The functional properties of synapses made by regenerated axons across spinal cord lesion sites in lamprey
David Parker
Abstract While the anatomical properties of regenerated axons across spinal cord lesion sites have been studied extensively, little is known of how the functional properties of regenerated synapses compared to those in unlesioned animals. This study aims to compare the properties of synapses made by regenerated axons with unlesioned axons using the lamprey, a model system for spinal injury research, in which functional locomotor recovery after spinal cord lesions is associated with axonal regeneration across the lesion site. Regenerated synapses below the lesion site did not differ from synapses from unlesioned axons with respect to the amplitude and duration of single excitatory postsynaptic potentials. They also showed the same activity-dependent depression over spike trains.However, regenerated synapses did differ from unlesioned synapses as the estimated number of synaptic vesicles was greater and there was evidence for increased postsynaptic quantal amplitude. For axons above the lesion site, the amplitude and duration of single synaptic inputs also did not differ significantly from unlesioned animals. However, in this case, there was evidence of a reduction in release probability and inputs facilitated rather than depressed over spike trains. Synaptic inputs from single regenerated axons below the lesion site thus do not increase in amplitude to compensate for the reduced number of descending axons after functional recovery. However, the postsynaptic input was maintained at the unlesioned level using different synaptic properties.Conversely, the facilitation from the same initial amplitude above the lesion site made the synaptic input over spike trains functionally stronger. This may help to increase propriospinal activity across the lesion site to compensate for the lesion-induced reduction in supraspinal inputs. The animal experiments were approved by the Animal Ethics Committee of Cambridge University.
Key Words: electrophysiology; lamprey; plasticity; regeneration; reticulospinal axon; spinal cord; spinal injury; synapse
Introduction
Regeneration remains the dominant approach in attempts to restore function after spinal cord injury (Ramer et al., 2014). The anatomical properties of regenerated axons (their number and projections) have been studied extensively in various systems, but their physiological properties have received relatively little attention. Given that these properties will determine the functional effect of any regenerated inputs it is important that they are considered, not least because this may help to explain the variable relationship between regeneration and recovery (Steward et al., 2012).
The small size of regenerated axons makes them difficult to study in the mammalian spinal cord. This study has used the lamprey, a model system for studying axonal regeneration and functional recovery after spinal cord lesions.Analyses in lamprey typically focus on the larger Müller reticulospinal axons as these allow stable intracellular recordings to be made from identified axons(Wood and Cohen, 1981; Hall et al., 1989; McHale et al., 1995; Oliphint et al.,2010; Zhang et al., 2011). These axons regenerate to make functional synaptic connections below lesion sites (Mackler and Selzer, 1987).
While regeneration is associated with functional recovery in the lamprey,regeneration is never complete (it ranges from 0-70%; McClellan, 1994) and regenerated axons project to ectopic locations (Wood and Cohen, 1981):regeneration thus does not ‘repair’ the spinal cord to its original state.Given that not all of the axons regenerate, for regeneration alone to equal recovery either means that in the unlesioned animal there are more axons than needed (i.e. there is a redundancy), or that there are other changes that allow the reduced number of axons to allow normal function despite the reduction in axon number after recovery. The latter seems more likely given that redundant descending inputs would be costly to develop and maintain,and given the evidence from the lamprey to the human spinal cord of functional changes below spinal cord lesion sites (Grasso et al., 2004; D’Amico et al., 2014; Parker, 2017).
Morphological analyses have shown that regenerated Müller axons make fewer synapses below the lesion site and that these synapses contain fewer vesicles and have smaller active zones than axons from unlesioned animals(Oliphint et al., 2010). Despite this, Müller axons evoke postsynaptic inputs that match those in unlesioned animals (Cooke and Parker, 2009). While this is unexpected given the morphological changes shown by Oliphint et al.(2010), there are compensations that can allow single axons to evoke the same postsynaptic input (Parker, 2017). While the large uniquely identifiable Müller axons are convenient targets for analyses, lesioning the medial column where they project does not abolish locomotion in functionally recovered animals. Lateral column axons, which are smaller and more numerous, seem to be more important for locomotor recovery (McClellan,1990; Chen et al., 2017).This analysis has focused on lateral tract axons, with the specific aim of determining how the properties of synapses made by regenerated axons compare to those made by axons in unlesioned animals, and the mechanisms of release. The results suggest that regenerated synaptic inputs to motor neurons below the lesion site match those in unlesioned animals, but that the input is evoked using different release properties. However, inputs to motor neurons from axons above the lesion site are altered to become functionally stronger than those in unlesioned animals.
