Degeneration of retinal ganglion cells in hypoxic responses: hypoxiainducible factor inhibition, a new therapeutic insight
Deokho Lee, Hiromitsu Kunimi, Kazuno Negishi, Toshihide Kurihara
Degeneration of retinal ganglion cells(RGCs) is one of the hallmarks of common optic neuropathies (Weinreb et al., 2014).Glaucoma, the most common optic neuropathy, is characterized by degeneration of RGCs. Acute angle-closure glaucoma is a serious ocular condition caused by a rapid increase in intraocular pressure(IOP) (Emanuel et al., 2014). One of the experimental models which could mimic this condition is a murine model of retinal ischemia/reperfusion (I/R) injury (Johnson and Tomarev, 2010). Retinal I/R injury can induce a rapid and transient elevation of IOP, which contributes to the degeneration of RGCs. Although understanding the pathophysiology of the degeneration of RGCs was considerably attempted, the major contributing pathways have not been yet elucidated (Calkins and Horner, 2012).
Over the past few years, our group has been focusing on the fundamental roles of hypoxia-inducible factor (HIF) in various ocular disorders and diseases such as agerelated macular degeneration, retinopathy of prematurity, diabetic retinopathy, ocular ischemic syndrome, and glaucoma (Lee et al., 2021a). HIF-1α has been known as a master regulator of oxygen homeostasis.Under normoxic conditions, HIF-1α is rapidly degraded by the ubiquitin-proteasome system. Hypoxic conditions stabilize HIF-1α and stabilized HIF-1α goes into the nucleus and works on the upregulation of various hypoxia-response genes, including vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT1), phosphoinositidedependent kinase-1 (PDK1), and BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3). This process seems to resolve the matter of oxygen homeostasis.However, the pathophysiology of various ocular disorders and diseases has been suggested to be involved in this process.
In this regard, we speculated that inhibition of HIF-1α and its target genes could be a promising therapeutic target for the degeneration of RGCs in glaucoma. After developing a murine model of retinal I/R injury by a transient elevation of IOP, we examined whether the administration of topotecan, a topoisomerase inhibitor/a potent HIF-1α inhibitor, could exert neuroprotective effects against retinal I/R injury (Kunimi et al., 2019a). Increases in HIF-1α expression were seen 6-24 hours after retinal I/R injury. Furthermore, increases in HIF-1α target gene expressions such asVegfa,Glut1, andPdk1were detected. In this system, the administration of topotecan showed the suppression in increased HIF-1α expression and its target gene expressions.Decreases in total retinal thickness, the number of Fluoro-Gold retrograde labeled-RGCs, and amplitudes of a-wave, b-wave,and visually evoked potentials were seen 7 days after retinal I/R injury (Kunimi et al.,2019a). These pathological outcomes were lessened by the administration of topotecan.With this together, we could find a tiny clue that pharmacological HIF inhibition may be involved in neuroprotection against degeneration of RGCs.
Next, we tried to find novel HIF inhibitors from natural plant and food extracts in terms of drug safety and accessibility (Kunimi et al., 2019b). We developed a dual-luciferase assay screening system for HIF activation and screened 238 natural plant and food extracts for HIF inhibition in NIH/3T3 (a murine fibroblast cell line), ARPE-19 (a human retinal pigment epithelial cell line), and 661W (a murine retinal ganglion precursorlike cell line) cells under a CoCl2(cobalt(II) chloride hexahydrate) pseudo-hypoxic oxidative stress condition and/or 1% O2hypoxic condition. After rigorous screening,we found that hydrangea extracts could have a potential to inhibit HIF activation.Furthermore, we found that halofuginone,a synthetic derivative of febrifugine (a naturally occurring alkaloid found in the root of hydrangea plants) could have strong inhibitory effects of HIF activation in all cell types (NIH/3T3, ARPE-19, and 661W) (Kunimi et al., 2019b). The treatment of halofuginone showed the suppression in increased HIF-1α expression under a CoCl2pseudo-hypoxic oxidative stress condition and 1% O2hypoxic condition in ARPE-19 and 661W cells. In the same murine model of retinal I/R injury above, we found that the administration of halofuginone showed the suppression in increased HIF-1α expression and its target gene expressions. Reduction in total retinal thickness as well as inner retinal thickness by retinal I/R injury was suppressed by the administration of halofuginone (Kunimi et al., 2019b). Furthermore, decreases in the number of Fluoro-Gold retrograde labeled-RGCs, and amplitudes of a-wave,b-wave, and visually evoked potentials were lessened by the administration of halofuginone. Taken together, we could confirm that pharmacological HIF inhibition(topotecan and/or halofuginone) could be beneficial for inner retinal protection against retinal I/R injury. However, we still need further investigations on this notion in that the pharmacological strategy may have a possibility on off-target effects of drugs themselves.
