Investigation on Thermal and Dimensional Stability of Epoxy Resin In-Situ Modified by Cyanate Ester Resin and Polydimethylsiloxane
ZHANG Zhiyun,HE Yannan,HOU Jinsen,ZHUANG Chun,TANG Jinmei,YU Zhiqiang
(1.Department of Materials Science,Fudan University,Shanghai 200433,China;2.Shanghai Composites Science &Technology Co.,Ltd.,Shanghai 201112,China)
Abstract:The thermal and dimensional stability of epoxy resin(EP)in-situ modified by cyanate ester(CE)and polydimethylsiloxane(PDMS)are investigated by means of experiments and numerical simulation.Thermal gravimetric analysis(TGA)and differential scanning calorimeter(DSC)are used to analyze the heat resistance of the modified EP.The dimensional stability is characterized by the volume shrinkage of the series PDMS/CE/EP obtained by the density method.The chemical structure of the PDMS/CE/EP is analyzed by Fourier transform infrared spectroscopy(FTIR).The results of TGA and DSC indicate that the thermal stability of PDMS/CE/EP decreases firstly and then increases with the increase in the amount of CE.The addition of PDMS shows a slight effect on the thermal stability.The 40% CE makes the blending system exhibit the lowest initial decomposition temperature,which reduces by 15.5% and 40.8% compared with pure EP and CE,respectively.The FTIR results suggested that the influence of CE on the thermal stability of the modified EP is mainly ascribed to the generation of oxazolidinone ring with low thermal stability and the increase in the triazine ring with high thermal stability.The volume shrinkage measurement results show that the introduction of CE and PDMS are both beneficial to the improvement of the dimensional stability of the blending systems.The in-situ addition of 80% CE shows the lowest volume shrinkage of 6.11%.The thermal stress distribution of PDMS/CE/EP generated during the solidification process is simulated by the finite element analysis.The results suggested that the introduction of 80% CE into EP results in the lowest thermal stress in the blending system,which indicates that the system has the lowest volume shrinkage,which agrees well with the experimental results.
Key words:epoxy resin and cyanate ester resin and polydimethylsiloxane(PDMS/CE/EP)blending system;insitu modification;thermal stability;volume shrinkage;finite element analysis
0 Introduction
Epoxy resin(EP)is a thermosetting material with excellent performance.It has good technologi‑cal properties,low curing shrinkage,and good me‑chanical properties.It is often used as the substrates of coating materials,composites materials,and semiconductor materials,and has been widely used in the automotive,aerospace,and mechanical fields.However,the cured EP has the disadvan‑tages of high brittleness,poor thermal stability,and low range.Many studies have been carried out on making up for the defects of EP above.It has been proven that the organic modification of molecular structure is an extremely effective method.
At present,the organic modification of EP has been fully developed.In order to improve the tough‑ness and usability of EP,JIANG et al.used diphe‑nylsilanediol and 3-amino-propyl ethoxysilane to modify the curing reaction process of EP to obtain the modified EP containing Si―O bond.The me‑chanical and thermal properties of the modified EP were improved,and the introduction of Si―O bond significantly enhanced its toughness.WANG et al.used polysulfone and graphene oxide to double toughening EP,and showed that under the common modification in the amount of 5% polysulfone and the amount of 0.2% graphene oxide,the toughen ef‑fect of EP was the best,the elongation at break in‑creased by 89.9%,while the thermal properties of EP were not affected.JI et al.synthesized a new trifluoromethyl polyimide(PIS)to improve the toughness of EP,and showed that the modified ep‑oxy resin with PIS had good thermal stability,the glass transition temperature increased from 35.9 °C to 146.4 °C,and the tensile strength increased from 40.4 MPa to 92.3 MPa.In addition,The plane strain fracture toughness(KIC)value increased from 0.86 MPa·mto 1.56 MPa·mwith obvious enhancement,and the fracture mode transformed from brittle fracture to ductile fracture with the change of the mass of trifluorommethyl polyimide.ZHOUet al.modified the EP for composite liquid oxygen(LOX)fuel tank by using phosphorus flame retardant(DOPO)and brominated EP(EX48).With the addition of DOPO and EX48,the process‑ability requirements of the hot melt prepregmet signif‑icantly increased with the increase in the viscosity of the modified epoxy resin.At the same time,the addi‑tion of DOPO and EX48 improved the tensile proper‑ties,bending properties,and fracture toughness of the EP at room temperature and 90 K.GU et al.modified the epoxy resin with epichlorohydrin grafted micro-nanopolystyrene(nano-G-Ps)and multiwalled carbon nanotubes(MWCNTs)by using the binary nano-filler strategy,and showed that,com‑pared with the pure EP,the tensile strength and bending strength of the(nano-G-Ps)=0.067 7%/(MWCNTs)=0.033 5% EP system were in‑creased by 37.6% and 34.4%,respectively.More‑over,the tensile toughness was increased by 379.2%,and the elongation at break was increased by 208.3%.At the same time,the electrical conduc‑tivity and mechanical properties of the system were also improved.