Materials and Methods
Animals and lesioning
Juvenile male and female adult lampreys (Petromyzon marinus) between 100-130 mm (n= 84) were purchased from commercial suppliers (Acme Lamprey,Harrison, ME, USA). Animals were maintained in aquaria at room temperature.To lesion the spinal cord, animals were anesthetized by immersion in MS-222 (Sigma, Welwyn Garden City, UK; 300 mg/mL, pH adjusted to 7.4). The spinal cord was exposed by making a dorsal incision approximately 1 cm below the last gill and was then completely transected using iridectomy scissors. The incision site was repaired with Vet Bond Tissue adhesive (3M Animal Care Products). Following transection, animals were kept at 21°C for 8-10 weeks (Cohen et al., 1999). Animals were examined after 8-10 weeks as at this time they show recovery of locomotor function (McClellan, 1994;Cohen et al., 1998). There are changes in electrophysiological properties at later time points (Becker and Parker, 2019), but later time points were not examined here. All experiments were conducted under the license of the UK Home Office (Animals Scientific Procedures Act 1986; Project license PPL 80/2417 (03/2011)) and with the approval of the Animal Ethics Committee of Cambridge University.
Electrophysiological recordings
Video and electromyogram recordings were made from randomly assigned control animals and animals 8-10 weeks after lesioning to assess the degree of recovery. The animals used here were assessed to have either recovered normal or near-normal locomotion (‘good’ recovery) or failed to show any recovery (‘poor’ recovery; see Hoffman and Parker, 2011 for details). The spinal cord was then isolated for intracellular recordings by anesthetizing animals in MS-222 and removing the spinal cord and notochord. The spinal cord was isolated from the notochord and placed ventral side up in a Sylgardlined Perspex experimental chamber where it was superfused with Ringer containing (in mM): 138 NaCl, 2.1 KCl, 1.8 CaCl2, 1.2 MgCl2, 4 glucose, 2 HEPES, 0.5 L-glutamine. The Ringer was bubbled with O2and the pH adjusted to 7.4 with 1 M NaOH. The experimental chamber was kept at 10-12°C.Calcium was reduced to 50% in low calcium Ringer and increased to 200% in high calcium Ringer; changes in Ringer glucose levels were made to maintain osmolarity.
Paired recordings were made from axons in the lateral tract and motor neurons using thin-walled micropipettes filled with 3 M potassium acetate and 0.1 M potassium chloride. The lateral tract in the lamprey spinal cord also contains axons from the giant interneurons which relay sensory input to the brainstem and reticulospinal neurons. Giant interneuron axons are located near the lateral edge of the spinal cord whereas the reticulospinal axons are in the inner two-thirds of the tracts (Rovainen, 1979): recordings were thus made on the medial edge of the lateral tract to avoid giant interneuron axons. Motor neurons were identified by recording orthodromic extracellular spikes in the corresponding ventral root following current injection into their somata. Axons were identified by recording antidromic extracellular spikes on the rostral end of the spinal cord following their stimulation, and by the absence of a slow afterhyperpolarization following the action potential.Monosynaptic connections were identified by their reliability and constant latency (i.e. the input did not fail or the latency change over the spike train)following presynaptic stimulation at 20 Hz (Berry and Pentreath 1976). To minimize potential differences due to the location of cells in different regions of the spinal cord, all experiments were performed in the rostral trunk region(i.e. the first 2 cm of the spinal cord immediately caudal to the last gill).Motor neurons were recorded approximately 2-3 segments above or below the lesion site (a segment is defined by the presence of a ventral root) in lesioned animals. Axons were recorded approximately 5 segments above the lesion site. An Axoclamp 2A amplifier (Axon Instruments, San Jose, CA, USA)was used for voltage recording and current injection. Where necessary, the membrane potential in control and altered Ringer solutions was kept constant by injecting depolarizing or hyperpolarising current using a single electrode discontinuous current clamp. Data were acquired, stored, and analyzed on a computer using an analogue-to-digital interface (Digidata 1200, Axon Instruments) and Axon Instruments software (pClamp 8).
Axon spikes were evoked either by injecting 1 ms depolarizing current pulses of 10-60 nA, or on the rebound from hyperpolarizing current pulses (2-5 ms,1-5 nA). Single synaptic inputs were evoked ten times at 0.1 Hz (no activitydependent plasticity occurs at this frequency; Brodin et al., 1994) and the input averaged to measure the properties of the connection. The plasticity of inputs during spike trains was examined using presynaptic stimulation at 20 Hz for 1 second (Brodin et al., 1994): a single input was evoked at 500 ms, 1,2, and 3 seconds after the end of the train to monitor the recovery from any plasticity. Ten spike trains evoked at 30 seconds intervals were averaged to determine the properties of the connection. The initial excitatory postsynaptic potentials (EPSPs) in the trains were also used as a measure of low frequencyevoked inputs.