To strengthen our notion further, we recently developed sensory retina-specificHif-1αconditional knockout (cKO) mice and started to genetically verify the pathological role of HIF in inner retinal degeneration by retinal I/R injury (Kunimi et al., 2021). Transgenic mice which express Cre recombinase with the Chx10 promoter (Chx10-Cre mice)were mated withHif-1αflox/floxmice in order to develop sensory retina-specificHif-1αcKO mice. Similar to our previous results of pharmacological inhibition, increases in HIF-1α expression and its target gene expressions by retinal I/R injury were suppressed in theHif-1αcKO retina. Inner retinal thinning and reductions in the number of Fluoro-Gold retrograde labeled-RGCs and amplitudes of visually evoked potentials were also suppressed in theHif-1αcKO retina. Taken together, we strongly ensure that HIF inhibition could be one of the possible therapeutic strategies on degeneration of RGCs in glaucoma. However,at the same time, we could not overlook the fundamental role of HIF in retinal function and homeostasis, as HIF is a strong regulator of various downstream genes associated with angiogenesis, cell proliferation/survival, and metabolism (Lee et al., 2021a). In this regard,we attempted to elucidate the specific HIF target gene candidates responsible for the onset of inner retinal degeneration. A lasercapture microdissection technique was applied to obtain the inner retina from the whole retina, and a quantitative polymerase chain reaction array was performed in the inner retina to find specific HIF target gene candidates involved in the onset of inner retinal degeneration (Kunimi et al., 2021).As the expression ofBnip3(a pro-apoptotic gene under hypoxia) was found to be highly induced by retinal I/R injury, we designed theBnip3knock-out (KO) strategy in the inner retina using the AAV2-CRISPR/Cas9 system. Inner retinal gene editing via AAV2 intraocular injection was well-developed andBnip3KO suppressed the pathological outcomes in the inner retina against retinal I/R injury. To explain the therapeutic effectsin vivo, we genetically madeHif-1αandBnip3KO cell lines from 661W cells and found cell death was ameliorated in those KO cells under a CoCl2pseudo-hypoxic oxidative stress condition and 1% O2hypoxic condition.Furthermore, a relationship on the HIF-1α/BNIP3 axis was strongly confirmed under the same conditions. Taken together, our stacking data have supported the notion that the HIF-1α or HIF-1α/BNIP3 pathway may have a pathological role in the inner retina under retinal I/R injury (Figure 1) (Kunimi et al., 2019a, b, 2021). We ensure that this notion can be linked with the development of new drugs for the degeneration of RGCs which can be referred to as one type of glaucoma.
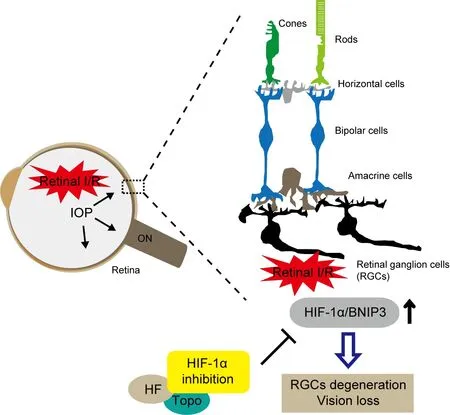
Figure 1 |A schematic of therapeutic approaches on pharmacological and genetic inhibition of HIF-1α/BNIP3 in amurine model of retinal I/R injury.Retinal I/R injury induces a transient abnormal elevation of IOP in the eye. In the retina, the HIF-1α/BNIP3 pathway is activated to cause degeneration of RGCs, which leads to vision loss. Pharmacological (halofuginone and topotecan)and genetic inhibition of HIF-1α/BNIP3 can suppress these pathological outcomes against retinal I/R injury. HF:Halofuginone; HIF: hypoxia-inducible factor; I/R: ischemia/reperfusion; IOP: intraocular pressure; ON: optic nerve; RGCs:retinal ganglion cells; Topo: topotecan.
Limitations and future directions: Our inhibition strategy was only tested in a murine model of retinal I/R injury by a transient elevation of IOP. There are other experimental models for optic neuropathies including murine models of optic nerve crush injury (Tang et al., 2011), N-methyl-Daspartate excitotoxicity (Christensen et al.,2019), and carotid artery occlusion (Lee et al., 2021b). Pharmacological and/or genetic inhibition of the HIF-1α/BNIP3 pathway in those models is desirable to generalize our notion more clearly. In fact, we shortly found that induction of the HIF-1α/BNIP3 pathway was suppressed by the consecutive administrations of fenofibrate in a murine model of acute retinal ischemia by carotid artery occlusion, and inner retinal functional protection was followed after that event (Lee et al., 2021c). Next, further experiments are highly needed to determine which stages of degeneration of RGCs could be effectively applied by our inhibition strategy. So far,we pre-treated the drugs to maximize the therapeutic effects when retinal I/R injury was induced (Kunimi et al., 2019a, b, 2021).Thus, post-treatment of the drugs should be tested to understand the intervention of the pathological process in the inner retina more clearly. Under HIF-1α target genes, BNIP3 may not be the only one that affects the onset of inner retinal degeneration based on our quantitative polymerase chain reaction array (Kunimi et al., 2021). Minor changes in several gene expressions such asGlut1andLgals3in the inner retina after retinal I/R injury should be taken into account for the future direction of our findings.
The present work was supported by Alcon Japan Research Grant (to TK). Furthermore, it was supported by Grants-in-Aid for Scientific Research (KAKENHI, number 18K09424 to TK, and 20K22692 to HK) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). At last, it was supported by Bayer Academic Support (to HK).
Deokho Lee, Hiromitsu Kunimi,
Kazuno Negishi, Toshihide Kurihara*
Department of Ophthalmology, Keio University School of Medicine, Shinanomachi, Shinjuku-ku,Japan
*Correspondence to: Toshihide Kurihara, MD,PhD, kurihara@z8.keio.jp.https://orcid.org/0000-0002-5457-2720(Toshihide Kurihara)
Date of submission: July 19, 2021
Date of decision: September 23, 2021
Date of acceptance: October 27, 2021
Date of web publication: February 28, 2022
https://doi.org/10.4103/1673-5374.335801
How to cite this article:Lee D, Kunimi H, Negishi K,Kurihara T (2022) Degeneration of retinal ganglion cells in hypoxic responses: hypoxia-inducible factor inhibition, a new therapeutic insight. Neural Regen Res 17(10):2230-2231.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?

