针对二尖瓣反流经右胸小切口或常规正中开胸术行二尖瓣修复术对心肺功能的影响
黄海涛,王 飞,丁胜光,夏春秋,仲崇俊,于晓强
南通大学附属第二医院,南通市第一人民医院胸心血管外科,江苏 南通 226001
Introduction
Minimal invasive cardiac surgery (MICS) has already appeared for two decades. With the development of open heart surgery and cardiopulmonary bypass technology, MICS was becoming mature gradually.Concerning surgery, access to Minimal invasive mitral valve surgery (MIMVS) can be subdivided into two groups: partial sternotomy and right thoracotomy,including the open and video-assisted methods, and minimal invasive surgical devices. Thus, MIMVS does not refer to a single procedure but rather to a group of methods aimed at decreasing surgical trauma, by minimizing the size of the incisions and avoiding full sternotomy without compromising surgical safety and efficiency[1].
Several studies have shown that MIMVS provided patients with greater satisfaction, maintaining the same quality and safety as the standard mitral valve surgery approach[2-3]. However, some other researches complained that MIMVS required a long learning curve and was associated with higher incidence of neurological events, aortic dissection, groin complications and higher rate of mitral valve replacement instead of mitral valve repair[4-5].
MIMVS through right mini-thoracotomy was performed in several medical centers at home and abroad; however, the procedure of right minithoracotomy for MR repair was not exactly the same.Comparison results of the clinical outcomes between MI and CFS for MR repair were reported rarely. In this study, clinical outcomes will be compared between patients with MR who received MI or CFS. The specific procedure of MI mitral repair will be described in detail. The safety and superiority of MI will be elucidated by comparing with CFS. Postoperative inflammatory reaction has a relationship with postoperative complications[6]. The clinical indicators of inflammatory reaction will be compared between two groups, and its correlation with cardiopulmonary function will be analyzed as well.
Methods
Patients and grouping: from 2007 to 2018, 96 patients with MR underwent CFS mitral repair (n=51)or MI mitral repair (n=45) at First People’s Hospital in Nantong, China. All patients took routine examinations such as blood routine tests, blood gas analysis, electrocardiogram, and echocardiogram.Patients with coronary artery disease were excluded(when age≥55, coronary artery angiography was performed regularly). All operations were mainly performed by surgeon Zhong CJ and Lu CX. Patients with lower BMI and better pulmonary function without strong pleural adhesions and severe COPD were recommended for MI. The study was approved by the ethical committee of the First People’s Hospital of Nantong, Nantong University, and individual consent form was achieved at the beginning of this study.
Surgical techniques
In CFS group, the procedure was same as described by Coutinho et al[1], in brief, which include: approach used conventional ascending aorta,superior and inferior vena cava cannulation, and mitral valve exposure with right atrium and atrial septum approach.
In MI group, patients were in a supine position with the right side of the chest slightly elevated (about 30 degrees). Patients were intubated with doublelumen endotracheal tube. Single lung ventilation was started when the thoracic cavity was open via the right fourth intercostal space incision (Fig. 1A 1#). Four ports were made in right chest wall after single ventilation (Fig. 1): one incision 4-5 cm in length in the fourth intercostal between the anterior and median axillary lines as the main operation ports (Fig. 1A 1#,a mini retractor was inserted to spread the intercostal space); one port about 1cm in the third intercostal anterior axillary lines as the observation port for thoracoscope (Fig. 1A 2#); one ports about 1 mm in length in the furth intercostal posterior axillary line for transthoracic Chitwood aortic cross-clamp, left ventricular vent catheter, carbon dioxide insufflator and atrium pull wire (Fig. 1A 3#); the puncture ports were made fifth posterior axillary line for pulling atrium (Fig. 1A 4#). A special retractor for left atrium was inserted from the 5# port in figure 1B.Cardiopulmonary bypass was all conducted with femoral artery cannula perfusion, long femoral venous cannula drainage (Fig. 1C 6# incision for femoral artery and venous), and direct transthoracic aortic clamping (Fig. 2A). Cardiac arrest was achieved by delivering antegrade cold crystalloid cardioplegia and later warm blood cardioplegia into the ascending aorta directly (Fig. 2A). The needle vent catheter was inserted from the incision (Fig. A1#). The other surgical steps were shown in (Fig.2).
General data collection and analysis
Data collected included: demographics, incision length, overall operative times, CPB time, aortic crossclamp time, blood loss during operation and postoperative day 1 (POD1), blood transfusion during and post-operation, intensive care unit stay, in-hospital stay, and mortality. Incision pain, systemic inflammatory response, and oxygen index on pre-, postoperative day,POD 1 to 5 was recorded.
Patients were routinely followed-up postoperation according to the following protocol: 1 monthly for 6 months, 3 monthly for 1 year, 6 monthly for 2 years,and yearly thereafter. The median follow-up time was 49 months (interquartile range 30-60 months) and the follow-up data were 98.2 % complete. Overall survival, reoperation and MR recurrence free survival was compared between two groups.