EPSP amplitudes were measured as the peak amplitude above the baseline immediately preceding the spike. At 20 Hz there was a little summation of EPSPs during spike trains and thus inputs could be measured without correction. The initial EPSP, the paired-pulse (PP) plasticity, and the plasticity over the 2ndto 5thspikes (Train2-5), the 6thto 10thspikes (Train6-10), and the 11thto 20thspikes in the train (Train11-20) were measured. Depression was defined as a reduction to at least 90% and facilitation to at least 110% of the initial EPSP amplitude: when depression or facilitation did not at least reach these levels the connection was classified as unchanged. Paired-pulse plasticity was expressed as EPSP2/EPSP1and plasticity over different regions of the spike train as EPSPTrain/EPSP1. Single, low frequency-evoked EPSPs were used to measure EPSP rise times and half-widths.
Statistical analysis
Values are presented as the mean ± SEM. Statistical significance was examined using two-tailed paired or independent samplest-tests, or one-way analysis of variance (ANOVA), and differences in the proportion of effects by the chisquare test. When a one-way analysis of variance was used, a Tukey’s test was used forpost hocanalysis of differences between groups.Nnumbers in the text refers to the number of connections examined (each connection reflects a new axon and motor neuron). Linear regression was also used to determine the correlations between various factors.Nnumber refers to the number of paired recordings. No more than three connections were examined in a single spinal cord. Statistical tests were only performed when the sample size allowed a power of at least 0.8: where aP-value was not reported the sample size was below that needed for this power.
Results
Basic synaptic properties
In lesioned animals, the EPSP amplitude, rise time, and half-width above (n=19 paired recordings) and below (n= 15 paired recordings) the lesion site did not differ significantly from unlesioned values (n= 62 paired recordings,P>0.05; Figure 1A-C). However, the paired-pulse ratio was significantly higher above the lesion site than for EPSPs in unlesioned animals or below the lesion site (P< 0.05; Figure 1B), an effect that can be associated with a lower release probability (Zucker and Regehr, 2002). EPSPs can also show an electrical component or be exclusively electrical. Morphological analyses of medial column Müller axons suggest a reduction of electrical connections after injury (Wood and Cohen, 1981), but the proportion of electrical versus nonelectrical synapses (number of connections with an electrical component/total number of connections) here did not differ significantly between unlesioned and lesioned animals above and below the lesion site (data not shown;P> 0.05). There were no changes in axonal action potential properties that could influence transmitter release (e.g. spike broadening; Parker et al.,1997; data not shown). These changes have been reported in regenerated axons in lamprey, but they have returned to unlesioned values at the time the analyses here were performed (McClellan et al., 2008).
Activity-dependent plasticity
Facilitation was the usual paired-pulse effect (i.e. the 2ndEPSP compared to the 1st) to 20 Hz stimulation in both lesioned and unlesioned animals.Plasticity over spike trains was assessed from the effect over the 11thand 20thEPSPs in response to 20 Hz stimulation (effects typically plateau over this part of the spike train; Figure 1D). In unlesioned axons, the average effect was depression to approximately 80% of the initial EPSP amplitude. However,individual connections varied (Brodin et al., 1994). In unlesioned animals, 30(of 62) connections depressed, 14 connections facilitated, 2 connections were biphasic, and 16 were unchanged (i.e. showed no activity-dependent plasticity over the spike train). In lesioned animals, below the lesion site, depression was again the usual effect (10 connections depressed, 2 facilitated, and 3 were unchanged). In contrast, above the lesion site facilitation was the most common effect over spike trains (Figure 1D; 12 connections facilitated, 4 connections depressed, and 3 were unchanged). While the proportions of the different types of activity-dependent plasticity did not differ in unlesioned and below lesion spinal cords, above the lesion site facilitating connections were significantly more common (P< 0.05).
When connections were separated by the type of activity-dependent plasticity, the degree of facilitation did not differ in unlesioned and above and below lesion spinal cords: facilitation developed to approximately 150%over Train11-20in all cases (Figure 1E and G), and did not differ significantly in unlesioned and above lesion axons (P> 0.05; the number of facilitating connections below the lesion site was too small to compare statistically).Depression also did not differ in unlesioned and above and below lesion spinal cords (Figure 1F and G), the magnitude not differing significantly in unlesioned and below lesion axons (P> 0.05; the number of depressing connections above the lesion site was too small to compare statistically).
In unlesioned animals there was no significant correlation between the initial EPSP amplitude and the paired-pulse (PP) ratio (r2= 0.02) or plasticity over the spike train (Train2-5plasticity (r2= 0.03), Train11-20plasticity (r2= 0.08);n=62; Figure 2A). The same applied to the above lesion PP ratio (r2= 0.02) and Train2-5(r2= 0.03), and Train11-20plasticity (r2= 0.07; Figure 2B). The initial EPSP amplitude thus did not predict the type of activity-dependent plasticity at these connections (a similar effect occurs at other excitatory synapses in the lamprey spinal cord). However, below the lesion site, while there was no significant relationship between the initial EPSP amplitude and the pairedpulse ratio (r2= 0.16), there were significant negative relationships with the Train2-5(r2= 0.23; data not shown) and Train11-20plasticity (r2= 0.29; Figure 2C). Larger initial EPSPs thus evoked greater depression, an effect that can be associated with release probability-dependent depression due to depletion or greater postsynaptic desensitization (Zucker and Regehr, 2002).
