Novel insights into the pathogenesis of tendon injury:mechanotransduction and neuroplasticity
Suellen Alessandra Soares de Moraes
Tendon pathology is characterized by damage to the tendon structural integrity with disruption of collagen fibers (Nourissat et al., 2015). Acute tendon injuries show a macroscopic discontinuity, ranging from partial to complete tendon rupture. They involve inflammation and lead to loss of motion.In chronic conditions (or tendinopathy),symptoms include changes in both locomotor and sensorial functions of the tendon (Nourissat et al., 2015; Scott et al., 2020). Inconsistency in terminology for cases of painful tendon disorders is found, but recently the term tendinopathy was established in consensus as preferable for cases with persistent tendon pain and loss of function related to mechanical loading. This term excludes a problem in clinical practice - i.e., specification of the presence of a particular pathological or biochemical process(Scott et al., 2020).
A healthy tendon is composed almost entirely of connective tissue, with a predominance of type I collagen organized in fibers arranged parallel to the muscle-tendon axis, and this organization is fundamental for the transmission of force and production of movement. The main cell type in tendons is the tenocyte, which is a fibroblast-like cell found in low density (Freedman et al., 2014; Nourissat et al., 2015).
After injury, tendon repair process involves successive and overlapping phases of inflammation, with cell migration, cell proliferation, and remodeling. Matrix metalloproteinases and cytokines such as vascular endothelial growth factor,epidermal growth factor, platelet-derived growth factor, and transforming growth factor β are overexpressed. The increase of vascular endothelial growth factor promotes angiogenesis and disturbs the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases. These events result in a healed tendon without the same chemical,mechanical and functional properties of a native uninjured tendon (van Sterkenburg and van Dijk, 2011; Freedman et al., 2014; Nourissat et al., 2015).
With the increase of new vessels, cell densities change completely by inflammatory cells migrating to tissue and by a new cell type arising in the tissue, the tendon stem cell(TSC). Consequently, the extracellular matrix(ECM) changes its composition to collagen type III, with fibril disorganization, increased proteoglycan and glycosaminoglycan content and increased noncollagenous ECM (Freedman et al., 2014). This disorganization in the ECM results in a key change in the location of nerves,which emit new branches into the mesh of ECM(Ackermann et al., 2003; Mendes et al., 2021).
The molecular and neural mechanisms controlling proliferation, migration, and differentiation of TSCs as well as pain and locomotor impairment are not well understood,but new insights have shown the relevant but yet ignored role of mechanotransduction and neuroplasticity.
Tendon is a mechanosensitive tissue that has specific mechanical properties able to respond and adapt to loads transmitted by muscles during mild or vigorous muscle contraction. Mechanical forces are perceived by tenocytes and TSCs as stimuli through diverse intermediaries and converted to biochemical signals, finally promoting a cellular response. In the tenocytes, mechanotransduction controls the shape and function of these cells and their surrounding ECM. Change in tension perceived by cells in a specific location can be transmitted to cells in other regions through signaling via connexins, integrins, G protein-coupled receptors, and growth factor receptor (Wang et al., 2020). In healthy tendons, the main response to load is an upregulation of insulinlike growth factor-I, which is associated with cellular proliferation and matrix remodeling(Nourissat et al., 2015; Wang et al., 2020).
Mechanical loading can influence both proliferation and differentiation of TSCs. Under specific conditions of molecular and load microenvironment, TSCs have the potential to differentiate into tenocytes, chondrocytes,osteocytes, and adipocytes. Would the nervous system play a role in this differentiation as well?Divergences in the level of tissue tension are detected by these signaling systems and affect the function and original characteristics of thetissue, resulting in definitive failures in healing(Nourissat et al., 2015; Wang et al., 2020).Thus, as in inflammation, mechanotransduction also has its own signaling, but we still need to understand how mechanical forces are converted to molecular, cellular, and functional processes.