Evaluation of perioperative cardiac function
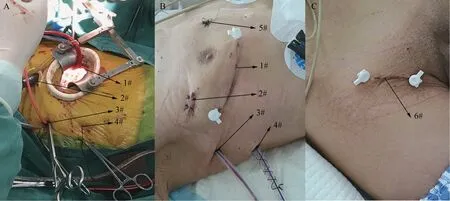
图1 手术中切口和通道及其作用Fig. 1 The incisions and ports performed during operation and their functions
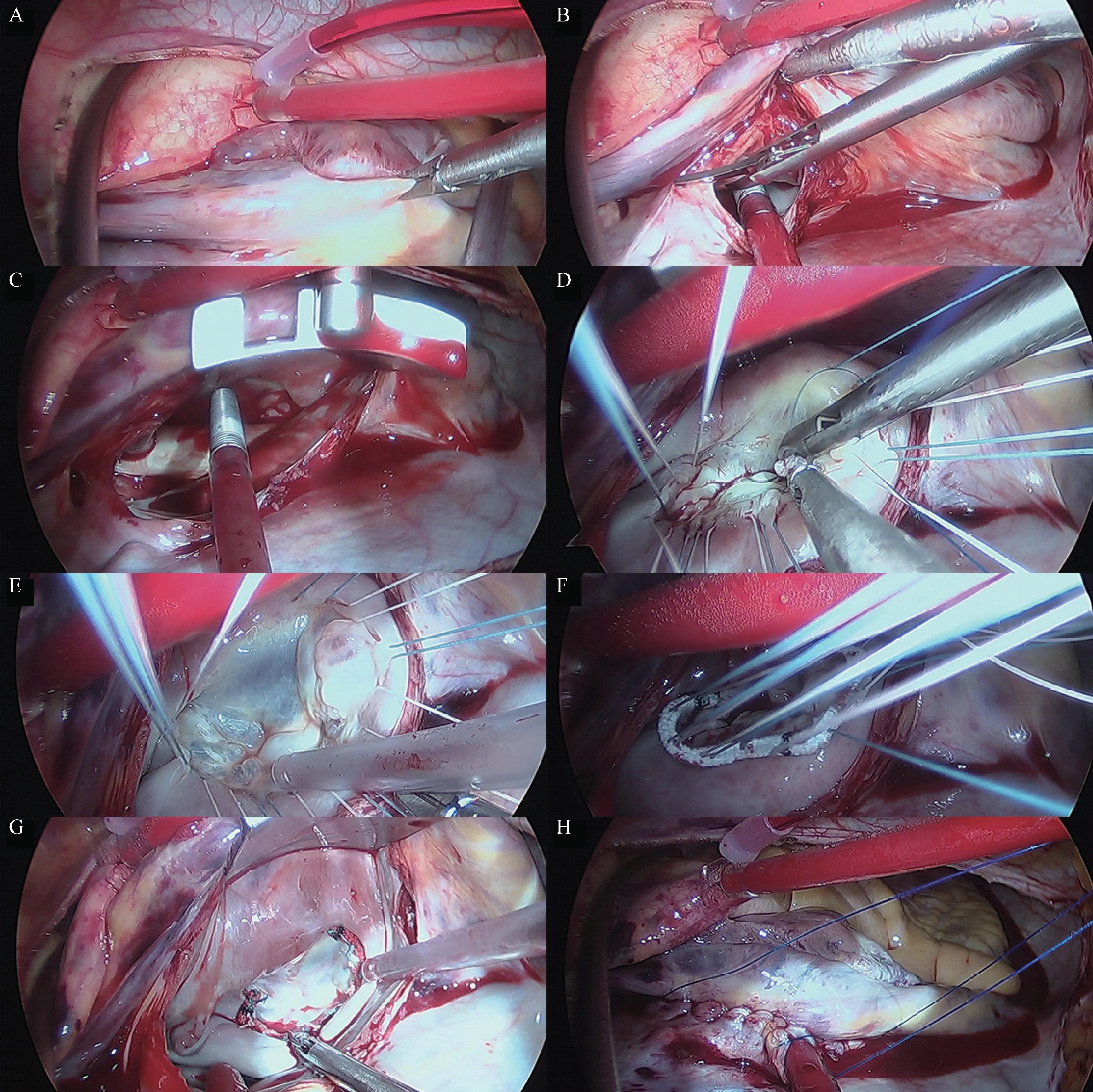
图2 胸腔镜辅助下的手术过程Fig. 2 The surgical procedure under thoracoscopy
All patients had a preoperative transthoracic echocardiography as well as at discharge.Transesophageal echocardiography device was placed before heparinization. Transthoracic echocardiography was performed preoperatively and postoperatively.Postoperative echocardiographic evaluations were performed before discharge and regularly every six to twelve months. The patients were followed at outpatient clinic in our hospital or related facilities. Patient clinical status and most recent echocardiographic results were obtained by telephone or outpatient interview. Ejection fraction (EF), left ventricular enddiastolic dimension (LVEDD), and MR grade were recorded. The severity of MR was graded following the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommendations[2]. Recurrent MR was fall into 4 grades: trivial, 1 of 4; mild, 2 of 4; moderate, 3 of 4; and severe, 4 of 4.