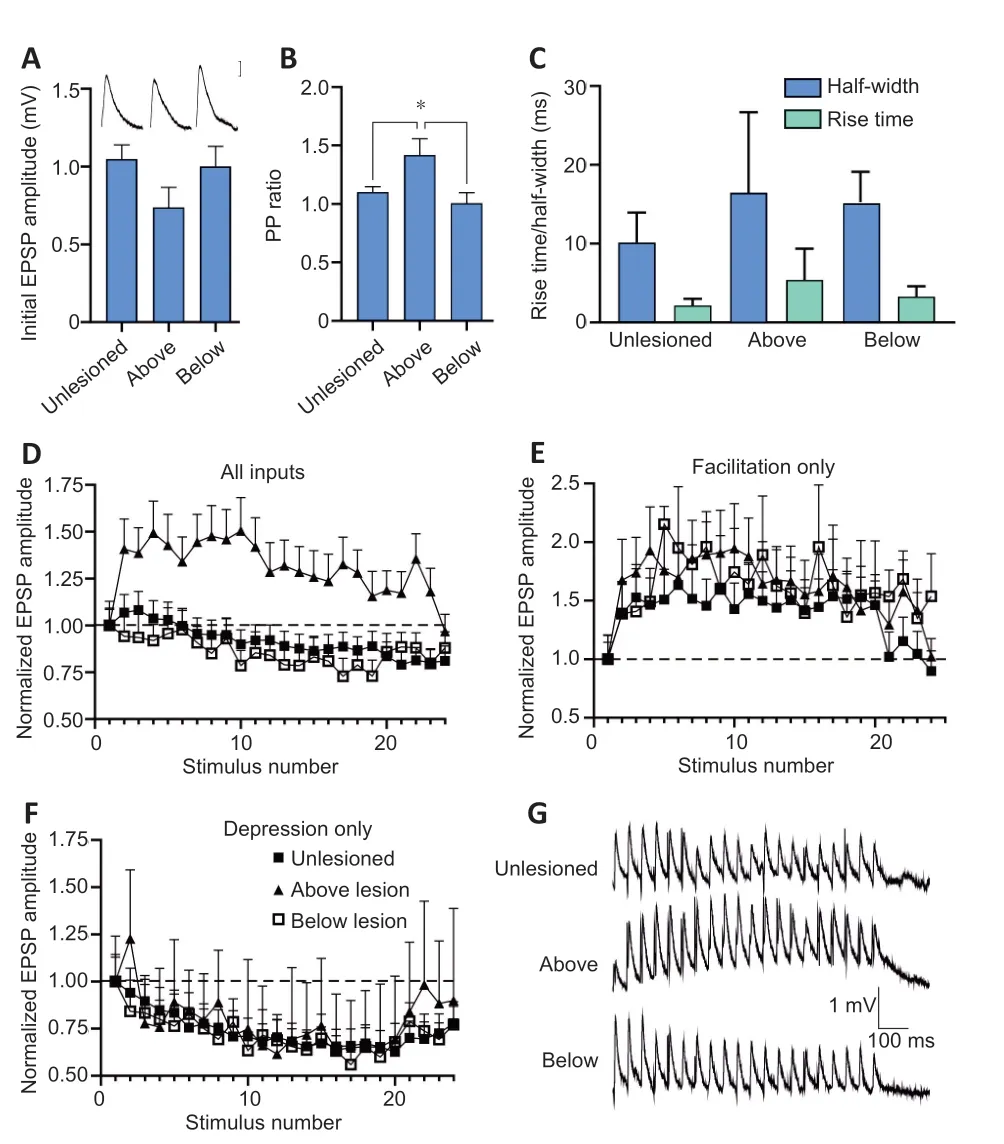
Figure 1 |Basic synaptic properties in unlesioned and lesioned spinal cords above and below the lesion site in lamprey.The amplitude (A), paired-pulse (PP) ratio (B), and half-width (C) of single low frequency-evoked excitatory postsynaptic potential (EPSPs). The inset in (A) shows averaged (n = 10 traces) low frequency-evoked EPSPs in the different conditions. (D)The averaged activity-dependent plasticity of all synaptic inputs in unlesioned, above,and below lesion cords over 20 Hz spike trains and the recovery of the effect after the end of stimulation (stimulus numbers 21-24). (E) Comparison of connections that only showed facilitation over spike trains. (F) Comparison of connections that only showed depression over spike trains. (G) Traces showing the average of 10 traces from single connections over spike trains in an unlesioned spinal cord, and above and below the lesion site. Data are expressed as the mean ± SEM. *P < 0.05 (one-way analysis of variance followed by Tukey’s post hoc test).