From the aforementioned literature,we can see that most of studies on the organic modification of EP at present are focused on the single toughening modification of EP,while the modification by cya‑nate ester(CE),especially the double modification by CE and polydimethylsiloxane(PDMS),is rarely reported.CE is a new type of high performance res‑in,and has excellent heat resistance and comprehen‑sive mechanical properties.PDMS siloxane has well toughness.It is expected to obtain a modified EP ma‑terial with high thermal stability and good dimension‑al stability by in-situ double modification of EP with cyanate and silicone.SHENG et al.modified the pure bisphenol E CE with alumina nanocomposites,and showed that the modified cyanate system could maintain good mechanical properties at 200 °C.LIN et al.prepared the PT-30/POSS nanocom‑posites by adding the 3-methylphenyl siloxane(POSS)to the CE(PT-30),and showed that the addition of POSS had a strong catalytic effect(reduc‑ing curing temperature and activation energy)on the curing reaction of PT-30.With the increase in the POSS content,the activation energy of PT-30 de‑creased constantly.At the POSS content of=5%,the catalytic effect of PT-30 was the best.HE et al.prepared a compound catalyst system of bisphenol A dicyanate(BADCy),bismaleimide(BMI),and di‑allyl phthalate(DAP),then developed a high-perfor‑mance matrix blend,and studied the thermal and me‑chanical properties of the blend system in detail.The results showed that the impact strength and bending strength of the system could be improved by adding appropriate amounts of DAP and BMI,but the ther‑mal stability of the system was lower than that of the unmodified BADCy resin.JIN et al.prepared the EP/graphene(GE)nanocomposites by a solution blending method after the surface modification of GE with silane coupling agent.The test results showed that the thermal stability,bending strength,elastic modulus,and impact strength of the modified EP sys‑tem were all significantly improved with the increase in the GE content.
In this paper,EP is modified by in-situ polymer‑ization of CE and PDMS to prepare the PDMS/CE/EP ternary copolymer system.The heat resistance and curing shrinkage of the copolymer system are studied by controlling the amount of the modified ma‑terial added.In addition,by establishing the finite ele‑ment model,the distribution of the thermal stress in the system at high temperature is analyzed,and the mechanism of the shrinkage rate change of the copo‑lymerization system is further analyzed.
1 Experiments
1.1 Materials and apparatuses
Bisphenol A(GC),epiclorohydrin(AR),eth‑yltriphenyl phosphorus bromide(98%),hydroxylterminated PDMS(40 MPa/s),and tin acetylaceto‑nate(99.9%)were purchased from Shanghai Alad‑din Biochemical Technology Co.,Ltd.Sodium hy‑droxide(AR)and triethylenetetramine(CP)were supplied by Sinopharm Chemical Reagent Co.,Ltd.CE(AR)was bought from Shanghai Lida Chemical Co.,Ltd.Cobalt acetyl acetone(97%)and nonyl‑phenol(GR)were purchased from Shanghai Macklin Biochemical Technology Co.,Ltd.Toluene(AR)was offered by Shanghai Dahe Chemicals Co.,Ltd.
The apparatuses used in the experiment are as follows:JB200-D strong power electric stirrer(Shanghai Suoying Instrument Equipment Co.,Ltd.),ZNCL-B heater(Shanghai Daojing Instru‑ment Co.,Ltd.),BSA-124S analytical balance(Sar‑torius Group Limited),RE-52A rotary evaporator(Shanghai Yarong Biochemical Instrument Facto‑ry),SHZ-III Circulating water multi-purpose vacu‑um pump(GongyiYuhua Instrument Co.,Ltd.),and DNG-9070A type electric constant temperature blast drying oven(Shanghai Jinghong Experimental Equipment Co.,Ltd.).
1.2 Preparation of experimental samples
The experiment is a ternary copolymerization re‑action of EP,CE,and PDMS,in which the EP is obtained by in-situ polymerization through a two-step method.The sample preparation steps are as follows.
First,add a certain proportion of bisphenol A and epichlorohydrin to a three-necked flask and stirred until homogeneous.Second,add catalyst in the mixture at 80 °C,and then reacts for 1 h.Third,add the amount of 5% sodium hydroxide solution,CE,and PDMS in turn,and continue to react for 1 h.After the reaction,add toluene to extract the liquid to get the resin extraction solution.Finally,remove the solvent from the extraction solution through a ro‑tary evaporator to get the copolymer resin,and add a certain amount of curing agent to cure at 80 °C.