Evaluation of perioperative pulmonary function
Blood gas test (PaO2/FiO2) was conducted preoperation, inoperation, and postoperation. Before operation, chest computer tomography and pulmonary function (forced expiratory volume in 1 second, Vital capacity) was conducted. Chest X-ray was performed 1-2 hours postoperation and POD5.
Statistical analysis
Mann-Whitney U and Pearson’s χ2tests were performed to test the difference between two groups. The statistical analysis was completed in SPSS®version 19(SPSS, Inc., Chicago, IL, USA), withP<0.05 indicating a statistically significant difference. Overall and disease-free survival was analyzed by the Kaplan–Meier method calculated from the date of operation until the date of death or date of recurrence, respectively.
Results
Demographic parameters
The demographics and operative data of the two groups were described in table 1 and 2. The CFS and MI groups were matched for age, sex, personal hobbies (alcohol and smoke) , preoperative cardiopulmonary function, blood gas analysis, blood routine test, except the BMI and the COPD comorbidities. The BMI in MI group was significantly lower than that in CFS group. More patients in CFS group combined with COPD. The data was shown in table 1.
Surgical and in-hospital outcomes
Compared with the CFS group, the length of incision in MI group was significantly shorter (P<0.0001). No significant difference was found on operation time, CPB time, and aortic cross-clamp time (P>0.05). The size of artificial ring and valvoplasty method of Carpentier-type leaflet resection was similar between two groups. Artificial chordae plasty was performed more frequently in CFS group(P=0.035). The amount of intraoperative blood loss and the incidence of postoperative blood transfusion were greater in CFS group. The drainage of thoracic in CFS group was more than that in MI group on POD 1 and 2. Atrial fibrillation ablation was performed in two groups regularly. The better AF ablation result was achieved in MI group (P=0.036). New onset of postoperative AF was significantly lower in MI group(P=0.039). No statistical difference was found in Reoperation for bleeding and sternal dehiscence between two groups. Two patients in CFS group need to reoperation for sternal dehiscence. Patients in MI group had less duration of stay in the intensive care unit and in-hospital (P=0.0001andP=0.039,respectively). The data was shown in table.2.
Inflammation, pain, and pulmonary function
More pulmonary complications were found in MI group (P=0.027). Eight patients in MI group had serious pulmonary effusion in chest X-ray (the typical chest X-ray was shown in Figure 3A and B); In CFS group, only two patients had moderate to serious pulmonary effusion in chest X-ray, most of the patients had satisfied chest X-ray (the chest X-ray was shown in Figure 3C and D).
The inflammatory response was lighter in CFS group than that in MI group on POD 1 and 2. Patients in MI group have significantly higher body temperature on POD 1 and 2, faster heart rate on POD 2, more white blood cell on POD 1, faster respiratory rate on POD 1, 2, worse oxygenation index (OI) on POD 0,1, 2; however, Patients in MI group have significantly lower body temperature on POD 3, slower heart rate on POD 3, less white blood cell on POD 2, slower respiratory rate on POD 3, 4, better oxygenation index(OI) on POD 4. Patients in two group had the equal degree chest pain on the first two days, but more obvious incision pain was expressed by patients in CFS group on POD 3, 4, 5. The data was shown in table.3.
Overall, free from reoperation and recurrent MR Sur‐vival
The median follow-up was 49 months (range 30-60 months; CSF: 53 months vs. MI: 45 months). No significant difference was found between two groups on overall survival (Fig. 4B, Log-Rank test:P=0.623),free from reoperation survival (Fig. 4D, Log-Rank test:P=0.592), and free from recurrent MR survival(Fig. 4F, Log-Rank test:P=0.658). A total of 12 patients (12.5%) died during follow-up (CFS: 7 vs.MI: 5). Recurrence MI was observed in 9 patients(9.4%: CFS; 5 vs. MI: 4) and reoperation was performed in 13 patients (13.5% CFS: 6 vs. MI: 7) in five years, and there was no statistical difference between two groups.
Discussion
Overall results of this study were summarized firstly. There was no significant difference on overallpostoperative survival rates, which indicated that the MI procedure was as safe as CFS intervention. No significant difference was found on survival rates of free from reoperation for MR; MR was detected before discharge and compared between two groups, the result had no statistic difference; no statistical difference was found on postoperative 5 years followup of recurrence MR between two groups, the later results showed that small incision for MV repairing could achieve identical therapeutic efficiency. Our results was similar with a recent meta-analysis, in which Sündermann et al[7]showed equivalent excellent short-term and mid-term results in MI for MR,compared with conventional surgery, with regard to mortality, and durability of the repair. Akowuah et al[8]also confirmed that access throughminithoracotomy does not interfere with quality and durability of MV repair.
