The integrative role of G proteincoupled receptor heterocomplexes in Parkinson’s disease
Dasiel O. Borroto-Escuela, Kjell Fuxe
In the 1950s to 1970s, the research on Parkinson’s disease (PD) and its treatment had mainly been focused on the Nigrostriatal dopamine (DA) neurons as the major site of degeneration in this disease.It contributed to the search for drugs that restored DA transmission in this pathway and contributed to the introduction of L-DOPA and DA receptor agonists in its treatment (Borroto-Escuela and Fuxe,2019).
An exciting development in the 1990s was the neuroprotective gene therapy for PD involving e.g., the use of lentivirus vectors.They were used for gene transfer of neurotrophic factors to the nigral-striatal DA neurons (Bjorklund et al., 2000).
In the period 1980 to 1993, the concept was introduced on the existence of allosteric receptor-receptor interactions i n G p ro te i n-co u p l e d re c e p to rs heteroreceptor complexes in the plasma membrane can represent an important molecular integrative mechanism (Fuxe and Borroto-Escuela, 2018). The agonist activation of one receptor protomer can induce an allosteric wave that can pass over the receptor interface to the other receptor protomer and modulate its recognition, signaling and trafficking as well as the density and composition of the heterodimer or high order heteroreceptor complex (Fuxe and Borroto-Escuela, 2018).The evidence was obtained mainly through use of biochemical and biophysical methods like e.g., coimmunoprecipitation,FRET and BRET, and proximity ligation assay (Fuxe and Borroto-Escuela, 2018).The allosteric mechanisms involved were complementary to the phosphorylation and dephosphorylation of receptors involving kinases and phosphatases,respectively.
The works of Lai et al. (2019), Flajolet et al. (2008) and Borroto-Escuela et al. (2012,2016, 2017) demonstrated that G proteincoupled receptor can form heteroreceptor complexes also with ionotropic receptors and with RTK receptors.
Perhaps the most interesting receptor complexes found in relation to PD are those found in the dorsal striatal-pallidal γ-aminobutyric acid (GABA) neurons, the first part of the indirect pathway mediating motor inhibition. Dopmaine D2 receptor(D2R) activation inhibits these GABA neurons and counteracts the inhibition of movements. Here, the adenosine A2A receptor (A2AR)-D2R heteroreceptor complexes plays a relevant role since the activation of the A2AR protomer with an A2AR agonist inhibits the D2R protomer signaling and increases motor inhibition(Fuxe et al., 2015).
The demonstration of the A2AR-D2R heteroreceptor complexes in the dorsal striatal-pallidal GABA neurons that enhance motor inhibition led to the development of A2AR antagonists like istradefylline, now introduced in treatment of PD, improving motor function (Kondo et al., 2015; Figure 1). As to future directions for A2AR based treatments in PD, it seems possible that A2AR antagonists can counteract the degeneration in PD based on the potential of A2AR to form complexes with alpha-synuclein.The activation of the A2AR protomer in the alpha-synuclein-A2AR complex can cause an increase in the alpha-synuclein aggregation and toxicity (Figure 1). It can take place through extracellular vesicle mediated volume transmission also involving internalization of such complexes into surrounding DA nerve terminals enhancing their degeneration since they are highly vulnerable to alpha synuclein aggregates (Surmeier et al., 2017).
There also likely exist in this motor inhibition pathway A2AR-mGluR5 heteroreceptor complexes and A2ARD2R-mGluR5 heteroreceptor complexes i n b a l a n c e w i t h t h e A 2 A R-D 2 R heteroreceptor complex (Borroto-Escuela and Fuxe, 2019; Figure 1). Combined activation of the A2AR and mGluR5 protomers enhances the activation of the dorsal-striatal-pallidal GABA neurons mediating motor inhibition involving increased inhibition of the D2R protomer recognition and signaling (Borroto-Escuela and Fuxe, 2019).It should also be noted that the D2R can form a heteroreceptor complex with NMDA receptors and inhibit its function in this pathway (Liu et al., 2006) which can contribute to the ability of the D2R protomer to reduce the activity of this pathway leading to reduced inhibition of movements. A2AR-D2R-NMDAR can also exist (Beggiato et al., 2021) which can further contribute to the ability of A2AR agonists to enhance motor activity by removal of the D2R inhibition of NMDA receptor signaling.