1.3 Characterization and test
The dynamic thermogravimetric analysis of the resin is carried out with the TGA-Q500 thermogravi‑metric analyzer(TGA)of TA Company in the Unit‑ed States.The heating atmosphere is nitrogen,the temperature ranges from 30 °C to 800 °C,and the heating rate is 10 °C·min.The infrared analysis of the resin is carried out with the Nicolet iS 10 Fourier transform infrared spectrometer(FTIR)of Thermo Fisher.The density of the copolymer resin after cur‑ing is measured with the DK-120S electronic density measuring instrument of Deka Precision Meter Co.,Ltd.,and the curing shrinkage of the copolymer resin is calculated according to the ISO 1675-1985 liquid resin density measurement standard and ISO 3521-1997 resin total shrinkage measurement standard.The total volume shrinkage of the resin is calculated as follows:
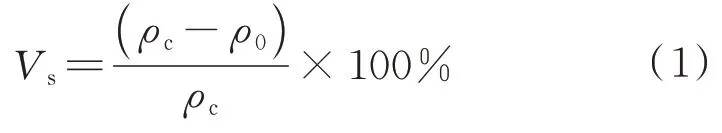
whereis the total volume shrinkage of the copoly‑mer resin,%.is the density of the copolymer resin after curing,g·mL.is the density of the copoly‑mer resin before curing,g·mL.
2 Numerical simulation
In this paper,ABAQUS,a mature commercial FEA software,is used to simulate the thermal stress distribution of the blending systems.According to the most important potential application of the blending system,the finite element model with a shape of paraboloid is constructed firstly.
As shown in Fig.1,in order to reduce the calcu‑lation amount reasonably and improve the calculation efficiency of the finite element model,a 1/4 symme‑try model is selected.The finite element meshes of the model are generated by using the 8-node thermal‑ly coupled brick element C3D8T with trilinear dis‑placement and temperature.Then,the mesh part is created,and the performance parameters of CE,EP,and polydimethylsiloxane shown in Tab.1 are assigned to different numbers of independent meshes random in the system according to the corresponding mass fractions of the related materials.
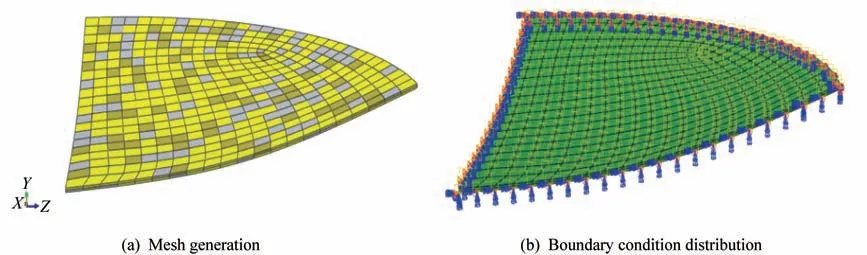
Fig.1 Construction of the finite element model for PDMS/CE/EP
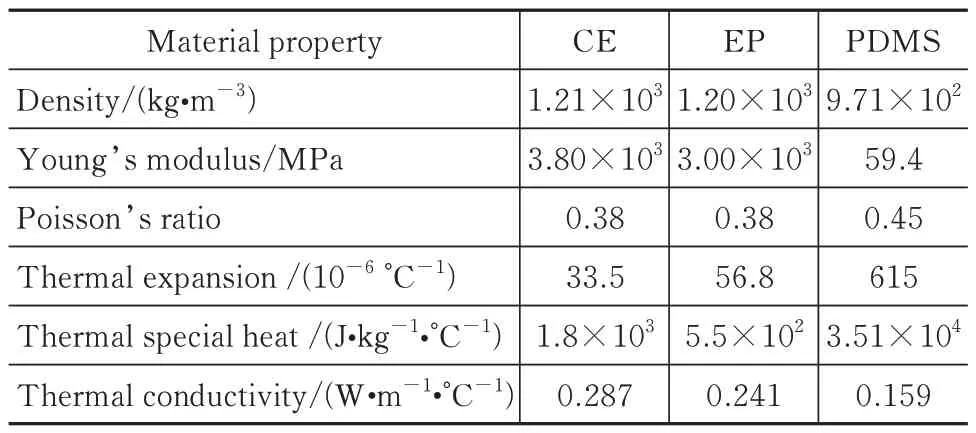
Tab.1 Material properties used in the finite element analysis
The boundary conditions are shown in Tab.2.Except of the necessary symmetry conditions,the initial temperature of the whole 1/4 model is set to be 25 °C,and the temperature of the concave surface of the paraboloid is set to be 100°C,so that there is a temperature difference in the production to obtain the corresponding thermal stress distribution.
Tab.2 TGA results of the copolymerization system
3 Results and discussion
3.1 Thermal stability analysis
3.1.1 Effects of CE dosage on the thermal stability of the copolymer system
In order to study the effect of the CE content on the thermal stability of the copolymer system,six groups of samples with the amounts of CE of 0%,20%,40%,60%,80%,and 100% are pre‑pared,among which 0% and 100 wt.% are only used as for comparison,and no PDMSis added in all the samples.The TGA curves of the six groups of samples are shown in Fig.2.The thermogravimetric analysis results of the six groups of samples are shown in Tab.2,whereandare the temper‑atures corresponding to the sampleweight loss rates of 5% and 10%,respectively,andis the final re‑sidual mass of the samples.The analysis curve based on the data results in Tab.2 is shown in Fig.3.In the process of thermogravimetric analysis,andcan be used to characterize the thermal stability of the copolymer.It can be seen that the larger the value is,the better the thermal stability of the copolymer is.Moreover,the residual mass can be used to charac‑terize the crosslinking degree of the copolymer.The higher the value is,the better the crosslinking degree of the copolymer is.