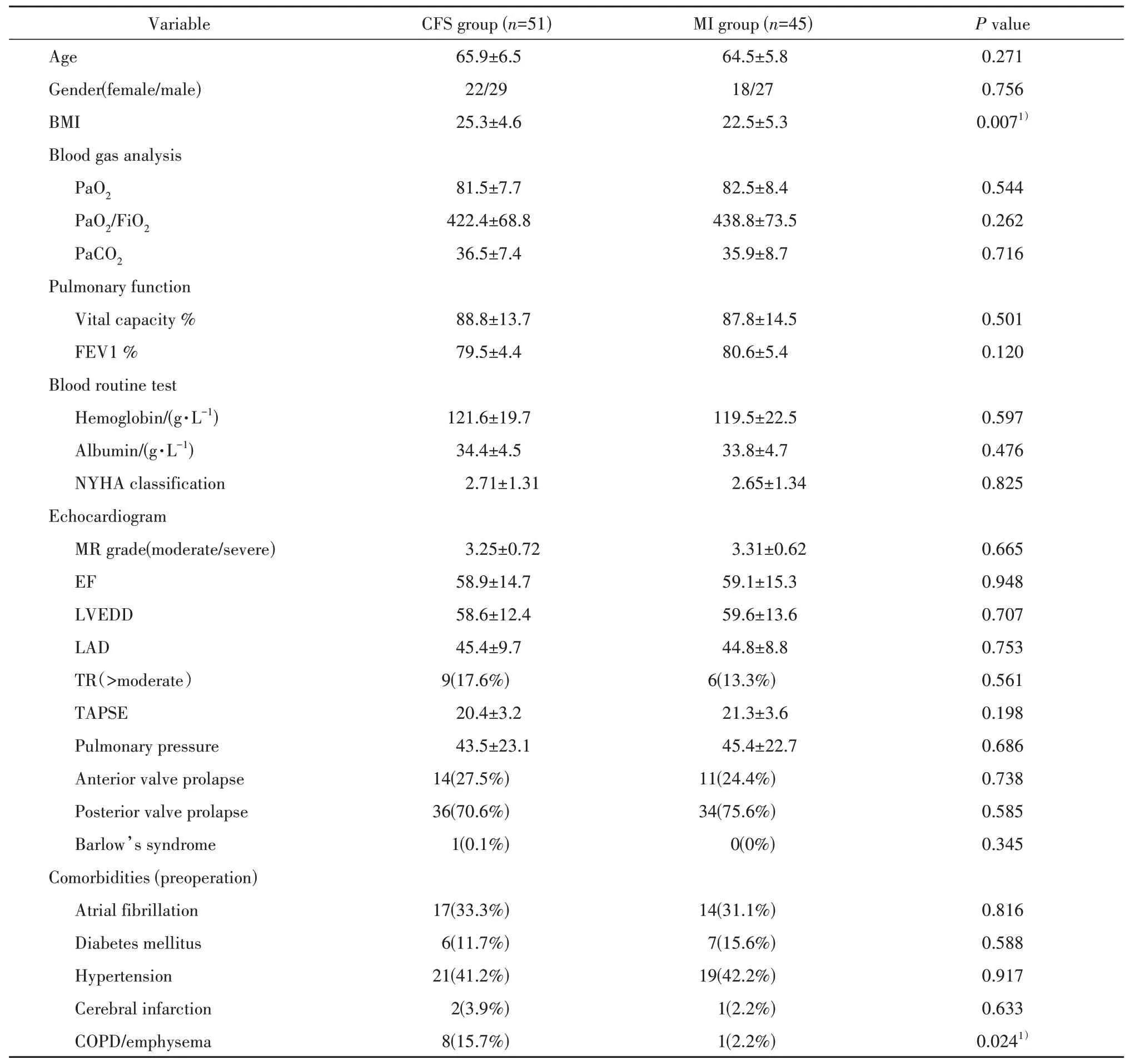
表1 患者一般资料Tab.1 Patients’ demographics
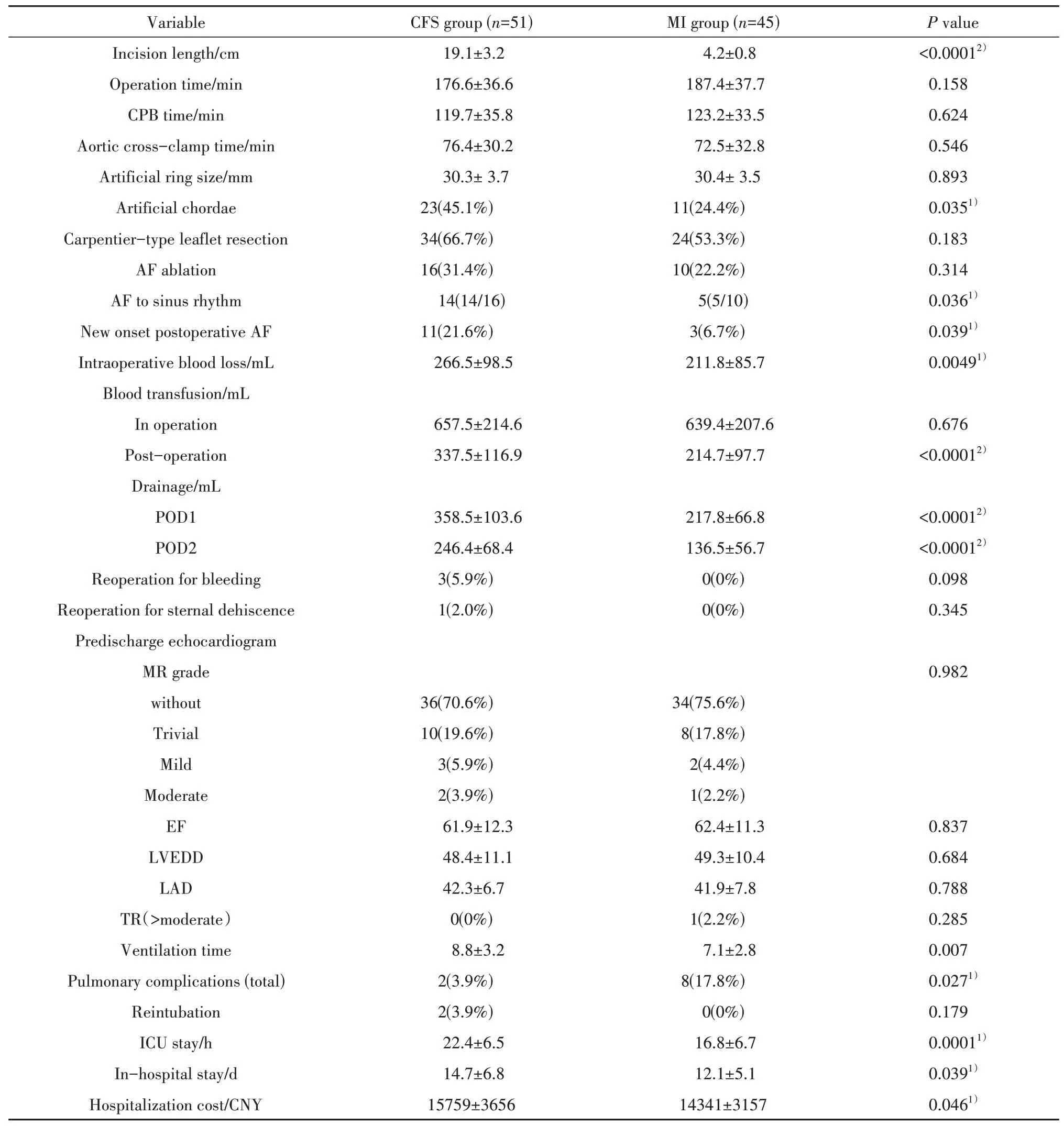
表2 手术资料Tab. 2 Operation outcomes
A minimally invasive surgical approach via right lateral thoracotomy was reported by Mohr et al as early as 1999[9]. Recently, Glauber described the technique in details[10]. In this study, the surgical procedure was also described carefully as well; the incisions and operative ports were showed in the Figure 1 in detail,and the principal procedures of MV repair were shown in Figure 2.
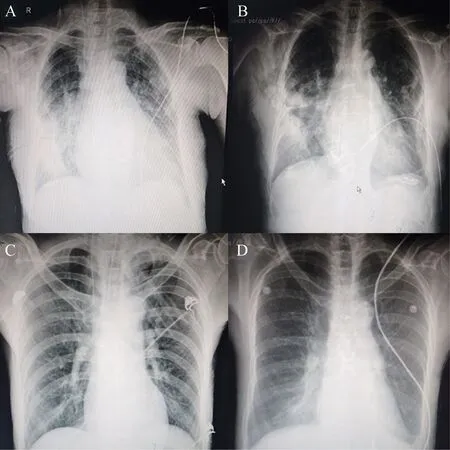
图3 术后及术后第5天的X线胸片Fig. 3 Chest radiographs after surgery and on postoperative day 5
From the Figure 1 and comparison with CFS group in table.2, the incision length(cm) in MI group was significantly shorter (19.1±3.2 vs. 4.2±0.8,P<0.0001). Consequently, postoperative complications caused by the position and size of incision were statistically different. In CFS group, the integrality of sternum was damaged, which caused the obvious bleeding even beginning from chest opening, more thoracic drainage and blood transfusion postoperation,the longer ventilation time and intensive care stay compared with MI group. Algarni et al also found that MIMVS was associated with reduced bleeding and blood transfusion, shorter ventilation time and intensive care stay, as well as with elimination of sternum-related morbidity and more rapid resumption of normal activity[11]. Moreover, Atluri et al[12]have reported that MIMVS was associated with lower hospital costs. In our study, the cost in CFS group was lower than that in MI group. The over-cost in CFS group was mainly expended by sterna fix system(sterna plate) and other expenses for more blood transfusion, longer stay in ICU and hospital and so on.
