on agricultural production under climate change., 111(9): 3239‒3244.
Fahad S, Bajwa A A, Nazir U, Anjum S A, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan M Z, Alharby H, Wu C, Wang D P, Huang J L. 2017. Crop production under drought and heat stress: Plant responses and management options., 8: 1147.
Farooq M, Hussain M, Wahid A, Siddique K H M. 2012. Drought stress in plants: An overview.: Aroca R. Plant Responses to Drought Stress. Berlin, Germany: Springer: 1‒33.
Franz S, Ehlert B, Liese A, Kurth J, Cazalé A C, Romeis T. 2011. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in., 4(1): 83‒96.
Fukao T, Bailey-Serres J. 2008. Submergence tolerance conferred byis mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice., 105: 16814‒16819.
Gao F M, Wen W E, Liu J D, Rasheed A, Yin G H, Xia X C, Wu X X, He Z H. 2015. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring., 6: 1099.
Gu Q H, Lin R L. 2010. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1., 108(4): 891‒897.
Gupta A, Rico-Medina A, Caño-Delgado A I. 2020. The physiology of plant responses to drought., 368: 266‒269.
Gupta B, Huang B. 2014. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization., 2014: 701596.
Gustin M C, Albertyn J, Alexander M, Davenport K. 1998. MAP kinase pathways in the yeast., 62(4): 1264‒1300.
Haak D C, Fukao T, Grene R, Hua Z H, Ivanov R, Perrella G, Li S. 2017. Multilevel regulation of abiotic stress responses in plants., 8: 1564.
Hadiarto T, Tran L S P. 2011. Progress studies of drought-responsive genes in rice., 30(3): 297‒310.
Hasanuzzaman M, Alam M M, Rahman A, Hasanuzzaman M, Nahar K, Fujita M. 2014. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (L.) varieties., 2014: 757219.
Hasanuzzaman M, Anee T I, Bhuiyan T F, Nahar K, Fujita M. 2019. Emerging role of osmolytes in enhancing abiotic stress tolerance in rice.: Hasanuzzaman M, Fujita M, Nahar K, Biswas J K. Advances in Rice Research for Abiotic Stress Tolerance. London, UK: Woodhead Publishing: 677‒708.
Heber U. 2002. Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants., 73: 223‒231.
Henry A, Cal A J, Batoto T C, Torres R O, Serraj R. 2012. Root attributes affecting water uptake of rice () under drought., 63(13): 4751‒4763.
Hou X, Xie K B, Yao J L, Qi Z Y, Xiong L Z. 2009. A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance., 106(15): 6410‒6415.
Hu Y, Wu Q Y, Peng Z, Sprague S A, Wang W, Park J, Akhunov E, Jagadish K S V, Nakata P A, Cheng N H, Hirschi K D, White F F, Park S. 2017. Silencing ofin rice improves drought stress tolerance by modulating ROS accumulation and stomatal closure., 7(1): 15950.
Huang L Y, Wang Y X, Wang W S, Zhao X Q, Qin Q, Sun F, Hu F Y, Zhao Y, Li Z C, Fu B Y, Li Z K. 2018. Characterization of transcription factor geneconferring drought tolerance in rice., 9: 94.
Jiang J J, Ma S H, Ye N H, Jiang M, Cao J S, Zhang J H. 2017. WRKY transcription factors in plant responses to stresses., 59(2): 86‒101.
Jung H, Chung P J, Park S H, Redillas M C F R, Kim Y S, Suh J W, Kim J K. 2017. Overexpression ofcauses regulation of, a calmodulin-like protein gene that enhances root growth and drought tolerance., 15(10): 1295‒1308.
Kartika K, Jun-Ichi S, Benyamin L, Shin Y, Andi W, Sabaruddin K, Laily I W, Erna S, Yoshihiro N. 2020. Morpho-physiological response ofto gradual soil drying., 27(1): 67‒74.