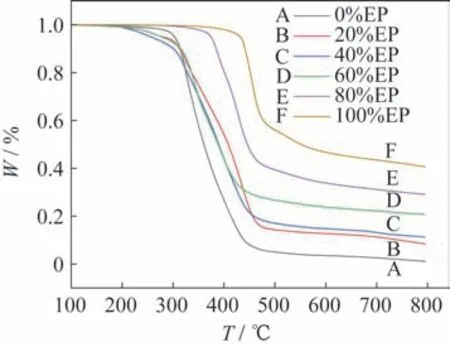
Fig.2 TGA curves of the copolymerization system with different dosages of CE
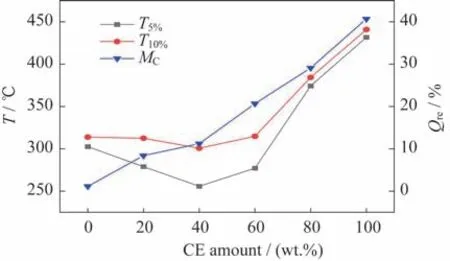
Fig.3 TGA results of the copolymerization system
From Fig.2,Fig.3,and Tab.2,it can be seen that the thermal stability of the copolymerization sys‑tem is significantly improved when the addition amount of CE is more than 60%,while does not change obviously when the addition amount of CE is less than 60%.This is because when the amount of CE is high,the majority of CE is polymerized to generate the triazine ring structure,and the triazine ring and epoxy group are co-polymerized to further generate the oxazolidinone five-membered ring struc‑ture.Both of them are heat-resistant groups,and could greatly improve the thermal stability of the co‑polymerization system.When the addition amount of CE is low,the formation of heat-resistant groups in the system is less,and thus the thermal stability is relatively low.Therefore,with the increase in the amount of CE,the thermal stability of the copolymer system increases on the whole.When the amount of CE is 80%,the thermal stability of the copolymer system is the highest,andandincrease by 19.2% and 18.4% compared with the copolymer without the addition of CE,respectively.
From Tab.2 and Fig.3,it can also be seen that the residual mass of the copolymer system increases constantly with the increasing addition of CE.This is mainly ascribed to the generation of the triazine ring structure with high structural symmetry and stability by CE during its polymerization.In addition,the co‑polymerization of CE and epoxy resin also generates the five-membered ring structure of oxazolidinone,which interweaves with each other and thus greatly enhances the crosslinking degree of the polymer net‑work.Therefore,with the increasing proportion of CE,the crosslinking degree of the copolymer system is improved,and thus the heat resistance of the copo‑lymerization system becomes better.At the CE con‑tent of 80%,the residual quality of the copolymeriza‑tion system with the highest crosslinking degree in‑creases by 96.1% compared with that without cya‑nate ester addition.
3.1.2 Effects of polydimethylsiloxane on the thermal stability of the copolymerization system
According to the results in Subsection 3.1.1,it can be seen that the thermal stability of the CE/EP resin copolymer system with the CE amount at 80%and the EP amount at 20% is the best.Therefore,this CE/EP resin copolymer system will be selected as the matrix for the further study in the next silicone modification process.In order to study the effects of the PDMS content on the thermal stability of the co‑polymer system,five groups of samples with the mass fractions of PDMS of 0%,5%,10%,15%,and 20% are prepared,and the addition amount of CE is 80% in all the CE/EP copolymer system.The TGA analysis results of the five groups of samples are listed in Tab.3,and the analysis curves drawn ac‑cording to the data in Tab.3 are shown in Fig.4.