Figure 2 |The correlation between the paired-pulse (PP) or Train11-20 plasticity and the initial excitatory postsynaptic potential (EPSP) amplitude.The correlation between the PP or Train11-20 plasticity and the initial EPSP amplitude in unlesioned spinal cords using linear regression (PP ratio (r2 = 0.02), Train2-5 plasticity (r2 =0.03), Train11-20 plasticity (r2 = 0.08); n = 62, P > 0.05; A), above the lesion site (PP ratio (r2= 0.02) and Train2-5 (r2 = 0.03), and Train11-20 plasticity (r2 = 0.07), P > 0.05; B), and below the lesion site (PP ratio r2 = 0.16, P > 0.05), there were significant negative relationships with the Train2-5 (r2 = 0.23; P < 0.05 data not shown) and Train11-20 plasticity (r2 = 0.29, P <0.05; C). Linear regression was used.
Release properties
Synaptic properties reflect the parameters of transmitter release, including the number of vesicles or vesicle release sites, the release probability, and the postsynaptic response to the transmitter (i.e. quantal amplitude; Zucker and Regehr, 2002). The number of vesicles was estimated from the model of Wang and Zucker (1998):
Nves=(V02τd)/(q(V0-V∞))
V0is the initial EPSP amplitude,τdthe inverse rate constant of EPSP decay(expressed as the number of presynaptic spikes needed for the EPSP to fall to1/eof the initial value),qthe mean quantal amplitude (assumed to be 0.1 mV), andV∞the EPSP amplitude at the plateau level of depression.Because this analysis depends on the rate of depression (Wang and Zucker,1998; Schneggenburger et al., 1999; Millar et al., 2002), it could only be applied to unlesioned and below lesion axons as the number of depressing connections was too small above the lesion site. The analysis also required that the input decreased to1/eof the initial value over the spike train. This often does not happen even over longer spike trains (> 50 EPSPs), probably due to the concomitant development of activity-dependent replenishment of the releasable pool over the spike train (Parker, 2000). As a result, the extrapolated exponential depression calculated from the initial depressing EPSPs in the train was used to determineτdandV∞to allow approximation of the vesicle pool. Any influence of replenishment would reduce the rate of rundown and give an over-estimation of the initial vesicle pool. This would apply equally to unlesioned and lesioned synapses unless there was a difference in the rate of replenishment between unlesioned and lesioned synapses (Parker, 2000), an effect that would be functionally equivalent to an increase or decrease in vesicle numbers if the rate of replenishment was faster or slower, respectively. In unlesioned animals, the mean number of vesicles was within the range of direct vesicle counts of 100-500 vesicles in the middle section of the vesicle pool from Müller axons in lamprey(Gustafsson et al., 2002). However, below the lesion site the estimated mean number of vesicles was almost 50% greater (P< 0.05; Figure 3A and B).
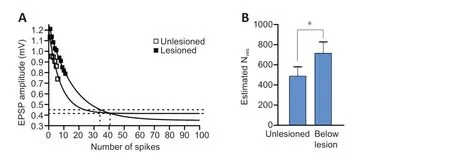
Figure 3 |Estimate of the number of vesicles (NVes) at connections in unlesioned spinal cords and connections below the lesion site in lamprey.(A) Graph showing the extrapolated exponential decay of the excitatory postsynaptic potential (EPSP) in unlesioned (n = 20 connections) and below lesion spinal cord (n = 10 connection), used to derive parameters to estimate NVes (see text for details). (B) Graph showing the NVes in unlesioned and below lesion connections. Data are expressed as the mean ± SEM. *P < 0.05 (independent samples t-test).
Presynaptic or postsynaptic parameters were assessed using the coefficient of variation (CV-2) to compare changes in mean and variance of the initial and 20thEPSPs in spike trains (Brock et al., 2020). The analysis assumes that parallel changes in synaptic amplitude and CV-2reflect presynaptic effects,while synaptic changes without a change in CV-2reflect postsynaptic effects(Faber and Korn, 1991). As the method is sensitive to noise (Brock et al.,2020), only connections with no spontaneous synaptic activity were analyzed.In unlesioned animals (Figure 4A), 3 of 6 facilitating connections had CV-2changes associated with presynaptic effects, 3 with postsynaptic or mixed pre and postsynaptic effects: for depressing connections 17 had CV-2changes consistent with a presynaptic effect, and 11 with postsynaptic/mixed effects.Above the lesion site, 4 of 8 facilitating connections had changes consistent with a presynaptic effect, 4 with mixed or postsynaptic effects (Figure 4B). Below the lesion site (Figure 4C), 4 connections showed CV-2changes consistent with presynaptic effects and 2 with mixed or postsynaptic effects.The proportions of presynaptic and postsynaptic/mixed-effects did not differ in the unlesioned or lesioned spinal cord (P> 0.05).