Finally, it is relevant to underline that A2AR-FGFR1 heteroreceptor complexes exist in the dorsal striatal-pallidal GABA neurons (Flajolet et al., 2008).The combined activation of these two protomers enhanced structural plasticity and extracellular signal regulated kinase activity associated with inhibition of firing of these neurons. Thus, this case had overactivity in the FGFR1 protomer. This complex may reduce the inhibitory impact of the A2AR protomer activation on the D2R protomer signaling in the A2AR-D2R heteroreceptor complex in terms of effects on firing of the striatal-pallidal GABA neurons mediating motor inhibition.
These examples serve to illustrate the dynamic integrative impact of multiple heteroreceptor complexes in the modulation of the firing of the dorsal striatal-pallidal GABA neurons of high relevance for understanding the motor dysfunctions in PD.
The authors have no affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
This work was supported by grants from the Swedish Medical Research Council(04X-715) to KF, by Olle Engkvists Stiftelse to KF and DOBE, and from Hjärnfonden(F02019-0296), Karolinska Institutet Forskningsstiftelser and EMERGIA 2020-39318 (Plan Andaluz de Investigación,Desarrollo e Innovación 2020) to DOBE.DOBE belongs to Academia de Biólogos Cubanos.
Dasiel O. Borroto-Escuela*, Kjell Fuxe*Department of Neuroscience, Karolinska Institutet,Biomedicum, Stockholm, Sweden
*Correspondence to: Dasiel O. Borroto-Escuela,PhD, dasiel.borroto.escuela@ki.se; Kjell Fuxe, PhD,kjell.fuxe@ki.se.
https://orcid.org/0000-0002-5736-373X(Dasiel O. Borroto-Escuela)https://orcid.org/0000-0002-5736-373X(Kjell Fuxe)
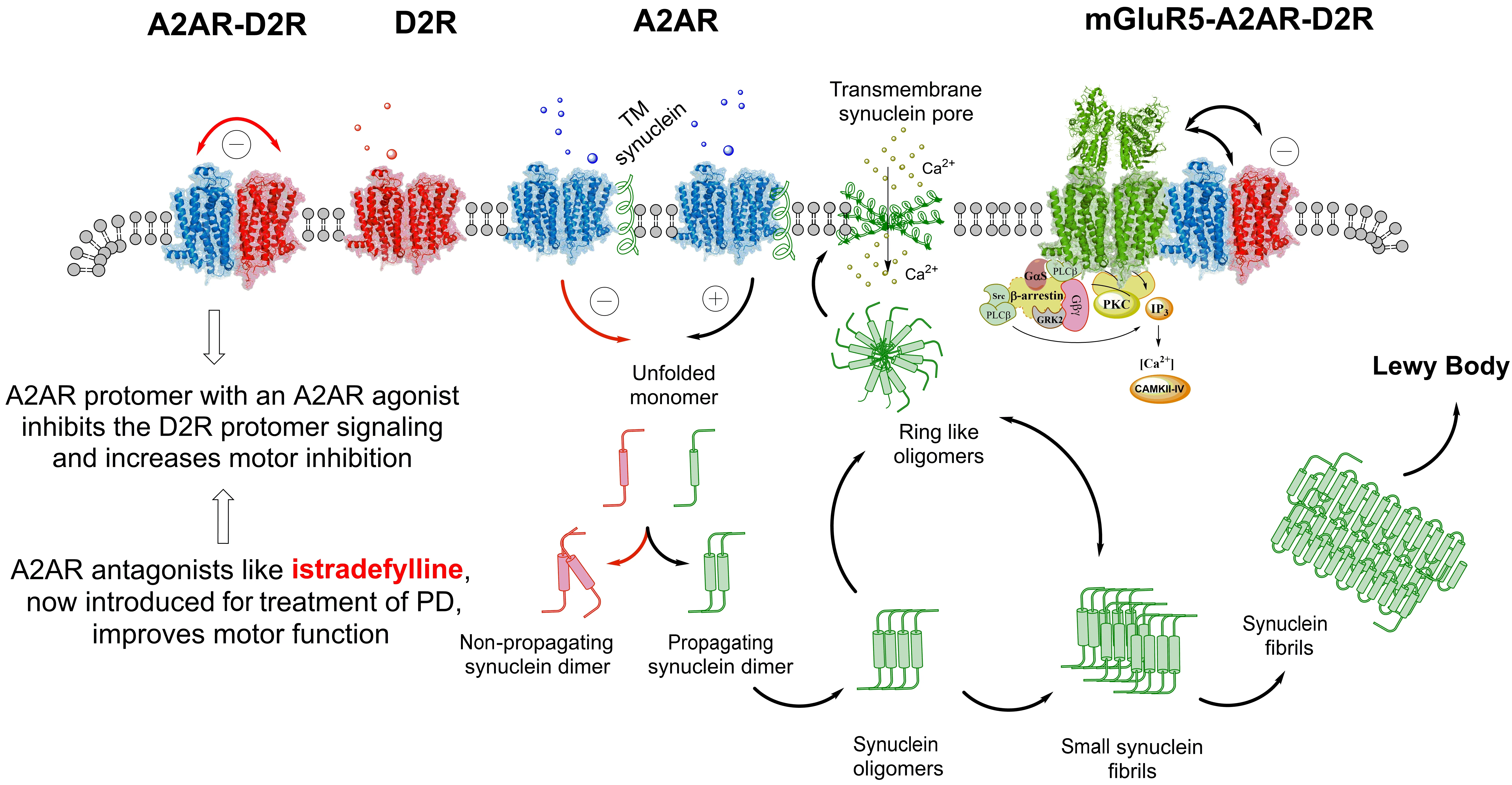
Figure 1 |Schematic diagram of a possible molecular mechanism by which alpha-synuclein monomers/oligomers/ synuclein fibrils can modulate the A2AR homoreceptor complexes and A2AR-D2R, mGluR5-A2AR-D2Rheteroreceptor complexes and their balance in the plasma membrane.It is proposed that monomeric alpha-synuclein TM peptides can become linked to the