Kim H, Lee K, Hwang H, Bhatnagar N, Kim D Y, Yoon I S, Byun M O, Kim S T, Jung K H, Kim B G. 2014. Overexpression ofin rice enhances drought tolerance, inhibits growth, and modulates gene expression., 65(2): 453‒464.
Khan A, Pan X D, Najeeb U, Tan D K Y, Fahad S, Zahoor R, Luo H H. 2018. Coping with drought: stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness., 51(1): 47.
Ku Y S, Sintaha M, Cheung M Y, Lam H M. 2018. Plant hormone signaling crosstalks between biotic and abiotic stress responses., 19(10): 3206.
Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. 2010. ABC transporter AtABCG25 is involved in abscisic acid transport and responses., 107(5): 2361‒2366.
Kusumi K, Hirotsuka S, Kumamaru T, Iba K. 2012. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein., 63(15): 5635‒5644.
Lazar A, Coll A, Dobnik D, Baebler S, Bedina-Zavec A, Zel J, Gruden K. 2014. Involvement of potato (L.) MKK6 in response toY., 9(8): e104553.
Lee D K, Jung H, Jang G, Jeong J S, Kim Y S, Ha S H, Do Choi Y, Kim J K. 2016. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance., 172(1): 575‒588.
Li J J, Guo X, Zhang M H, Wang X, Zhao Y, Yin Z G, Zhang Z Y, Wang Y M, Xiong H Y, Zhang H L, Todorovska E, Li Z C. 2018.confers drought tolerance via modulating ABA signaling and proline biosynthesis., 270: 131‒139.
Li Y, Zhou Y, Guo S W, Shen Q R. 2007. Effects of different N forms on root morphology and water absorption of lowland and upland rice plants., 21(3): 294‒298. (in Chinese with English abstract)
Liu Y K, He C Z. 2017. A review of redox signaling and the control of MAP kinase pathway in plants., 11: 192‒204.
Lou D J, Wang H P, Liang G, Yu D Q. 2017. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice., 8: 993.
Ma S Q, Tang N, Li X, Xie Y J, Xiang D H, Fu J, Shen J Q, Yang J, Tu H F, Li X H, Hu H H, Xiong L Z. 2019. Reversible histone H2B monoubiquitination fine-tunes abscisic acid signaling and drought response in rice., 12(2): 263‒277.
Mahrookashani A, Siebert S, Hüging H, Ewert F. 2017. Independent and combined effects of high temperature and drought stress around anthesis on wheat., 203(6): 453‒463.
Mehrotra R, Bhalothia P, Bansal P, Basantani M K, Bharti V, Mehrotra S. 2014. Abscisic acid and abiotic stress tolerance: Different tiers of regulation., 171(7): 486‒496.
Mishra R, Joshi R K, Zhao K J. 2018. Genome editing in rice: Recent advances, challenges, and future implications., 9: 1361.
Mittal S, Mallikarjuna M G, Rao A R, Jain P A, Dash P K, Thirunavukkarasu N. 2017. Comparative analysis of CDPK family in maize,, rice, and sorghum revealed potential targets for drought tolerance improvement., 5: 115.
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance., 7(9): 405‒410.
Mori I C, Murata Y, Yang Y Z, Munemasa S, Wang Y F, Andreoli S, Tiriac H, Alonso J M, Harper J F, Ecker J R, Kwak J M, Schroeder J I. 2006. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure., 4(10): e327.
Muthayya S, Sugimoto J D, Montgomery S, Maberly G F. 2014. An overview of global rice production, supply, trade, and consumption., 1324(1): 7‒14.
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer C H. 2002. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration?, 89(7): 841‒850.
Ogata T, Ishizaki T, Fujita M, Fujita Y. 2020. CRISPR/Cas9- targeted mutagenesis ofconfers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice., 15(12): e0243376.
Papanatsiou M, Petersen J, Henderson L, Wang Y, Christie J M, Blatt M R. 2019. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth., 363: 1456‒1459.