As the CV-2method relies on certain conditions and assumptions to identify a pre or postsynaptic locus of change (Faber and Korn, 1991; Brock et al.,2020), release properties were also examined using the variance-mean method (Clements and Silver, 2000). This examines postsynaptic potentials in normal, high, and low calcium Ringer. Unless the release probability is low(< 0.3), the relationship of the EPSP variance and mean in different calcium Ringers results in a parabolic relationship from which release parameters can be estimated (Clements and Silver, 2000 for details):Nmin, the number of release sites is determined from the width of the parabola;p, the probability of release from the curvature; andq, the postsynaptic quantal amplitude from the initial slope (Figure 5Ai).
The analysis again only includes connections that were stable throughout the experiment and in which EPSP amplitudes could be measured unequivocally(i.e. without contamination by an electrical component to the EPSP or spontaneous synaptic inputs). This was a significant issue, as the lateral tract axons are relatively small and recordings stable enough to allow the effects of the different calcium Ringer solutions to be examined were not common, and low calcium Ringer often increased background synaptic inputs, presumably through a reduction of surface screening (Piccolino and Pignatelli, 1996). This meant that thennumber of fully analyzed connections is small (this is not uncommon for variance-mean analyses; see for example, Mitchell and Silver,2000; Kazama and Wilson, 2008; Lawrence et al., 2015; Malagon et al., 2016).In regenerated axons that evoked depression below the lesion site,Nminwas generally reduced (n= 3; Figure 5Bi), consistent with the sparser anatomical connectivity reported for medial column axons (Oliphint et al., 2010), butpwas unchanged compared to synapses in unlesioned cords (n= 3; Figure 5Bii), an effect consistent with the similar activity-dependent depression over spike trains (Figure 1F and G). However,qwas increased (n= 3; Figure 5Biii),suggesting a potential change in postsynaptic response to the transmitter.Analyses of facilitating connections above the lesion site consistently showed a linear rather than parabolic relationship between the variance and mean(n= 4; Figure 5Ai). This is indicative of a low release probability (< 0.3;Clements and Silver, 2000), and is consistent with the higher PP ratio and the development of facilitation above the lesion site (Figure 1D). The absence of the parabolic relationship prevented estimation ofNminandp, but the slope of the relationship was not increased compared to unlesioned animals,suggesting against a change in q above the lesion site (Clements and Silver,2000).

Figure 4 |The analysis of the plasticity over the spike train (EPSP20/EPSP1) and the inverse of the coefficient of variation (CV-220/CV-21) for unlesioned (A), above lesion (B),and below lesion connections (C).Note that the x-axis shows the plasticity over the spike train and the y-axis the change in the coefficient of variation. A presynaptic change in plasticity is indicated by values falling either on or below (for depression) or above (for facilitation) the diagonal line,a postsynaptic change when values fall on the horizontal dashed line (i.e. no change in the CV-220/CV-21 despite a change in the EPSP), and both presynaptic and postsynaptic changes for depression and facilitation when values fall above and below the diagonal line, respectively (see Faber and Korn, 1991). Data are expressed as the mean ± SEM.CV-2: Coefficient of variation; EPSP: excitatory postsynaptic potential.
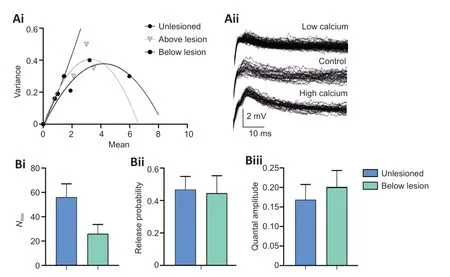
Figure 5 |Analysis of synaptic release properties.A variance-mean analysis of connections in unlesioned spinal cords, and above and below the lesion site (Ai, see text for details). (Aii) Sample traces in an unlesioned spinal cord in normal and low and high calcium Ringer. (Bi-Biii) The estimated number of release sites (Nmin; Bi), release probability (Bii), and quantal amplitude (Biii) of unlesioned(n = 3) and below lesion (n = 3). Data are expressed as the mean ± SEM.
Influence of degree of recovery
The analysis above only considered properties in animals that recovered well. Poorly recovered lampreys show an altered relationship between regenerated inputs and spinal cord excitability compared to animals that recover well: in good recovery, there is a positive relationship between the amount of regeneration and the sub-lesion excitability, but in poor recovery there is a negative correlation (Hoffman and Parker, 2011), suggesting that the interaction of regenerated synapses and their spinal cord targets,rather than just the presence of regeneration, is important to recovery. An obvious consideration in determining the functional relevance of the synaptic properties examined here is to examine the properties of regenerated synapses in animals that recovered poorly. However, while this has been done for other aspects after lesioning in lamprey (Hoffman and Parker, 2011;Becker and Parker, 2015), this was difficult in this analysis as poor recovery tends to be associated with the absence of regeneration, which limited the sample size of paired recordings. However, synaptic properties were examined in animals that recovered poorly despite the presence of regeneration. The number of these animals, and the sample size of connections, is low and thus the data is preliminary. Compared to the amplitude of connections in animals that recovered well (Figure 1A), connections below the lesion site had smaller amplitudes than connections in animals that showed good recovery (n= 4;Figure 6A), usually depressed to a greater extent, and uncharacteristically for these synapses tended to exhibit failures. Above the lesion site (n=7), connections again showed facilitation albeit from smaller initial EPSP amplitudes than connections that recovered well (Figure 6A and B).