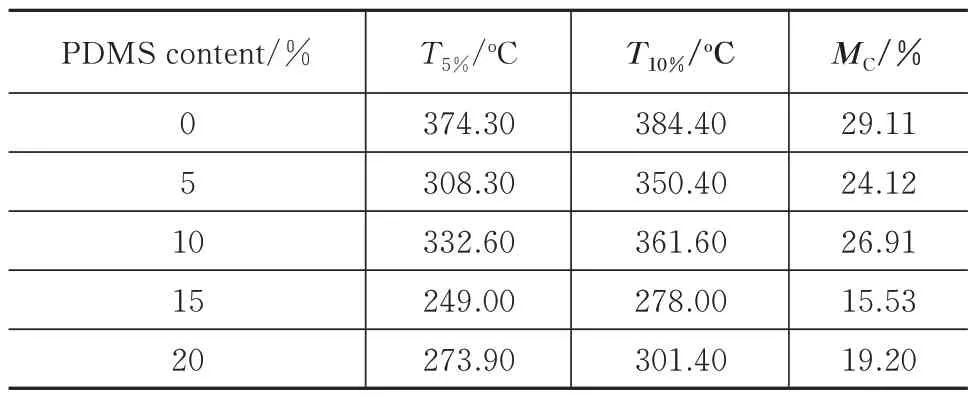
Tab.3 TGA results of the copolymerization system

Fig.4 TGA results of the copolymerization system with different dosages of PDMS
From the results in Tab.3 and Fig.4,it can be seen that when PDMS is added,the thermal stability and crosslinking degree of the system decrease com‑pared with that without PDMS.The reduction in the thermal stability of the 5% and 10% groups with less organic silicon addition is lower than that of the 15%and 20% groups with more organic silicon addition.This reason might be that since the organic silicon content is too low in the 5% and 10% groups,the number of organic silicon molecules into the copoly‑merization system is not enough to make them effec‑tively crosslinked with the copolymer.At the same time,the amount of organic silicon in the 15% and 20% groups is excess compared with the above two groups,and thus many organic silicon molecules do not participate in the copolymerization and remain in the system.Moreover,these two parts of the organic silicon resin will both automatically reunion.When the agglomeration size reaches a certain degree,the performance of the copolymerization system is re‑duced.In addition,it is confirmed that the effect of the excess silicone on the thermal properties of the system is greater than that of a small amount of sili‑cone(see Tab.3 and Fig.4).However,when the amount of organic silicon is increased from 5% to 10% or from 15% to 20%,the thermal stability and crosslinking degree of the copolymerization system are improved,indicating that the crosslinking reac‑tion still occurs in the copolymerization system.How‑ever,by comparing Tab.2 with Tab.3,it can be seen that owing to the influence of CE,the residual mass characterizing the crosslinking degree of the co‑polymer system with different amounts of organic sili‑con does not increase compared with the pure EP.Therefore,it is suggested that the decline of the sys‑tem performance after adding organic silicon is within an acceptable range.Meanwhile,according to the subsequent studies,although the thermal stability of the copolymer system with silicone is reduced to a certain extent,its curing shrinkage rate is greatly im‑proved.
3.2 FTIR analysis
In order to further explore the effects of compo‑nents on the thermal stability of the copolymerization system and the reaction mechanism during the modifi‑cation process,the infrared analysis of the six groups of samples in Fig.2 and another six groups of sam‑ples with 20% organic silicon addition is performed after the heat treatment from room temperature to 300 °C to eliminate the interference of the non-com‑ponent factors such as impurities.At the same time,according to Tab.2 and Fig.3,300 °C is exactly close to the initial decomposition temperature of the four groups of samples when the dosage of CE is 0%,20%,40%,or 60%.Therefore,it is conve‑nient to analyze the components of each sample at this temperature.The infrared spectra of the above two sets of samples after the heat treatment are shown in Fig.5 and Fig.6,respectively.
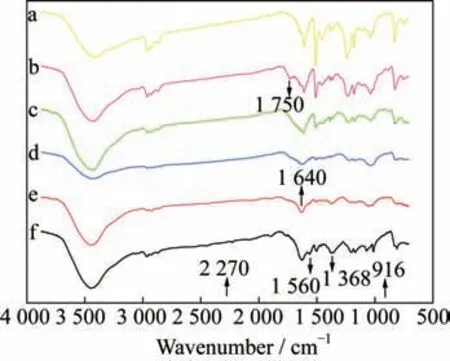
Fig.5 FTIR spectra of the copolymerization system after the heat treatment with 0% CE(a),20% CE(b),40% CE(c),60% CE(d)80% CE(e),and 100%CE(f)
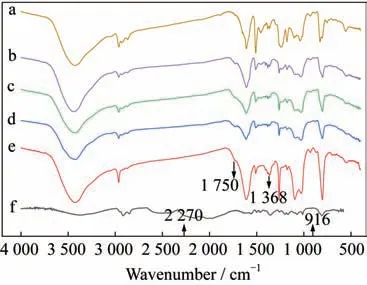
Fig.6 FTIR spectra of the copolymerization system after the heat treatment with 0% CE(a),20% CE(b),40% CE(c),60% CE(d)80% CE(e),and 100%CE(f)
According to Fig.5 and Fig.6,the wave num‑ber of 2 270 cmis the characteristic peak of the CE group,which does not appear in the spectra of the two sets of samples,indicating that all the CE groups have participated in the reaction of the copoly‑merization process.The characteristic peak of the ep‑oxy structure is found to be 916cm,which appears in the three groups of spectra with the CE dosages of 0%,20%,and 40% in both Fig.5 and Fig.6,indi‑cating that there are still some un-reacted epoxy groups in these samples,and the existence of the ep‑oxy groups with high activity and poor heat resis‑tance reduces the thermal stability of the copolymers.In addition,1 368 cmand 1 560 cmare the char‑acteristic peaks of the triazine ring structure,which appear in the 80% and 100% spectra in the two fig‑ures.The triazine ring structure with strong heat re‑sistance is beneficial to improve the thermal stability of the copolymers.At the same time,the characteris‑tic peak of the oxazoline structure with the wavenum‑ber of 1 640 cmappears in all the spectra of the co‑polymerization system,indicating that the copoly‑merization reaction truly takes place between the EP and the CE.Then,the characteristic peak of oxa‑zolidinone corresponding to 1 750 cmappears in the 20% and 60% spectra of Fig.5.The spectra of the copolymerization system of Fig.6 further prove that the CE successfully modifies the EP.