A2AR homoreceptor complex and modulate the A2AR function. Under the modulation of the monomeric alpha-synuclein peptides the A2AR antagonist(e.g., istradefylline) may favour the formation of non-propagating alpha-synuclein dimers (pathway highlighted in red).Instead, the A2A receptor agonist induced A2AR activation (pathway highlighted in green) may in the alpha-synuclein-A2AR complex produce signals that favour the propagation of alpha-synuclein dimers/oligomers into small and large synuclein aggregates that accumulate in Lewy bodies. Ring-like synuclein oligomers can also be formed, which enter the plasma membrane and there produce beta sheet structures that associate and produce pores through which calcium ions may pass. The A2AR-D2R heteroreceptor complexes plays a relevant role since the activation of the A2AR protomer with an A2AR agonist inhibits the D2R protomer signaling and increases motor inhibition. There also likely exist in this motor inhibition pathway A2AR-mGluR5 heteroreceptor complexes and A2AR-D2R-mGluR5 heteroreceptor complexes in balance with the A2AR-D2R heteroreceptor complexes. A2AR: Adenosine A2A receptor; D2R: dopmaine D2 receptor;PD: Parkinson’s disease; TM: transmembrane.
Date of submission: July 23, 2021
Date of decision: September 3, 2021
Date of acceptance: October 4, 2021
Date of web publication: February 28, 2022
- 中国神经再生研究(英文版)的其它文章
- Functional in vivo assessment of stem cell-secreted prooligodendroglial factors
- iGluR expression in the hippocampal formation, entorhinal cortex,and superior temporal gyrus in Alzheimer’s disease
- Exploiting Caenorhabditis elegans to discover human gut microbiotamediated intervention strategies in protein conformational diseases
- N-methyl-D-aspartate receptor functions altered by neuronal PTP1B activation in Alzheimer’s disease and schizophrenia models
- Aminopeptidase A and dipeptidyl peptidase 4: a pathogenic duo in Alzheimer’s disease?
- Ubiquitin homeostasis disruption,a common cause of proteostasis collapse in amyotrophic lateral sclerosis?