Figure 6 |Differences in synaptic properties in animals that recovered well or poorly in lamprey.(A) The excitatory postsynaptic potential (EPSP) amplitude below the lesion site in good and poor recovery (n = 4). (B) The activity-dependent plasticity of connections above and below the lesion site in animals that recovered well or poorly. Data are expressed as the mean ± SEM.
Discussion
This analysis has examined the properties of regenerated axons in the lamprey. Synaptic inputs from lateral column reticulospinal axons below the lesion site matched those from unlesioned axons in terms of their amplitude and activity-dependent plasticity. However, despite the same output being generated, release properties differed. In contrast, connections made by axons above the lesion site differed from those in the unlesioned spinal cord as they showed facilitation that developed from the same initial EPSP amplitude and would thus be functionally stronger.
Regeneration remains the dominant approach in spinal cord injury research.Analyses focus on the anatomical properties of regenerated axons (e.g. their number, location, and how far they regenerate below the lesion site), and the correlation of regeneration with recovery. However, it is not enough just for axons to regenerate: functional recovery requires that they make appropriate connections with targets below the lesion site. Differences in the release properties that determine the features of these connections could thus contribute to the variable influence of regeneration on recovery seen in experimental model systems like the lamprey (Parker, 2017) and clinical trials of regeneration (Steward et al., 2012).
It is difficult to examine synaptic properties directly to compare effects in unlesioned and lesioned spinal cords. A range of approaches was used here,including direct measurements of basic synaptic properties, the estimation of vesicle numbers, comparison of presynapticversuspostsynaptic mechanisms(CV-2), and comparison of release properties (variance-mean analysis).While these are commonly used approaches, they have limitations and determining the mechanisms of release at central synapses is still difficult(Lanore and Silver, 2016; Pulido and Marty, 2017). Changes in postsynaptic response can be assessed simply and directly by comparing the response to exogenously applied glutamate in functionally isolated cells (e.g. in the presence of TTX; Parker et al., 1997), but this only works for comparisons of the same cell under different conditions (e.g. before and after application of a neuromodulator), not for comparing effects in different spinal cords.The variance-mean approach makes fewer assumptions than traditional quantal mechanisms (Clements and Silver, 2000), including the CV-2analysis(Faber and Korn, 1991), and has been applied at a range of central synapses(Lanore and Silver, 2016). However, the analysis needs relatively long-term stable recordings from relatively small presynaptic axons to allow the changes in Ringer calcium levels and evoked EPSPs that are not contaminated by spontaneous background inputs, requirements that limit the sample size.However, the results of the various analyses tended to be consistent with each other, providing support for the general conclusions.
The same amplitude and activity-dependent properties of regenerated axon inputs to motor neurons below the lesion site as unlesioned animals suggest against an increase in the synaptic strength of individual axons to compensate for the overall reduction of the descending input after recovery from a spinal cord lesion (fully recovered animals show at least a 30% reduction in the number of axons; Cohen et al., 1988; McClellan, 1990). However, the same synaptic effect is evoked by different synaptic mechanisms. The different relationship between the initial EPSP amplitude and the paired-pulse ratio in below lesion synapses suggests a change in presynaptic release (that larger initial EPSPs evoked greater depression may reflect a larger quantal content with a concomitant increase in vesicle depletion and depression or greater postsynaptic desensitization; Zucker and Regehr, 2002), while the variancemean analysis suggests an increase in the postsynaptic response (quantal amplitude,q) below the lesion site, an effect that is consistent with previously identified sub-lesion functional changes (Parker, 2017). This effect could scale up regenerated inputs to allow them to match the properties of unlesioned synapses despite the reduced number of release sites suggested by the reducedNminfrom the variance-mean analysis, and the potential for a reduced number of synaptic contacts from morphological analyses of regenerated Müller axons below the lesion site (Oliphint et al., 2010). The increase in the estimated vesicle numbers below the lesion site could also help to strengthen each contact. The combination of these presynaptic and postsynaptic mechanisms below the lesion site may account for the lack of a difference in the proportions of putative presynaptic and postsynaptic in the CV-2 analysis.Why should different release properties be used to generate the same synaptic output below the lesion site? The maintenance of unlesioned properties could obviously reflect the need for inputs of a certain magnitude to effectively activate sub-lesion networks. The difficulties of recapitulating the original development of these axons and their synaptic contacts in a mature nervous system may account for the smaller and limited number of synaptic contacts below the lesion site (Oliphint et al., 2010). Each contact made by a single axon could be made stronger by increasing the transmitter release probability, but unless transmitter replenishment was also more efficient this could lead to a release probability-dependent increase in depression that would weaken inputs over spike trains (Zucker and Regehr, 2002). Instead of strengthening the connection, an increase in release probability alone would thus redistribute the input over the spike train, from being initially stronger to being subsequently weaker than unlesioned inputs, depending on how many inputs were evoked. Maintaining high levels of release and the associated need for increased transmitter clearance and replenishment would also invoke a significant energy cost (Howarth et al., 2012), which may be an issue for axons that are still regenerating and given the compromised state of the lesioned spinal cord. The scaling of the synapse through a postsynaptic increase in quantal amplitude and presynaptic increase in vesicle numbers suggested here could maintain the pre-lesion synaptic amplitude at the unlesioned value over spike trains despite each axon potentially making fewer synaptic contacts, without the energetic costs of upregulating transmitter release and replenishment mechanisms. The various changes in cellular and synaptic properties seen below the lesion site in various model systems from lamprey to mammals could make the spinal cord below the lesion site more excitable (D’Amico et al., 2014; Parker, 2017). These postsynaptic changes may better compensate for the reduced descending input below lesion sites than a presynaptic modification of the regenerated axons, as postsynaptic cells will be able to modify their response to synaptic inputs depending on the integrated descending input that they receive (Parker and Grillner, 2000).