Answering the previous question on the role played by the nervous system in TSC differentiation: as stem cells, TSCs are responsive not only to the mechanical stimulus,but also present the capacity to express many kinds of receptors and respond to substances like neurotransmitters, including glutamatergic and endocannabinoid systems.
Glutamate is classically an excitatory neurotransmitter of the central nervous system, but now we know this molecule is relevant to the comprehension of pain and metabolism in many tissues. An increase in levels of this molecule was found in painful tendons with chronic degeneration. In the literature, evidence support that tenocytes and TSCs can synthesize glutamate as well as express glutamate receptors, transporters like N-methyl-D-aspartate R1, metabotropic glutamate receptor 4, metabotropic glutamate receptor 7, and vesicular glutamate transporter 2, which promote effects that include cell death and modulation of protein synthesis. Although studies have shown that the use of N-methyl-Daspartate antagonists can reduce pain elicited by glutamate, the mechanisms that regulate this process are still little known in the tendon,but the involvement of the glutaminergic system is clear (Franklin et al., 2014; Spang et al., 2017).
The cannabinoid system is effective for the relief of a variety of pain types, and, in tendon injury, chronic pain is very common and poses a challenge to establish a satisfactory treatment.Interestingly, a recent paper indicates the expression of cannabinoid receptor 1 in cells of healthy or injured Achilles tendon, showing overexpression in the tendinosis group when compared with the healthy group. Despite this, the function of cannabinoid receptor 1 in tenocytes is still uncertain (Björklund et al.,2011).
I n a d d i t i o n t o t h e u n e x p e c t e d mechanotransduction and signaling, other variable to be considered is neuroplasticity.Knowledge on the involvement of the nervous system in tendon repair is recent, with identification of new sensory and sympathetic nerve fibers growing from the paratenon to the proper tendon along with the neovessels and with the release of regulatory substances which acts on cell proliferation, angiogenesis,and nociception (Ackermann et al., 2003).Sensory neuropeptides as substance P,calcitonin gene-related peptide and galanin are already presented in several studies related to nociception. In this regard, substance P, and calcitonin gene-related peptide levels were closely related to the thermal sensitivity while mechanical sensitivity seems related to the rate of change of galanin in a model of tendon rupture (Ackermann et al., 2003).
The growth of new nerves into the disorganized collagen mesh favors the consolidation of tissue architecture that is unfavorable to the original function of the tendon to receive and transmit high levels of tension, since sensory neuropeptides start to contribute permanently to the tissue dynamics - a process called neurogenic inflammation (van Sterkenburg and van Dijk, 2011).
Recognition of peripheral neural plasticity generated new questions, leading to the identification of the participation of other nervous system levels and neurotransmitters in the control of tendon repair. A recent study developed by our group showed that, at the spinal cord level, neural plasticity also occurs after tendon damage. We have analyzed whether glial reactivity in the spinal cord corresponding to innervation of the injured Achilles tendon would occur, since such a reactivity had already been described after both peripheral nerve and articular injury.Furthermore, we have also investigated whether there would be a relationship between mechanical sensitivity and functional capacity.This was the first description of an association of increased astrocytic reactivity in the spinal cord with increased mechanical sensitivity and impaired locomotion in the acute phase of tendon injury, with only reactivity and sensitivity being re-established in the chronic phase (Mendes et al., 2021).
Understanding the actual roles of glial cells in tendon injury is in progress, especially moved by their recent recognition as important regulators of neuronal functions, either for benefits or harms. The occurrence of central glial reactivity is largely known in models of central and peripheral neural injury, and it is characterized by morphological, physiological and molecular changes in glial cells. Reactive astrocytes are classified into A1 and A2 types,developing antagonist functions. A1 astrocytes secrete neurotoxins involved in rapid induction of death of neurons and oligodendrocytes,while A2 astrocytes promote neuronal survival and tissue repair (Li et al., 2019). In non-neural injuries, the occurrence of glial reactivity in the spinal cord is strongly related to sensory alterations and can even influence the synthesis and release of neuromodulators directly in thetissue. In the case of the tendon, this can occur through the very nervous branches present in the original tissue of the lesion, while the above-mentioned receptors to neuropeptides and neurotransmitters are expressed by the tenocyte itself (Li et al., 2019; Mendes, 2021).The contribution of the nervous system to tendon modulation may help explain why, in the condition of tendinous denervation, there is a reduction in the load to failure for the injured tendon and histological changes compatible with tendinopathy in healthy denervated tendons (El-Habta et al., 2018). Additionally, it may elucidate why relaxing the muscle - and consequently reducing tissue tension - by the use of botulinic toxin reduces neuropathic pain (Egeo et al., 2020). Would there be some relationship between glial reactivity, pain,and mechanotransduction processes? Figure
1 shows an overview of these components involved.