According to the results of the infrared analysis,we can conclude that the thermal stability of the copo‑lymer system is affected by the groups in the compo‑nents.Meanwhile,the thermal stability trend is con‑sistent with the results of the thermal analysis in Sub‑section 3.1.1.However,by comparing Fig.5 with Fig.6,the distributions of the characteristic peaks of the related structures shown in the two figures are ba‑sically the same,except for the difference in the peak strength,which indicates that the addition of silicone does not generate new groups in the copolymerization system.Nevertheless,according to the research re‑sults in Subsection 3.1.2,the thermal stability and the crosslinking degree of the copolymer system are indeed changed after the addition of silicone.With the addition,the copolymer system is modified main‑ly by physical blending,and the blending structure formed between the resins in the modification process could affect the thermal stability of the system.In general,the modification process of EP by CE and organosilicon is successful,and the addition of them both affects the thermal stability of the system though the CE improves and the organosilicon reduces.
3.3 Characterization of the curing shrinkage
3.3.1 Effects of PDMS on the curing shrinkage of the ternary copolymer system
In order to study the effects of the amount of PDMS on the curing shrinkage of the ternary copolymer sys‑tem,five groups of samples with the amount of sili‑cone of 0%,5%,10%,15%,and 20% are pre‑pared,and the addition amount of CE resin is 80% in all the CE/EP blending systems.The results of cur‑ing shrinkage of the five groups of samples are shown in Fig.7.
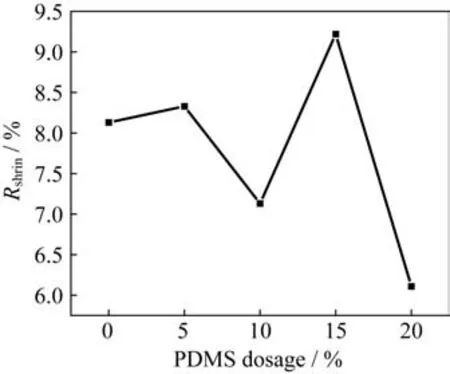
Fig.7 Curing shrinkage curve of the copolymerization system with different dosages of PDMS
From Fig.7,it can be seen that when the amount of silicone is more than 5%,the curing shrinkage of the copolymerization system shows a de‑creasing trend on the whole.When the addition amount of silicone is 5%,the curing shrinkage of the system increases slightly.According to the analysis results in Subsection 3.1.2,a lower addition amount of silicone will reduce the modification effect of the system.When the amount of organic silicon increases to 10%,the curing shrinkage of the system decreas‑es obviously.This is attributed to the good toughness of the polydimethylsiloxane that the curing shrinkage of the EP/cyanate resin system can be reduced by us‑ing the PDMS as a low shrinkage additive.Howev‑er,when the amount of the organic silicon reaches 15%,the curing shrinkage rate of the copolymer sys‑tem is the maximum.This is mainly caused by the poor compatibility between the PDMS and the EP/CE system.The related studiesshow that when the PDMS is used for the simple physical modifica‑tion of EP,the interaction between the PDMS and the EP is poor,and phase separation will appear ob‑viously.Moreover,the non-uniform distribution of PDMS in the copolymer system directly leads to the unsatisfactory modification results.When the amount of PDMS is 20%,the curing shrinkage of the copoly‑mer system is the smallest,i.e.,6.11%,which re‑duces by 24.8% compared with that without PDMS addition.In general,the addition of silicone has a great effect on reducing the curing shrinkage of the copolymer system.
3.3.2 Effects of CE on the curing shrinkage of the ternary copolymer system
In order to study the effects of the dosage of CE on the curing shrinkage of the ternary copolymer sys‑tem,six groups of samples with the cyanate dosages of 0%,20%,40%,60%,80%,and 100%(ratio of CE to EP)are prepared,and the amount of PDMS is 20% in the all samples.The results of the curing shrinkage of the six groups of samples are shown in Fig.8.