The variance-mean analysis and paired-pulse ratio suggest that the release probability is reduced at synapses between lateral tract axons and motor neurons above the lesion site; this is consistent with the increased PP ratio and facilitation over spike trains in this region. However, the reduction of release probability was not associated with a significant reduction of the initial EPSP amplitude in the spike trains, which would be expected of a reduction in release probability alone, suggesting that other factors are also altered.This could reflect a concomitant increase inq(see Bevan and Parker, 2004 for an example), but the variance-mean and CV-2analyses suggest against this. It could alternatively reflect an increase in vesicle numbers (Bevan and Parker, 2004): this will need anatomical analyses as the method used here for estimating vesicle numbers is based on the rate of depression and thus cannot be applied to facilitating connections. Irrespective of the mechanism,facilitation without a significant reduction of the initial EPSP amplitude would make the summed input above the lesion site functionally stronger than connections in the unlesioned spinal cord or below the lesion site, a compensation that may allow stronger propriospinal activity across the lesion site (Courtine et al., 2008).
Regeneration is never complete in lamprey, varying between 0-70% of the unlesioned value (Cohen et al., 1988; McClellan, 1994). That the same functional output can be generated despite a reduction in descending input either suggests that some of the descending input is redundant, which seems unlikely given the resources that would have to be dedicated to developing and maintaining these connections, or that there is some compensation for the reduction. Each regenerated axon could have increased its postsynaptic effect in proportion to the reduction of the total descending input to maintain the same overall sub-lesion synaptic input, but functionally stronger connections occurred above, not below the lesion site where a compensatory increase would be expected. However, increasing the amplitude of regenerated inputs below the lesion site to maintain the total descending synaptic at the unlesioned value would not necessarily be an effective compensation as it would not reflect the specific functional roles of inputs to different spinal cord regions. If only 50% of the axons regenerated, doubling the amplitude of each would lead to the same summed descending input, but doubling inputs to some regions would not necessarily compensate for zero inputs to other regions.
While regeneration remains a major focus of spinal injury research and overcoming the inhibition of regeneration in mammals has been a great success, regeneration alone will not be enough for the recovery of function after spinal cord injury. The functional efficacy of regenerated inputs is not only dependent on the properties of the synaptic connections they make but also on the properties of the neurons and circuits that they connect to(Ullström et al., 1999). There are changes in these properties after spinal cord injury in systems from lampreys to humans (Grasso et al., 2004; Parker, 2017).Understanding the functional consequences of regeneration and to improving function after spinal cord injury will require a focus on how regenerated interact with altered spinal cord properties above and below the lesion site.
Limitations
The analysis of release properties is difficult to determine, and the measures used here are subject to several caveats. A number of different approaches have been used, and while none is ideal, the results from these analyses generally support each other. In addition, the analysis only considers properties at one-time point after the lesion, and while animals typically recover locomotor function at this time, other properties show changes at later times, which suggests that the effects seen here are not necessarily the final changes.
Conclusion
Regenerated synapses below lesion sites in the lamprey show changes in their release properties. These are presumably important to the successful recovery of locomotor function after lesioning. In addition, synapses above the lesion site also show functional changes, which may be a compensation for the reduced descending input below the lesion site.
Author contributions:Study design, experiment implementation, data analysis, and manuscript writing: DP. The author read and approved the final version of the manuscript.
Conflicts of interest:The author declares no conflict of interest.
Author statement:This paper has been posted as a preprint on bioRxiv with doi: 10.1101/2021.06.21.449247, which is available from: https://www.biorxiv.org/content/10.1101/2021.06.21.449247v1.full.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?