Although there are many gaps in the specific mechanisms underlying tendon repair, the primary clinical goal of conservative tendon injury treatment is to reduce pain, mainly by using anti-inflammatory drugs. However, the use of drugs in conservative approaches does not generate resolute results. Another form of clinical intervention comprises reconstructive surgery, which is normally combined with the use of drugs. Independently of clinical management of tendon injury, the weakened tendon tissue is subjected to re-rupture and thus still constitutes a challenge for the recovery process (Freedman et al., 2014; Nourissat et al.,2015).
Therapeutic exercises are also among the conservative strategies, especially the protocols based on eccentric strength training and combination of eccentric and concentric contractions, which have shown beneficial results for pain reduction, improvement in functionality, and improvement in structural aspects (Gärdin et al., 2010; Stasinopoulos et al., 2013; Beyer et al., 2015). The benefits of this type of intervention are possible because the mechanical tension generated and transmitted with controlled exercises cause physical disturbance in the cells that constitute the tissue and trigger responses that promote structural changes, which can lead to tissue repair and remodeling (Martino et al., 2018).
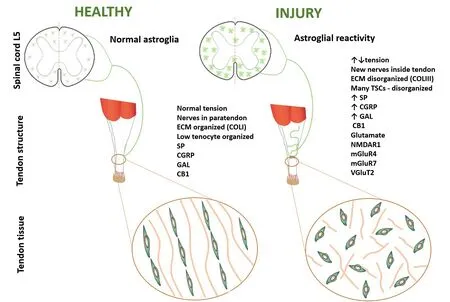
Figure 1 |An overview of the involvement of glial reactivity, mechanotransduction, and substances related to painin the pathogenesis of tendon injury.CB1: Cannabinoid receptor 1; CGRP: calcitonin gene-related peptide; COLI: type I collagen; COLIII: type III collagen;ECM: extracellular matrix; GAL: galanin; mGluR4: metabotropic glutamate receptor 4; mGluR7: metabotropic glutamate receptor 7; NMDAR1: N-methyl-D-aspartate receptor 1; SP: substance P; TSC: tendon stem cell; vGluT2: vesicular glutamate transporter 2.
Finally, even though the inflammatory process resulting from tendon injury has a great clinical impact, the new evidence presented above indicate that mechanotransduction and neural plasticity are relevantly involved in tissue repair and should also be considered during patient management.
The present work was supported by a grant from Pró-Reitora de Pesquisa e Pós-Graduação/UFPA to SASM.
Suellen Alessandra Soares de Moraes*Faculdade de Fisioterapia e Terapia Ocupacional,Universidade Federal do Pará, Belém, Pará, Brazil
*Correspondence to:Suellen Alessandra Soares de Moraes, PhD,sualessandra@yahoo.com.br.https://orcid.org/0000-0001-8616-6885(Suellen Alessandra Soares de Moraes)
Date of submission: August 14, 2021
Date of decision: September 27, 2021
Date of acceptance: November 5, 2021
Date of web publication: February 28, 2022
https://doi.org/10.4103/1673-5374.335802
How to cite this article:de Moraes SAS (2022)Novel insights into the pathogenesis of tendon injury: mechanotransduction and neuroplasticity.Neural Regen Res 17(10):2223-2224.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?