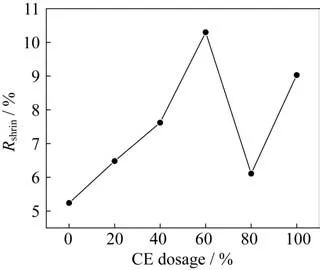
Fig.8 Shrinkage curve of the copolymerization system with different dosages of CE
From Fig.8,it can be seen that without consid‑ering the two groups of binary copolymerization sys‑tem with the CE content of 0% and 100%,when the amount of cyanate increases,the curing shrinkage of the ternary copolymer increases.When the dosage of CE is 60%,the curing shrinkage of the system reach‑es the maximum.According to the analysis of the free volume theory,we can obtain that the curing shrinkage of the simple polymer is mainly caused by the resin molecular chain change from the chaotic state to the ordered state and the temperature change in the curing process from high temperature to room temperature.However,in the copolymerization sys‑tem studied in this paper,with the increase in the CE,the molar ratio of CE to EP gradually approach‑es 1∶1,which means that the degree of copolymeriza‑tion of the system in the curing process is increasing.Therefore,the generation of the curing shrinkage of the copolymerization system is also affected by the copolymerization process,which generates many rings among the molecular groups,shortens the dis‑tance between the molecular groups to the distance between the bond lengths,and thus greatly reduces the molecule volume.As a result,the curing shrink‑age rate of the copolymer system increases with the increase in the CE content.Nevertheless,when the cyanate content increases to 80%,the curing shrink‑age rate of the system reduces to the lowest,i.e.,6.11%.From the above analysis,we can conclude that the molar ratio of CE and EP has exceeded 1∶1,the copolymerization part in the curing process de‑creases,and thus the shrinkage rate of the copolymer‑ization system begins to decrease.Therefore,on the basis of the experimental results shown in Fig.8,al‑though the addition of cyanate will increase the cur‑ing shrinkage of the copolymer,controlling the amount of cyanate could ensure that the curing shrink‑age of the copolymer is within an acceptable range,and the minimum curing shrinkage is 6.11% at the dosage of CE of 80%,which is reduced by 40.7%compared with the maximum curing shrinkage.
4 Finite element analysis
The distribution of thermal stress in the blend‑ing system represents the degree of uniformity of the material to some extent.The more uniform distribu‑tion of the components in the materials can make the PDMS/CE/EP form the interpenetration network structure more effectively,which will further reduce the internal free volume between the macromolecules of the copolymer and thus reduce the volume shrink‑age of the blending systems.Therefore,in order to further investigate the mechanism of the volume shrinkage of different PDMS/CE/EP blending systems,the thermal stress distribution of the system is characterized by the finite element analysis.
The variation of the thermal stress distribution of the PDMS/CE/EP system versus the amount of CE is shown in Fig.9.The amount of PDMS is 20% in all models.It can be seen that when the amount of cyanate ester increases from 0% to 80%,the stress concentration of the blending system first increases and then decreases.It can be seen from Fig.9(e)that when the amount of cyanate ester reaches 80%,the stress distribution is the most uni‑form.This variation trend can be seen more obvious‑ly in Fig.10,where the blending system with 80%CE exhibist the lowest maximum von Mises thermal stress,while the blending system with 100% CE shows the most intense thermal stress concentration.The main reason for these phenomena is that the in‑troduction of a small content of cyanate ester with high Young’s modulus causes an intense stress con‑centration in some regions of the blending system.When the amount of CE further increases,the ther‑mal specific heat increases,the coefficient of thermal expansion decreases,and thus the distribution of the thermal stress of the blending system with the main proportion of CE becomes more uniform.However,when there is no PDMS in the blending system,ow‑ing to the lack of transition between CE with high modulus and PDMS with poor mechanical proper‑ties,there will be a very serious stress concentra‑tion,i.e.,the distribution of them is uneven,which is not conducive to the reduction of the volume shrinkage.This is consistent with the previous experi‑ment.In addition,it can be seen that the PDMS/CE/EP system with(CE)=60% CE exhibits higher volume shrinkage than the PDMS/CE/EP system with any other mass fraction CE in experiments.However,the phenomenon is not obvious in the finite element analysis.This is mainly ascribed to that the chemical reaction in the blending systems is not taken into account in the finite element analysis.As men‑tioned above,since the equivalent ration of epoxy res‑in to CE in the system with(CE)=60% CE is roughly 1∶1,the chemical reaction degree of them is deep.Therefore,the simulation underestimates the stress concentration in the PDMS/CE/EP system with(CE)=60% CE.In general,the finite element analysis results are in reasonable agreement with the experimental data.