Quan R D, Wang J, Hui J, Bai H B, Lü X L, Zhu Y X, Zhang H W, Zhang Z J, Li S H, Huang R F. 2018. Improvement of salt tolerance using wild rice genes., 8: 2269.
Ramegowda V, Basu S, Krishnan A, Pereira A. 2014. Riceis required for drought tolerance and grain yield under normal and drought stress conditions., 166(3): 1634‒1645.
Rebouillat J, Dievart A, Verdeil J L, Escoute J, Giese G, Breitler J C, Gantet P, Espeout S, Guiderdoni E, Périn C. 2009. Molecular genetics of rice root development., 2(1): 15‒34.
Sahebi M, Hanafi M M, Rafii M Y, Mahmud T M M, Azizi P, Osman M, Abiri R, Taheri S, Kalhori N, Shabanimofrad M, Miah G, Atabaki N. 2018. Improvement of drought tolerance in rice (L.): Genetics, genomic tools, and the WRKY gene family., 2018: 3158474.
Santosh Kumar V V, Verma R K, Yadav S K, Yadav P, Watts A, Rao M V, Chinnusamy V. 2020. CRISPR-Cas9 mediated genome editing of(OsDST) gene in indica mega rice cultivar MTU1010., 26(6): 1099–1110.
Saud S, Fahad S, Chen Y J, Ihsan M Z, Hammad H M, Nasim W, Amanullah Jr, Arif M, Alharby H. 2017. Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky bluegrass plants., 8: 983.
Schulz P, Herde M, Romeis T. 2013. Calcium-dependent protein kinases: Hubs in plant stress signaling and development., 163(2): 523‒530.
Serraj R, McNally K L, Slamet-Loedin I, Kohli A, Haefele S M, Atlin G, Kumar A. 2011. Drought resistance improvement in rice: An integrated genetic and resource management strategy., 14(1): 1‒14.
Shi S J, Li S G, Asim M, Mao J J, Xu D Z, Ullah Z, Liu G S, Wang Q, Liu H B. 2018. Thecalcium-dependent protein kinases (CDPKs) and their roles in plant growth regulation and abiotic stress responses., 19(7): 1900.
Silva D V, Cabral C M, Ferreira E A, de Carvalho F P, dos Santos J B, Dombroski J L D. 2018. Anatomical adaptations to different soil moisture contents in palisade grass and smooth pigweed., 65(4): 306‒313.
Tabassum J, Ahmad S, Hussain B, Mawia A M, Zeb A, Luo J. 2021. Applications and potential of genome-editing systems in rice improvement: Current and future perspectives., 11(7): 1359.
Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. 2015. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants., 6: 84.
Ullah A, Sun H, Yang X Y, Zhang X L. 2017. Drought coping strategies in cotton: Increased crop per drop., 15(3): 271‒284.
Usman B, Nawaz G, Zhao N, Liao S Y, Liu Y G, Li R B. 2020. Precise editing of thegene by RNA-guided Cas9 nuclease confers enhanced drought tolerance and grain yield in rice (L.) by regulating circadian rhythm and abiotic stress responsive proteins., 21(21): 7854.
Verma V, Ravindran P, Kumar P P. 2016. Plant hormone-mediated regulation of stress responses., 16: 86.
Wang B, Zhong Z H, Wang X, Han X Y, Yu D S, Wang C G, Song W Q, Zheng X L, Chen C B, Zhang Y. 2020. Knockout of thetranscription factor causes drought and heat sensitivity in rice., 21(7): 2288.
Wang H Z, Zhang L H, Ma J, Li X Y, Li Y, Zhang R P, Wang R Q. 2010. Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage., 9(5): 633‒641.
Wang Y, Lu Y Y, Guo Z Y, Ding Y F, Ding C Q. 2020., a TFL1-like gene, responses to drought stress and regulates rice flowering transition., 13(1): 70.