The patients suffered the chest incision pain nearly the same in two groups in first few days after surgery. The reasons may be as follow: 1. The right thoracic drainage tube should be placed for patients in MI group (the right thoracic plural cavity was opened), and the right thoracic drainage tube moved following respiratory motion which stimulates the pleura to cause chest pain. 2. Part of intercostal nerve was damaged when the right minithoracotomy was performed, which could cause obvious chest pain. In CFS group, sternum was fixed by steel wire and steel plate. The sternum was fixed very firm, and only the peripheral nerve was damaged by the medium sternotomy. So sometimes the incision pain in CFS group was not obvious on the contrary. But for elder women, right minithoracotomy showed obvious advantages for these patients suffered with osteoporosis obvious. Two patients’ sternums in CFS group were cut by the fixing steel wire, which caused thoracic instability; consequently, cardiopulmonary function was affected significantly. Both patients need debridement and sternum fixation again. The patient's suffering, hospital stay, and expenses were increased involuntarily.
The effect on the pulmonary function: in MI group, the patients need double lumen endotracheal intubation; only left lung ventilation with right lung collapse during the surgery led to the excessive ventilation of left lung; and the right lung was squeezed frequently during the operation; as previous explanation, patients in MI group suffered the equivalent chest pain, which decreased the efficiency of coughing and expectoration. For the reasons, the postoperative pulmonary function in MI group was worse than that in CFS group, and the incidence of pulmonary exudates was higher, and the inflammatory response was more obvious in the short time after surgery (explained in the following parts). The right pleural effusion will occur in some patients after moving the thoracic drainage tube. We prefer to keep a small diameter drainage tube with negative pressure ball for more few days (Fig.1B 3#), which is removedwhen the drainage fluid is less than 50 mL/24 h.
表3 两组术后炎症、疼痛评分及氧合指数比较(±s)Tab. 3 Postoperative inflammation, pain score, and oxygenation index compared between two groups (±s)
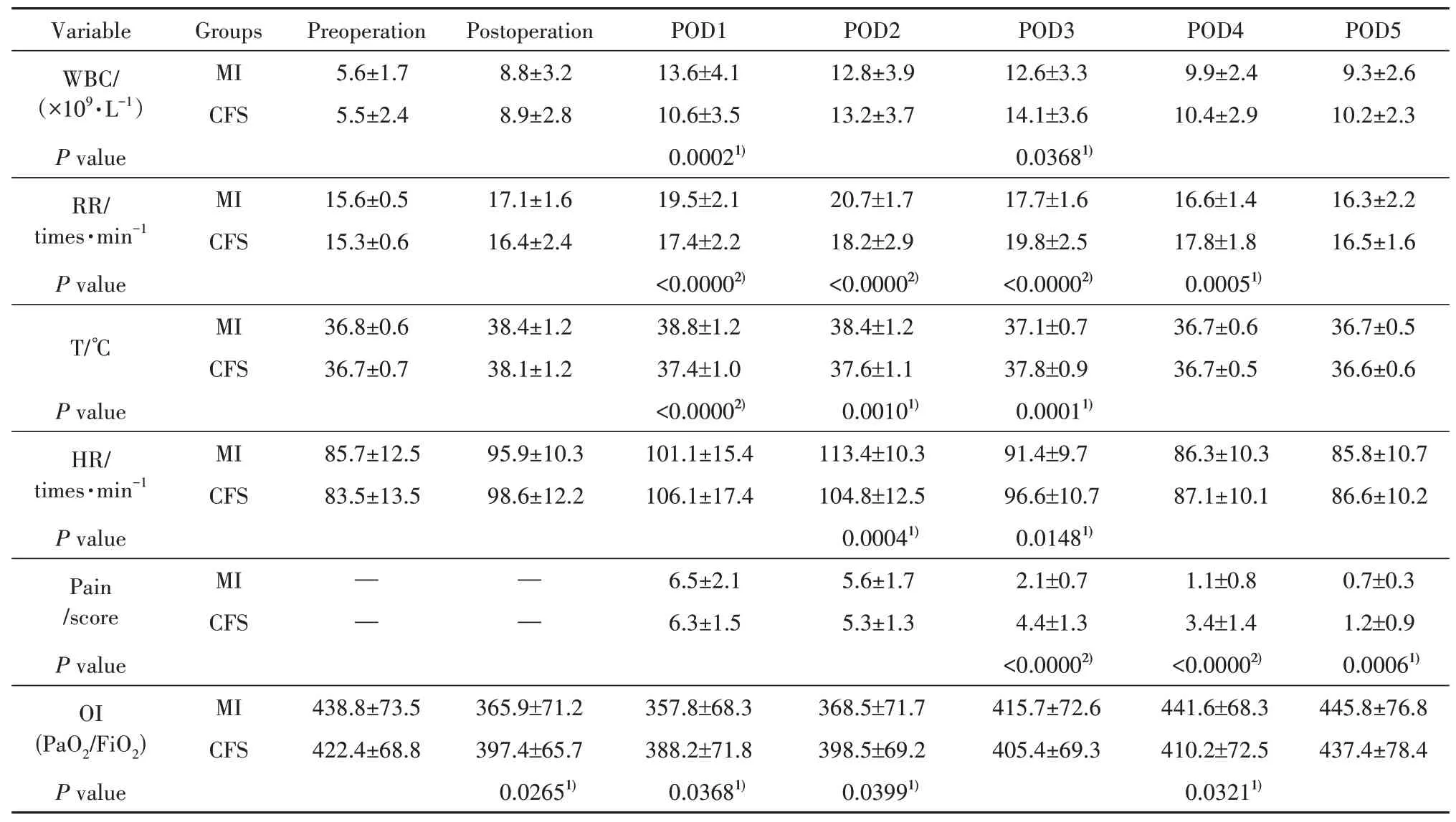
表3 两组术后炎症、疼痛评分及氧合指数比较(±s)Tab. 3 Postoperative inflammation, pain score, and oxygenation index compared between two groups (±s)