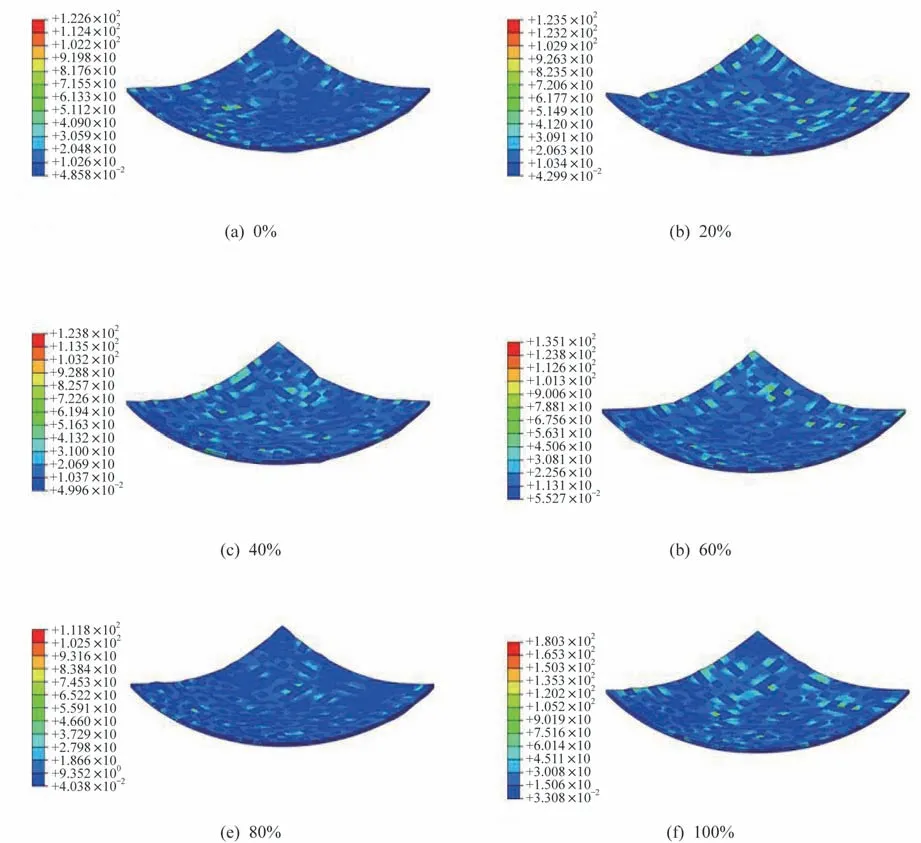
Fig.9 Distribution of thermal stress of the PDMS/CE/EP system
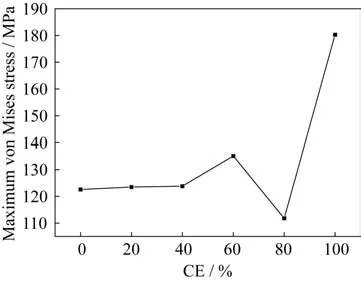
Fig.10 Distribution of the maximum von Mises thermal stress of the PDMS/CE/EP system vs.versus the mass fraction of CE
5 Conclusions
CE and PDMS are used to modify the epoxy resin by in-situ polymerization,and the PDMS/CE/EP ternary copolymer system is prepared.The ef‑fects of the amount of the modified components on the heat resistance and curing shrinkage of the modi‑fied copolymer system are studied.The main results are as follows.
1)The thermal stability of the copolymer sys‑tem increases with the addition of CE.When the amount of CE is 80%,the thermal stability of the co‑polymer system is the highest,andandin‑creased by 19.2% and 18.4% compared with those without CE.Meanwhile,the crosslinking degree of the copolymerization system is improved with the in‑crease in the amount of CE,and the crosslinking degree is the highest when the amount of CE increas‑es to 80%,and the residual mass of the copolymer‑ization system increases by 96.1% compared with that without CE.In addition,the thermal stability and crosslinking degree of the copolymer system with the addition of organic silicon decrease,but the de‑crease degree is within the acceptable range.
2)The curing shrinkage of the copolymer sys‑tem decreases with the increase in the amount of PD‑MS on the whole.When the amount of PDMS is 20%,the curing shrinkage of the copolymer system is the smallest,i.e.,6.11%,which is reduced by 24.8% compared with that without PDMS.More‑over,the addition of CE will increase the curing shrinkage of the copolymer,but controlling the amount of CE could ensure that the curing shrinkage of the copolymer is within an acceptable range.When the amount of CE is 80%,and the curing shrinkage of the copolymer reaches its minimum,i.e.,40.7%lower than its maximum curing shrinkage.
3)The numerical simulation results indicate that the PDMS/CE/EP system with 80% CE and 20% PDMS exhibit the most uniform distribution of thermal stress,and the maximum von Mises ther‑mal stress in this system is also the lowest,which means that the distribution of different resins in the blending system is the most uniform,the free vol‑ume between the macromolecules in this system is the smallest,and thus the volume shrinkage is the lowest.
In general,according to all the experimental re‑sults,combined with the comprehensive analysis of the thermal stability and curing shrinkage rate of the copolymer system,the best ternary copolymer sys‑tem is obtained when 20% of organic silicon is added into the CE/EP matrix in which the amount of CE is 80% and the amount of the EP is 20%.
- 上海航天的其它文章
- 序言
- 企业简介
- Microstructure and Property Analysis of AerMet100 Steel Manufactured by In-situ Rolling Mixed Wire Arc Additive Manufacturing
- Linear Shaped Charge Cutting Property and Charge Cutting Mechanism of Mg-Gd-Y-Zn Alloy
- Effect of Isothermal Forging on the Microstructure and Mechanical Properties of Mg-8Gd-3Y-0.5Zr Alloy
- Fabrication and Investigation on Double-Hollow Shell CoFe Oxide@TiO2 Nanocube with Superior Electromagnetic Properties