Wang Z, Wang F X, Hong Y C, Yao J J, Ren Z Z, Shi H Z, Zhu J K. 2018. The flowering repressor SVP confers drought resistance inby regulating abscisic acid catabolism., 11(9): 1184‒1197.
Wei S Y, Hu W, Deng X M, Zhang Y Y, Liu X D, Zhao X D, Luo Q C, Jin Z Y, Li Y, Zhou S Y, Sun T, Wang L Z, Yang G X, He G Y. 2014. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility., 14: 133.
Wu D, Guo Z L, Ye J L, Feng H, Liu J X, Chen G X, Zheng J S, Yan D M, Yang X Q, Xiong X, Liu Q, Niu Z Y, Gray A P, Doonan J H, Xiong L Z, Yang W N. 2019. Combining high-throughput micro-CT-RGB phenotyping and genome-wide association study to dissect the genetic architecture of tiller growth in rice., 70(2): 545‒561.
Xiong H Y, Yu J P, Miao J L, Li J J, Zhang H L, Wang X, Liu P L, Zhao Y, Jiang C H, Yin Z G, Li Y, Guo Y, Fu B Y, Wang W S, Li Z K, Ali J, Li Z C. 2018. Natural variation inincreases drought tolerance in rice by inducing ROS scavenging., 178(1): 451‒467.
Xu Z Z, Jiang Y L, Jia B R, Zhou G S. 2016. Elevated-CO2response of stomata and its dependence on environmental factors., 7: 657.
Yamaguchi-Shinozaki K, Shinozaki K. 2006. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses., 57: 781‒803.
Yang S Y, Hao D L, Jin M, Li Y, Liu Z T, Huang Y N, Chen T X, Su Y H. 2020. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice., 20(1): 143.
You J, Chan Z L. 2015. ROS regulation during abiotic stress responses in crop plants., 6: 1092.
Zafar S A, Zaidi S S E, Gaba Y, Singla-Pareek S L, Dhankher O P, Li X Y, Mansoor S, Pareek A. 2020. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing., 71(2): 470‒479.
Zaman Q U, Li C, Cheng H T, Hu Q. 2019. Genome editing opens a new era of genetic improvement in polyploid crops., 7(2): 141‒150.
Zhang A, Ren H M, Tan Y Q, Qi G N, Yao F Y, Wu G L, Yang L W, Hussain J, Sun S J, Wang Y F. 2016. S-type anion channels SLAC1 and SLAH3 function as essential negative regulators of inward K+channels and stomatal opening in., 28(4): 949‒955.
Zhang H, Li Y Y, Zhu J K. 2018. Developing naturally stress- resistant crops for a sustainable agriculture., 4(12): 989‒996.
Zhang J H, Schurr U, Davies W J. 1987. Control of stomatal behaviour by abscisic acid which apparently originates in the roots., 38(7): 1174‒1181.
Zhou H, He M, Li J, Chen L, Huang Z F, Zheng S Y, Zhu L Y, Ni E D, Jiang D G, Zhao B R, Zhuang C X. 2016. Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediatedediting system., 6: 37395.
Zhou H Y, Ren S Y, Han Y F, Zhang Q, Qin L, Xing Y. 2017. Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in., 18(8): 1766.
14 April 2021;
2 August 2021
Muhammad Abdul Rehman Rashid (rashidpbg@hotmail.com)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.08.001
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Screening of Rice Germplasm and Processing Methods to Produce Low Glycemic Rice
- Cloning and Functional Analysis of Calcineurin Subunits A and B in Development and Fecundity of Nilapavata lugens (Stål)
- Comparisons of Metabolic Profiles for Carbohydrates, Amino Acids, Lipids, Fragrance and Flavones During Grain Development in indica Rice Cultivars
- Pectin Methylesterases Enhance Root Cell-Wall Phosphorus Remobilization in Rice
- Genetic Improvement of Rice for Bacterial Blight Resistance: Present Status and Future Prospects
- Improving Rice Blast Resistance by Mining Broad-Spectrum Resistance Genes at Pik Locus