注:1)P<0.05, 2)P<0.0001, CFS:常规全胸骨切开术;MI:微创;WBC:白细胞;RR:呼吸速率;T:温度;HR:心率;POD:术后1 d;OI:氧合指数;PO2:氧的分压;FiO2:吸入氧浓度。Notes:1)P<0.05, 2)P<0.0001, CFS: Conventional full sternotomy; MI: Minimal invasive; WBC: White blood cell; RR: Respiratory rate; T:Temperature; HR: Heart rate; POD: Postoperative day; OI: Oxygenation index; PaO2: Partial pressure of oxygen; FiO2: Fraction of inspired oxygen.
?
In the present study, results also suggested that, in the first two days after surgery, patients in MI group suffered obvious inflammatory response compared with CFS group. The reasons included the poor pulmonary function caused by collapse and compression of right lung, and right thoracic drainage tube. However, two days later, the indicator of inflammation response was improved significantly in MI group. The reason was that form the third day after surgery, when the right thoracic drainage tube was removed, the chest pain was relieved significantly on most of the patients. In addition, the integrity of chest wall was damaged mildly, patients in MI group were willing to off-bed early, and cough actively.Consequently, hospitalization time reduced, and overall cost decreased too. On the contrary, 3 days after surgery,patients in CFS group suffered from obvious incision pain caused by coughing. Sternum, rib-vertebral joint,sternal-rib, sternoclavicular joint were damaged when the sternum was spread by a sternal retractor. Even fractures of sternum happened in some patients with osteoporosis. Patients in this group might suffer from pulmonary infection by poor expectoration, incisional infection, fat liquefaction, and non-healing, which led to significant inflammatory responses.
Recent data from the STS Database showed that 32.2% of patients presented to MV surgery have AF and concomitant AF ablation in this setting was performed in 61.5% of patients[13].AF ablation was performed on most of the patients at the same time during surgery (16/17 in CFSvs.10/14 in MI,P>0.05). In this study, AF ablation was performed regularly by combination of single polar and bipolar ablation in CFS group; the single polar ablation was always used in MI group; and left auricle was sewn from the left atrium in two groups. The result of AF ablation (AF to sinus rhythm postoperation) in CFS was superior to that in MI group. Patients with AF of relatively long duration before surgery have been associated with a reduced likelihood of ablation success[14]. Unfortunately, the first attack time of AF preoperation was unclear, which was an important influence factor on the result of AF ablation.
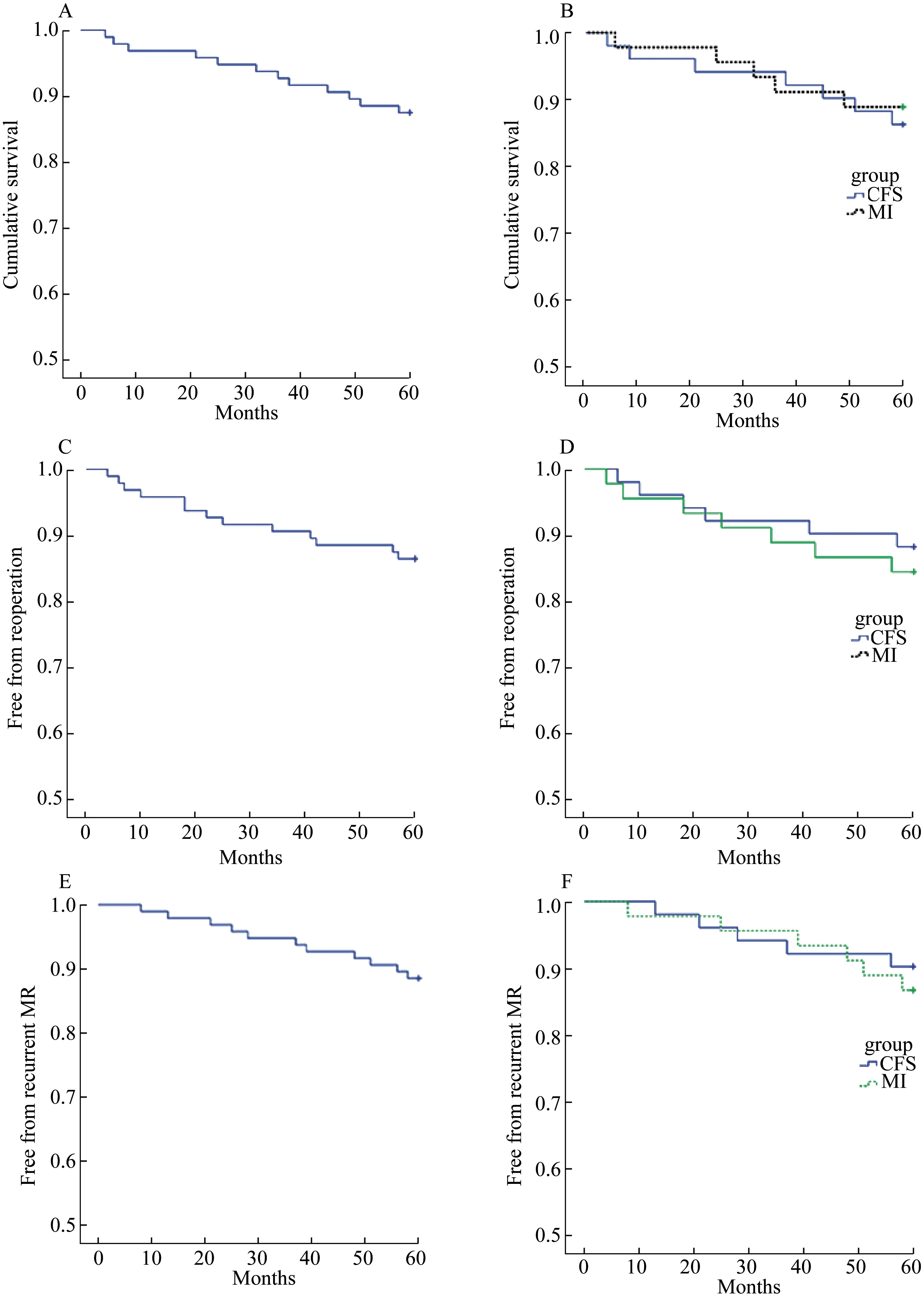
图4 随访两组5年生存率、免再次手术率、无二尖瓣反流复发率Fig.4 Survival, survival free from reoperation, and survival free from recurrent MR in five years in two groups
Postoperative atrial fibrillation (POAF) is a common complication following cardiac surgery. The new onset postoperative AF in MI group was lower than that in CFS group (21.6%vs.6.7%,P<0.05). Preoperative,intraoperative, and postoperative variables all affect the incidence of postoperative AF[15]. Left atrial enlargement was a powerful predictor of post-operative AF[16].Postoperative factors associated with AF included respiratory failure, pleural or pericardial effusions,transfusions, prolonged ventilation time, and so on[17].In our group, no difference was found on the size of left atrium between two groups. In our opinion, the pulmonary complication and inflammatory response in CFS 2 days after surgery may be the powerful influence factors.Previous studies also showed that anti-inflammatory therapy significantly reducesd the incidence of POAF following cardiac surgery[18,19,20]. Most of the postoperative AF happened on the POD 2 and 3, which further indicated the importance of the above two factors.
The main limitation of this research was the retrospective character. Patients in two groups had a certain bias on the preoperative data, especially the BMI and pulmonary situation. The patients with higher BMI and COPD were advised to undergo conventional surgery. The difference of preoperative data in two groups inevitably had a certain affect on the following study. The data of the first attack time of AF preoperation was unavailable, and the long-term follow-up result of the AF ablation was missing too.
Conclusions
MI mitral valve repair is a safe and reproducible approach associated with low perioperative mortality andmorbidity. MI for MR can be performed in the vast majority of patients with MR.

